Abstract
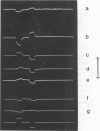
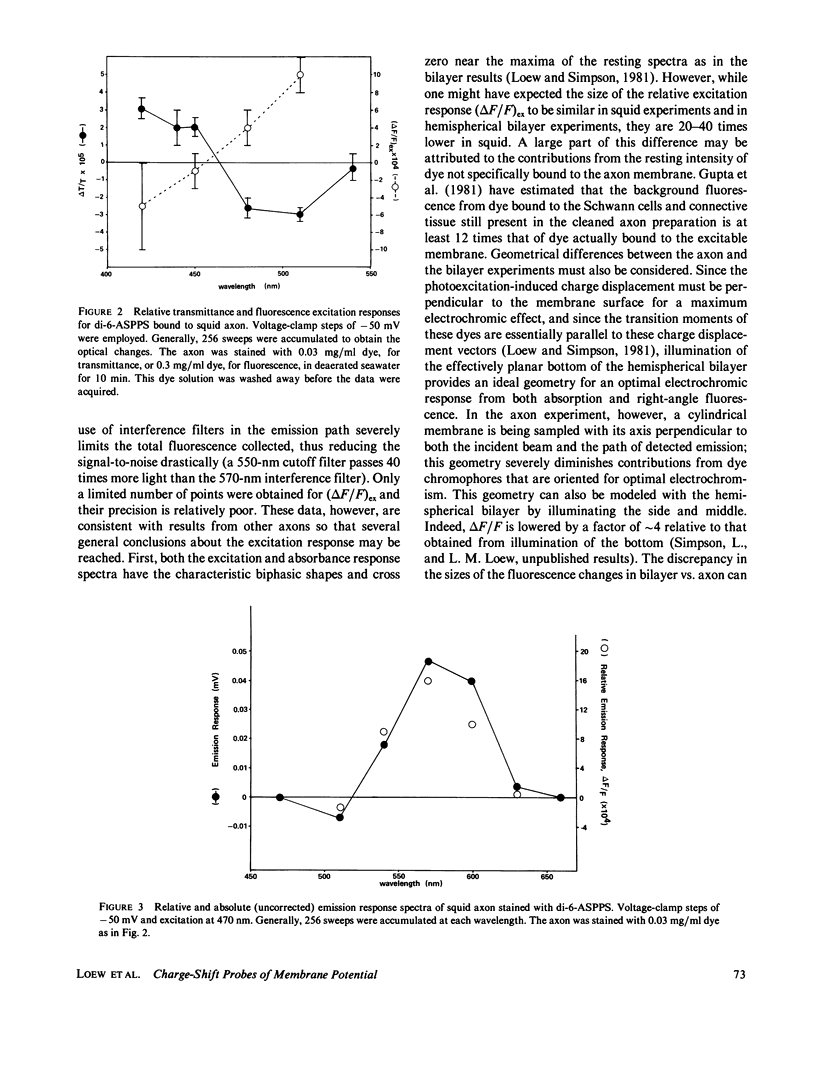
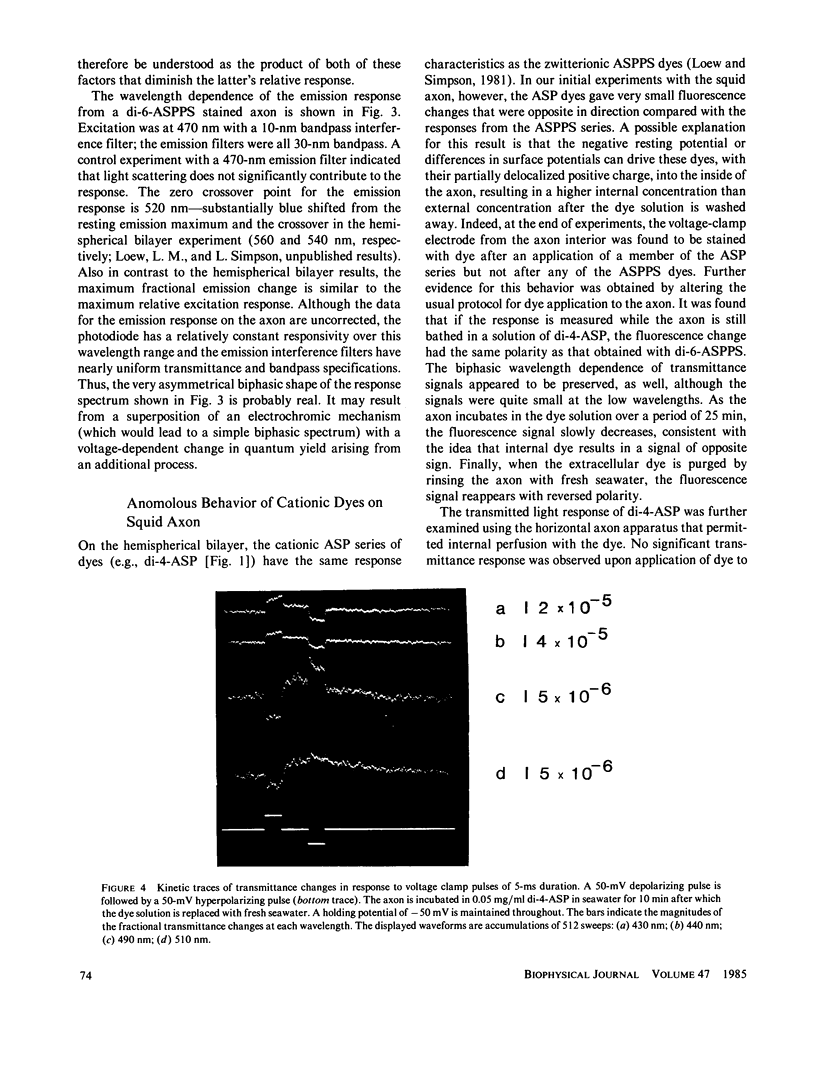
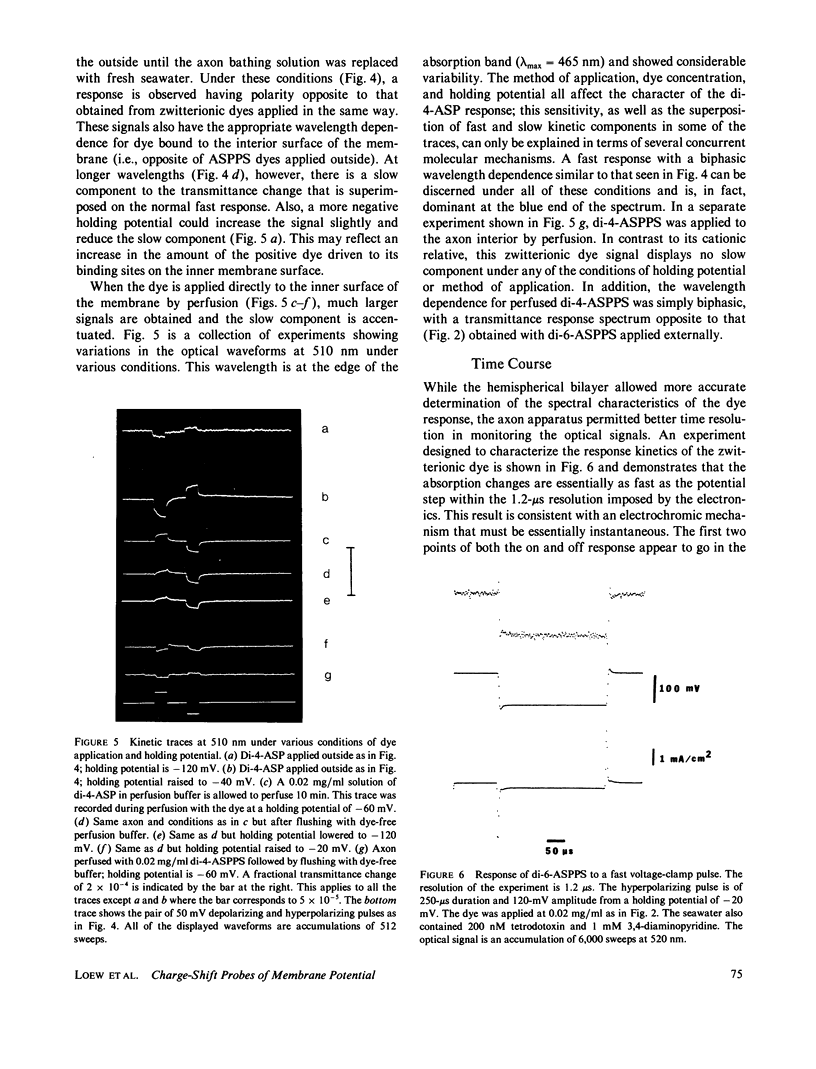
The characteristics of transmittance and fluorescence changes of 4-(p-aminostyryl)-1-pyridinium dyes in response to voltage-clamp pulses on the squid giant axon were examined. A zwitterionic styryl dye displays transmittance and excitation spectra on the voltage-clamped squid axon with shapes similar to those previously measured on a model membrane system and consistent with a postulated electrochromic mechanism. The speed of the transmittance response is faster than 1.2 microseconds. The size of the fluorescence change is a factor of 40 lower than on the model membrane; this diminution can be rationalized in terms of the background fluorescence from Schwann cells and the nonoptimal geometric arrangement of the axon membrane. When the emission spectrum is dissected from the excitation response, a nonelectrochromic component is found. This component might result from molecular motion during the excited state lifetime. A positively charged dye permeates the axon membrane and displays complex response waveforms dependent on the method of application and the axon holding potential. This contrasts markedly with model membrane results where the behavior of the cationic and zwitterionic dyes were indistinguishable.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Taylor R. E., Fernández J. M. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J Gen Physiol. 1982 Jan;79(1):21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. B., Cohen L. B., Macagno E. R., Orbach H. The number and size of neurons in the CNS of gastropod molluscs and their suitability for optical recording of activity. Brain Res. 1983 May 5;266(2):305–317. doi: 10.1016/0006-8993(83)90662-5. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Fine A., Farber I. C., Hildesheim R. Fluorescence monitoring of electrical responses from small neurons and their processes. Biophys J. 1983 May;42(2):195–198. doi: 10.1016/S0006-3495(83)84386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Hildesheim R., Farber I. C., Anglister L. Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J. 1982 Sep;39(3):301–308. doi: 10.1016/S0006-3495(82)84520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Salzberg B. M., Grinvald A., Cohen L. B., Kamino K., Lesher S., Boyle M. B., Waggoner A. S., Wang C. H. Improvements in optical methods for measuring rapid changes in membrane potential. J Membr Biol. 1981 Feb 15;58(2):123–137. doi: 10.1007/BF01870975. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Bonneville G. W., Surow J. Charge shift optical probes of membrane potential. Theory. Biochemistry. 1978 Sep 19;17(19):4065–4071. doi: 10.1021/bi00612a030. [DOI] [PubMed] [Google Scholar]
- Loew L. M. Design and characterization of electrochromic membrane probes. J Biochem Biophys Methods. 1982 Aug;6(3):243–260. doi: 10.1016/0165-022x(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Simpson L. L. Charge-shift probes of membrane potential: a probable electrochromic mechanism for p-aminostyrylpyridinium probes on a hemispherical lipid bilayer. Biophys J. 1981 Jun;34(3):353–365. doi: 10.1016/S0006-3495(81)84854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Reichardt L. F. Species-specific effects on the optical signals of voltage-sensitive dyes. J Membr Biol. 1979 Aug;48(4):343–356. doi: 10.1007/BF01869445. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Bezanilla F. An optical determination of the series resistance in Loligo. J Gen Physiol. 1983 Dec;82(6):807–817. doi: 10.1085/jgp.82.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKII, WATANABE A., TAKENAKA T. Resting and action potential of intracellularly perfused squid giant axon. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1177–1184. doi: 10.1073/pnas.48.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warashina A. Further studies on absorption changes arising in dye-stained nerves during excitation. J Membr Biol. 1980;53(3):207–213. doi: 10.1007/BF01868826. [DOI] [PubMed] [Google Scholar]