Abstract
Several suggestions have been made about the functional significance of dendritic spines in connection with synaptic plasticity. We investigated transient electrical behavior of spines with bulbous terminals in neurons with arbitrary dendritic geometries. It is shown that postsynaptic potential transform caused by a synapse on a spine can be resolved into a product of two transfer functions and the synaptic input current transform. The first transfer function was determined to be independent of the spine. The second transfer function represents the straightforward attenuation effect of the spine, which determines the effective synaptic current reaching the parent dendrite. Using what is known of the size and the shape of spines from histology, we conclude that almost all of the synaptic current flow into the parent dendrite, and that therefore the straightforward attenuation effect is negligible. Consequently, when the synaptic current remained unaltered, as was the case for a large synaptic resistance as compared with the spine stem resistance, a morphological change of the spine did not produce an effective change in the postsynaptic potential. On the other hand, when the synaptic resistance is compared with the spine stem impedance, the morphological change of the spine might induce changes of the synaptic current and the postsynaptic potential.
Full text
PDF


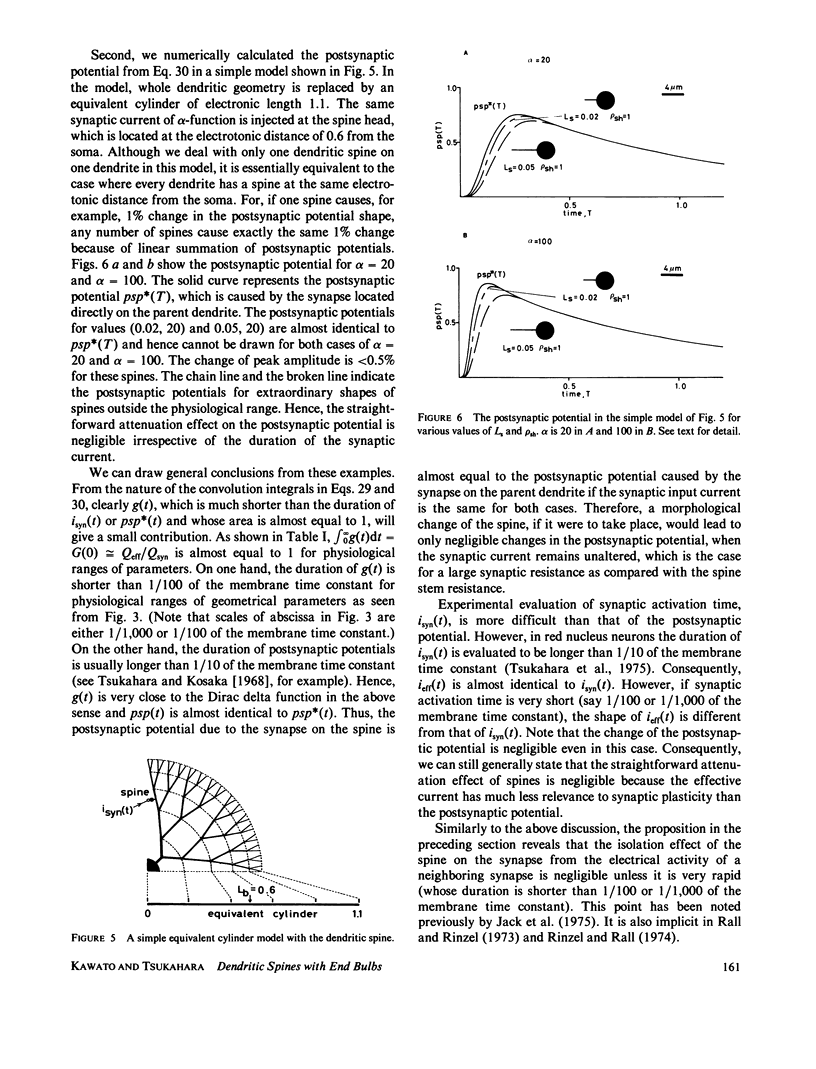




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Crill W. E. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol. 1974 Jun;239(2):325–345. doi: 10.1113/jphysiol.1974.sp010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz E. G., Cowan J. D. Transient potentials in dendritic systems of arbitrary geometry. Biophys J. 1974 Sep;14(9):661–689. doi: 10.1016/S0006-3495(74)85943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG H. T. Cortical neurons with particular reference to the apical dendrites. Cold Spring Harb Symp Quant Biol. 1952;17:189–202. doi: 10.1101/sqb.1952.017.01.019. [DOI] [PubMed] [Google Scholar]
- Fifková E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977 Apr;6(2):211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Horwitz B. An analytical method for investigating transient potentials in neurons with branching dendritic trees. Biophys J. 1981 Oct;36(1):155–192. doi: 10.1016/S0006-3495(81)84722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. Morphological variations in the dendritic spines of the neocortex. J Cell Sci. 1969 Sep;5(2):509–529. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- Kawato M., Tsukahara N. Theoretical study on electrical properties of dendritic spines. J Theor Biol. 1983 Aug 21;103(4):507–522. doi: 10.1016/0022-5193(83)90280-1. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Schottler F., Oliver M., Lynch G. Brief bursts of high-frequency stimulation produce two types of structural change in rat hippocampus. J Neurophysiol. 1980 Aug;44(2):247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970 Apr;127(4):321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys J. 1973 Jul;13(7):648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinzel J., Rall W. Transient response in a dendritic neuron model for current injected at one branch. Biophys J. 1974 Oct;14(10):759–790. doi: 10.1016/S0006-3495(74)85948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol. 1965 Sep;28(5):908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Hultborn H., Murakami F., Fujito Y. Electrophysiological study of formation of new synapses and collateral sprouting in red nucleus neurons after partial denervation. J Neurophysiol. 1975 Nov;38(6):1359–1372. doi: 10.1152/jn.1975.38.6.1359. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Kosaka K. The mode of cerebral excitation of red nucleus neurons. Exp Brain Res. 1968;5(2):102–117. doi: 10.1007/BF00238700. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Murakami F., Hultborn H. Electrical constants of neurons of the red nucleus. Exp Brain Res. 1975 Jul 11;23(1):49–64. doi: 10.1007/BF00238728. [DOI] [PubMed] [Google Scholar]
- Valverde F. Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res. 1967;3(4):337–352. doi: 10.1007/BF00237559. [DOI] [PubMed] [Google Scholar]


