Abstract
Ion translocation in red cell anion exchange is assumed to occur by means of an alternating access mechanism, in which a critical binding site for the transported ion alternates between two conformational states, each accessible from only one side of the membrane. If this alternating site is located within the transport protein at some distance from one or both surfaces of the membrane, an access channel is required to connect the alternating site to the adjacent bulk solution. This automatically leads to inhibition of transport at high concentrations of the transported ion because release of the ion from the alternating site can occur only via unoccupied channel sites.
Full text
PDF
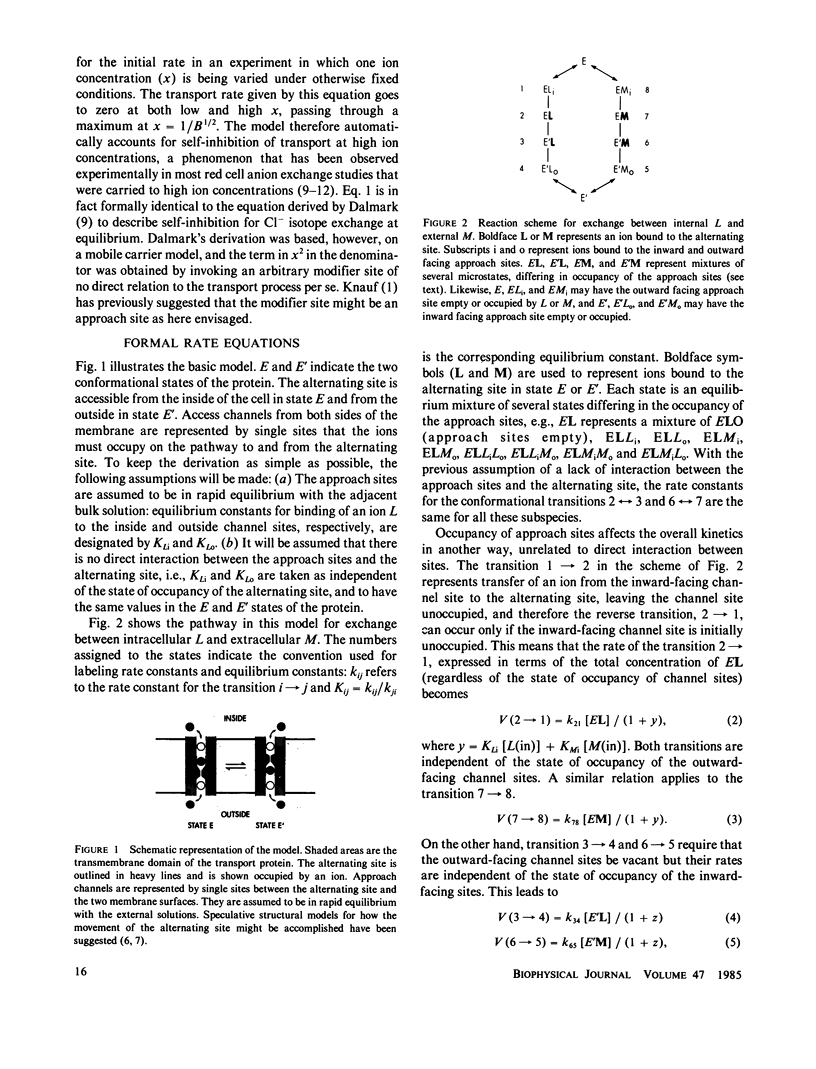




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalmark M. Effects of halides and bicarbonate on chloride transport in human red blood cells. J Gen Physiol. 1976 Feb;67(2):223–234. doi: 10.1085/jgp.67.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn R. B., Fröhlich O. Asymmetry in the mechanism for anion exchange in human red blood cell membranes. Evidence for reciprocating sites that react with one transported anion at a time. J Gen Physiol. 1979 Sep;74(3):351–374. doi: 10.1085/jgp.74.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Yee V. J., Warnock D. G. Mixed type inhibition of the renal Na+/H+ antiporter by Li+ and amiloride. Evidence for a modifier site. J Biol Chem. 1983 Aug 25;258(16):9710–9716. [PubMed] [Google Scholar]
- Läuger P. A channel mechanism for electrogenic ion pumps. Biochim Biophys Acta. 1979 Mar 23;552(1):143–161. doi: 10.1016/0005-2736(79)90253-0. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Kuo S., Cantley L. C. Evidence that inhibitors of anion exchange induce a transmembrane conformational change in band 3. J Biol Chem. 1983 Feb 10;258(3):1785–1792. [PubMed] [Google Scholar]
- Rao A., Martin P., Reithmeier R. A., Cantley L. C. Location of the stilbenedisulfonate binding site of the human erythrocyte anion-exchange system by resonance energy transfer. Biochemistry. 1979 Oct 16;18(21):4505–4516. doi: 10.1021/bi00588a008. [DOI] [PubMed] [Google Scholar]
- Schnell K. F., Besl E., von der Mosel R. Phosphate transport in human red blood cells: concentration dependence and pH dependence of the unidirectional phosphate flux at equilibrium conditions. J Membr Biol. 1981;61(3):173–192. doi: 10.1007/BF01870522. [DOI] [PubMed] [Google Scholar]
- Schnell K. F., Gerhardt S., Schöppe-Fredenburg A. Kinetic characteristics of the sulfate self-exchange in human red blood cells and red blood cell ghosts. J Membr Biol. 1977 Jan 28;30(4):319–350. doi: 10.1007/BF01869675. [DOI] [PubMed] [Google Scholar]
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. doi: 10.1146/annurev.bi.52.070183.002115. [DOI] [PubMed] [Google Scholar]
- Tanford C. Simple model for the chemical potential change of a transported ion in active transport. Proc Natl Acad Sci U S A. 1982 May;79(9):2882–2884. doi: 10.1073/pnas.79.9.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Translocation pathway in the catalysis of active transport. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3701–3705. doi: 10.1073/pnas.80.12.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villereal M. L. Sodium fluxes in human fibroblasts: kinetics of serum-dependent and serum-independent pathways. J Cell Physiol. 1981 Aug;108(2):251–259. doi: 10.1002/jcp.1041080215. [DOI] [PubMed] [Google Scholar]


