Abstract
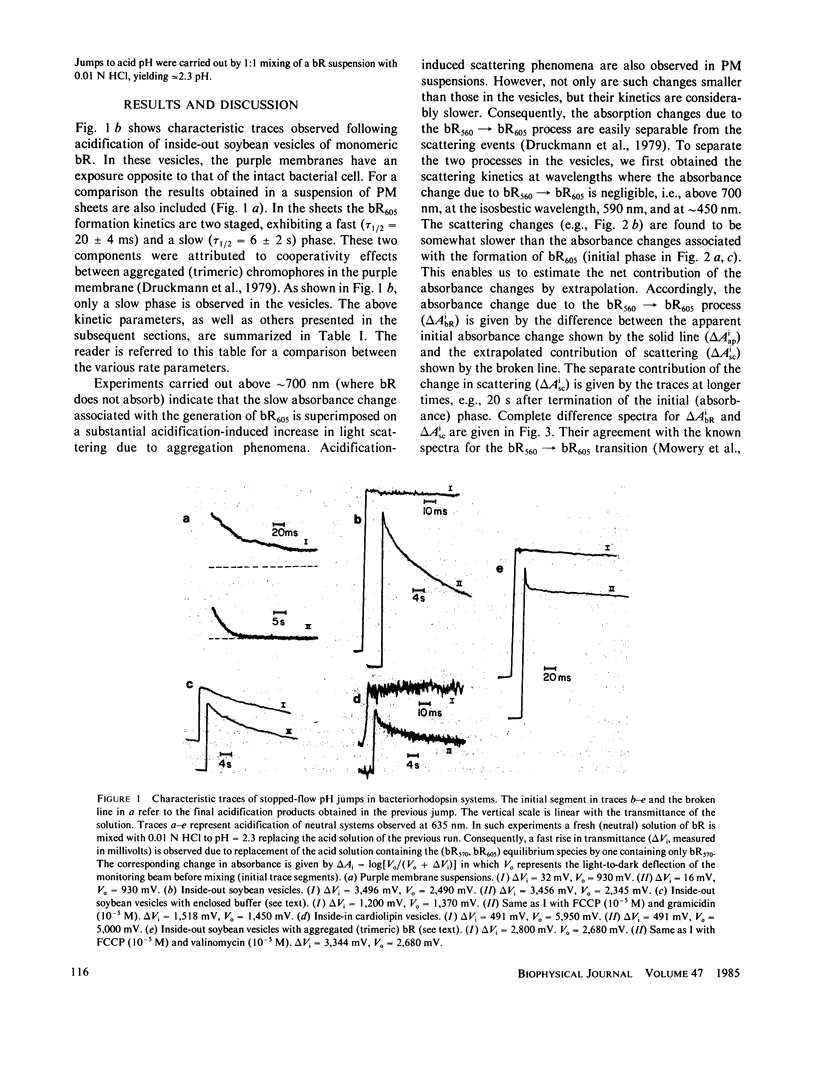
The direction of the accessibility to protons of the binding site in bacteriorhodopsin is of primary importance in elucidating the proton-pump mechanism. The problem is approached via the pH-dependent equilibrium bR560 in equilibrium bR605 in vesicles with preferentially oriented purple membranes. Fast acidification (stopped-flow) experiments with inside-out, monomeric, bR vesicles were carried out with and without a buffer enclosed in the vesicle interior. The results, showing a buffer-induced delay in the formation of bR605, indicate that the binding site is accessible to protons from the inside of the vesicles. We arrive at this conclusion also by working with inside-out trimeric vesicles in the presence and in the absence of H+ (and K+) ionophores. The results suggest that in Halobacterium halobium, the binding site and thus the retinal Schiff base are exposed to the outside of the cell. This conclusion is consistent with a pumping mechanism based on a light-induced pK change.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadio R., Stoeckenius W. Effect of protein-protein interaction on light adaptation of bacteriorhodopsin. Biochemistry. 1980 Jul 8;19(14):3374–3381. doi: 10.1021/bi00555a043. [DOI] [PubMed] [Google Scholar]
- Druckmann S., Samuni A., Ottolenghi M. Dynamics of pH-induced spectral changes in bacteriorhodopsin. Biophys J. 1979 Apr;26(1):143–145. doi: 10.1016/S0006-3495(79)85241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G. E., Gray C. P., Wildenauer D., Khorana H. G. Orientation of bacteriorhodopsin in Halobacterium halobium as studied by selective proteolysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5426–5430. doi: 10.1073/pnas.74.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe M., Teathera R. M., Overath P., Knobling A., Oesterhelt D. Direction of proton translocation in proteoliposomes formed from purple membrane and acidic lipids depends on the pH during reconstitution. Biochim Biophys Acta. 1977 Mar 1;465(2):415–420. doi: 10.1016/0005-2736(77)90092-x. [DOI] [PubMed] [Google Scholar]
- Lind C., Höjeberg B., Khorana H. G. Reconstitution of delipidated bacteriorhodopsin with endogenous polar lipids. J Biol Chem. 1981 Aug 25;256(16):8298–8305. [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Racker E. A new procedure for the reconstitution of biologically active phospholipid vesicles. Biochem Biophys Res Commun. 1973 Nov 1;55(1):224–230. doi: 10.1016/s0006-291x(73)80083-x. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Warshel A., Ottolenighi M. Kinetic and spectroscopic effects of protein-chromophore electrostatic interactions in bacteriorhodopsin. Photochem Photobiol. 1979 Aug;30(2):291–293. doi: 10.1111/j.1751-1097.1979.tb07149.x. [DOI] [PubMed] [Google Scholar]


