Abstract
We develop a thermodynamic calculus for the modeling of cell adhesion. By means of this approach, we are able to compute the end results of competition between the formation of specific macromolecular bridges and nonspecific repulsion arising from electrostatic forces and osmotic (steric stabilization) forces. Using this calculus also allows us to derive in a straightforward manner the effects of cell deformability, the Young's modulus for stretching of bridges, diffusional mobility of receptors, heterogeneity of receptors, variation in receptor number, and the strength of receptor-receptor binding. The major insight that results from our analysis concerns the existence and characteristics of two phase transitions corresponding, respectively, to the onset of stable cell adhesion and to the onset of maximum cell-cell or cell-substrate contact. We are also able to make detailed predictions of the equilibrium contact area, equilibrium number of bridges, and the cell-cell or cell-substrate separation distance. We illustrate how our approach can be used to improve the analysis of experimental data, by means of two concrete examples.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Beukers H., Deierkauf F. A., Blom C. P., Deierkauf M., Riemersma J. C. Effects of albumin on the phagocytosis of polystyrene spherules by rabbit polymorphonuclear leucocytes. J Cell Physiol. 1978 Oct;97(1):29–36. doi: 10.1002/jcp.1040970105. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
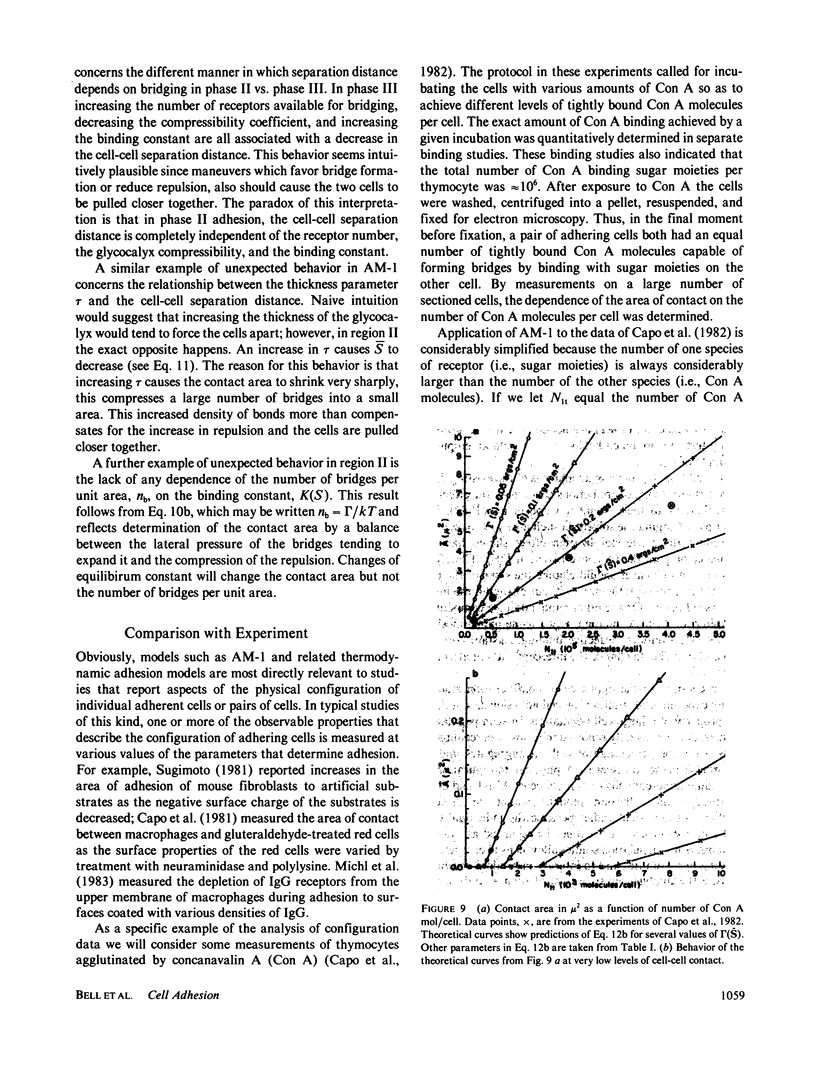
- Capo C., Garrouste F., Benoliel A. M., Bongrand P., Ryter A., Bell G. I. Concanavalin-A-mediated thymocyte agglutination: a model for a quantitative study of cell adhesion. J Cell Sci. 1982 Aug;56:21–48. doi: 10.1242/jcs.56.1.21. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Evans E., Leung A. Adhesivity and rigidity of erythrocyte membrane in relation to wheat germ agglutinin binding. J Cell Biol. 1984 Apr;98(4):1201–1208. doi: 10.1083/jcb.98.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Studies on the mechanism of cell attachment to a substratum with serum in the medium: further evidence supporting a requirement for two biochemically distinct processes. Arch Biochem Biophys. 1974 Dec;165(2):524–530. doi: 10.1016/0003-9861(74)90278-1. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Letter to the editor: Fusion of Sendai viruses with model membranes. J Mol Biol. 1974 Aug 15;87(3):625–628. doi: 10.1016/0022-2836(74)90107-7. [DOI] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Bell G. I., Silverstein S. C. Fc receptor modulation in mononuclear phagocytes maintained on immobilized immune complexes occurs by diffusion of the receptor molecule. J Exp Med. 1983 Jun 1;157(6):2121–2139. doi: 10.1084/jem.157.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir S., Andersen M. Van der Waals interactions between cell surfaces. J Membr Biol. 1977 Feb 24;31(1-2):1–18. doi: 10.1007/BF01869396. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A. Long-range physical forces in the biological milieu. Annu Rev Biophys Bioeng. 1973;2:221–255. doi: 10.1146/annurev.bb.02.060173.001253. [DOI] [PubMed] [Google Scholar]
- Poo M. M., Poo W. J., Lam J. W. Lateral electrophoresis and diffusion of Concanavalin A receptors in the membrane of embryonic muscle cell. J Cell Biol. 1978 Feb;76(2):483–501. doi: 10.1083/jcb.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A., Lloyd C. W., Thom D. Control of grip and stick in cell adhesion through lateral relationships of membrane glycoproteins. Nature. 1977 May 12;267(5607):124–128. doi: 10.1038/267124a0. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hellio R. Electron-microscope study of Dictyostelium discoideum plasma membrane and its modifications during and after phagocytosis. J Cell Sci. 1980 Feb;41:75–88. doi: 10.1242/jcs.41.1.75. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Evidence for another cell-adhesion molecule in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6561–6565. doi: 10.1073/pnas.79.21.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suezaki Y., Go N. Fluctuations and mechanical strength of alpha-helices of polyglycine and poly(L-alanine). Biopolymers. 1976 Nov;15(11):2137–2153. doi: 10.1002/bip.1976.360151104. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y. Effect on the adhesion and locomotion of mouse fibroblasts by their interacting with differently charged substrates. A quantitative study by ultrastructural method. Exp Cell Res. 1981 Sep;135(1):39–45. doi: 10.1016/0014-4827(81)90297-4. [DOI] [PubMed] [Google Scholar]
- Torza S., Mason S. G. Coalescence of two immiscible liquid drops. Science. 1969 Feb 21;163(3869):813–814. doi: 10.1126/science.163.3869.813. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Schnaar R. L., Kuhlenschmidt M. S., Schmell E., Lee R. T., Lee Y. C., Roseman S. Adhesion of hepatocytes to immobilized sugars. A threshold phenomenon. J Biol Chem. 1979 Nov 10;254(21):10830–10838. [PubMed] [Google Scholar]


