Abstract
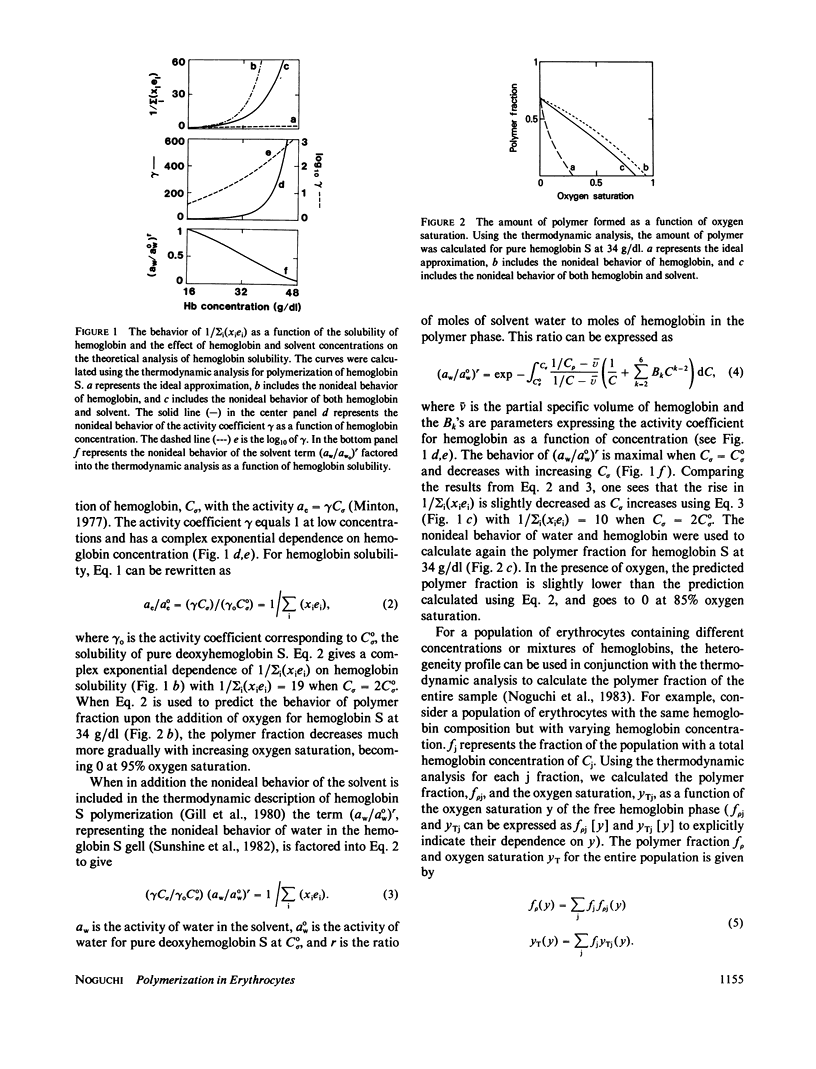
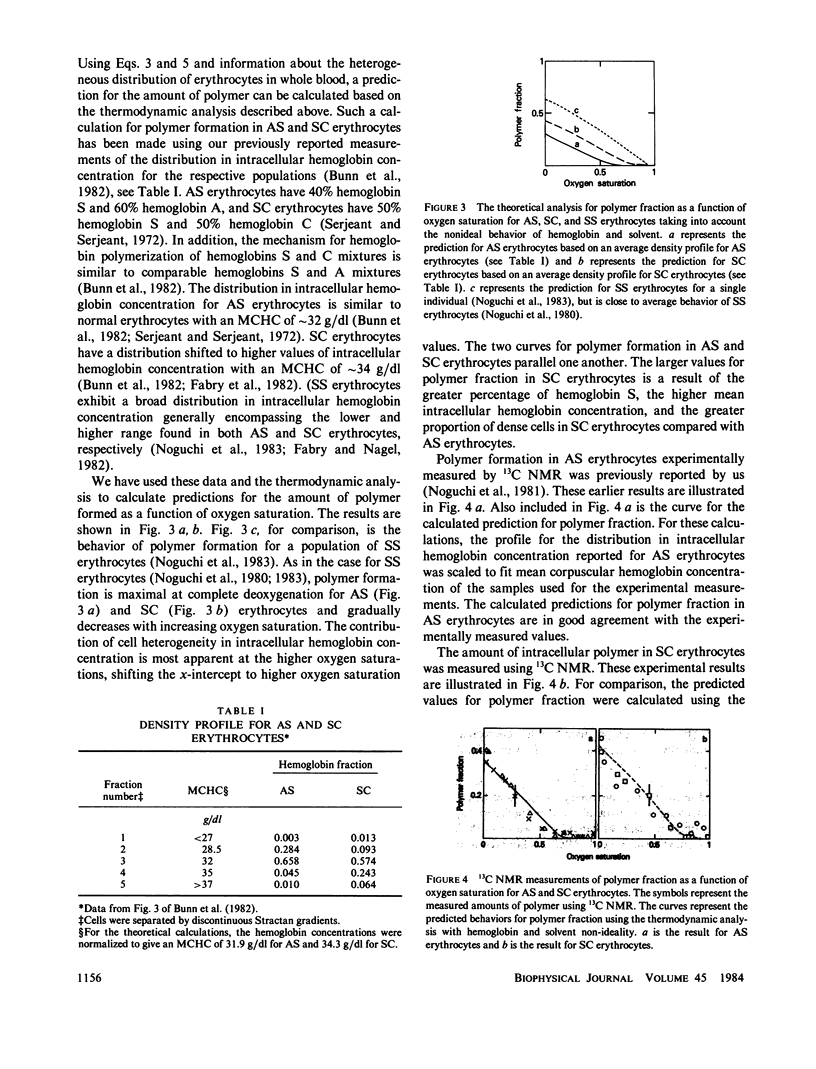
We analyzed the effects of protein and water nonideality and of erythrocyte heterogeneity on the polymerization of hemoglobin S in cells where there were significant amounts of non-S hemoglobins, sickle trait (AS), and SC disease. For AS erythrocytes, the calculated predicted results were in good agreement with measured polymer formation as previously reported (Noguchi C.T., D.A. Torchia, and A.N. Schnechter, 1981, J. Biol. Chem. 256:4168-4171). Throughout much of the physiologically relevant oxygen saturation region, polymer was not formed in AS erythrocytes. Measurements of polymer formation in SC erythrocytes as a function of oxygen saturation using 13C NMR are reported here and also are in good agreement with the calculated predicted results. As in sickle (SS) erythrocytes, polymer can be detected in SC erythrocytes in the region above 60% oxygen saturation. The increased polymer formation in SC erythrocytes as compared with AS erythrocytes can be explained in terms of hemoglobin composition and concentration in SC erythrocytes, with the concomitant increase in the proportion of dense cells. These findings provide a basis for understanding the pathophysiology of sickle cell and of SC disease, in contrast to benign sickle trait, in terms of intracellular polymer formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunn H. F., Noguchi C. T., Hofrichter J., Schechter G. P., Schechter A. N., Eaton W. A. Molecular and cellular pathogenesis of hemoglobin SC disease. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7527–7531. doi: 10.1073/pnas.79.23.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- Fabry M. E., Kaul D. K., Raventos-Suarez C., Chang H., Nagel R. L. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest. 1982 Dec;70(6):1315–1319. doi: 10.1172/JCI110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., Nagel R. L. The effect of deoxygenation on red cell density: significance for the pathophysiology of sickle cell anemia. Blood. 1982 Dec;60(6):1370–1377. [PubMed] [Google Scholar]
- Gill S. J., Spokane R., Benedict R. C., Fall L., Wymann J. Ligand-linked phase equilibria of sickle cell hemoglobin. J Mol Biol. 1980 Jun 25;140(2):299–312. doi: 10.1016/0022-2836(80)90107-2. [DOI] [PubMed] [Google Scholar]
- Hofrichter J. Ligand binding and the gelation of sickle cell hemoglobin. J Mol Biol. 1979 Mar 5;128(3):335–369. doi: 10.1016/0022-2836(79)90092-5. [DOI] [PubMed] [Google Scholar]
- Minton A. P. A thermodynamic model for gelation of sickle-cell hemoglobin. J Mol Biol. 1974 Feb 5;82(4):483–498. doi: 10.1016/0022-2836(74)90243-5. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Non-ideality and the thermodynamics of sickle-cell hemoglobin gelation. J Mol Biol. 1977 Feb 15;110(1):89–103. doi: 10.1016/s0022-2836(77)80100-9. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Determination of deoxyhemoglobin S polymer in sickle erythrocytes upon deoxygenation. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5487–5491. doi: 10.1073/pnas.77.9.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Intracellular polymerization of sickle hemoglobin. Effects of cell heterogeneity. J Clin Invest. 1983 Sep;72(3):846–852. doi: 10.1172/JCI111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Polymerization of hemoglobin in sickle trait erythrocytes and lysates. J Biol Chem. 1981 May 10;256(9):4168–4171. [PubMed] [Google Scholar]
- Ross P. D., Briehl R. W., Minton A. P. Temperature dependence of nonideality in concentrated solutions of hemoglobin. Biopolymers. 1978 Sep;17(9):2285–2288. doi: 10.1002/bip.1978.360170920. [DOI] [PubMed] [Google Scholar]
- Rossi-Bernardi L., Perella M., Luzzana M., Samaja M., Raffaele I. Simultaneous determination of hemoglobin derivatives, oxygen content, oxygen capacity, and oxygen saturation in 10 microliters of whole blood. Clin Chem. 1977 Jul;23(7):1215–1225. [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E. A comparison of erythrocyte characteristics in sickle cell syndromes in Jamaica. Br J Haematol. 1972 Aug;23(2):205–213. doi: 10.1111/j.1365-2141.1972.tb03473.x. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Ferrone F. A., Eaton W. A. Oxygen binding by sickle cell hemoglobin polymers. J Mol Biol. 1982 Jun 25;158(2):251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]
- Sutherland J. W., Egan W., Schechter A. N., Torchia D. A. Carbon-13-proton nuclear magnetic double-resonance study of deoxyhemoglobin S gelation. Biochemistry. 1979 May 1;18(9):1797–1803. doi: 10.1021/bi00576a025. [DOI] [PubMed] [Google Scholar]


