Abstract
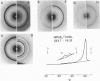
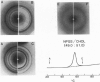
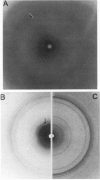
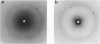
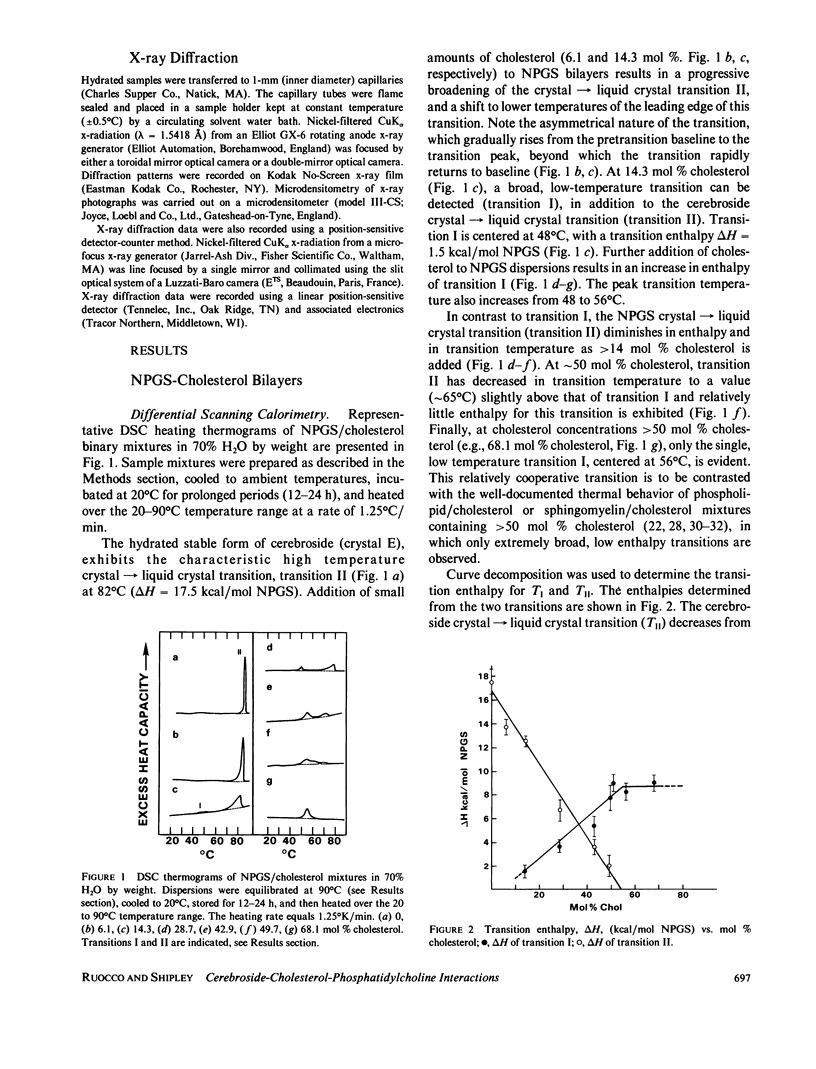
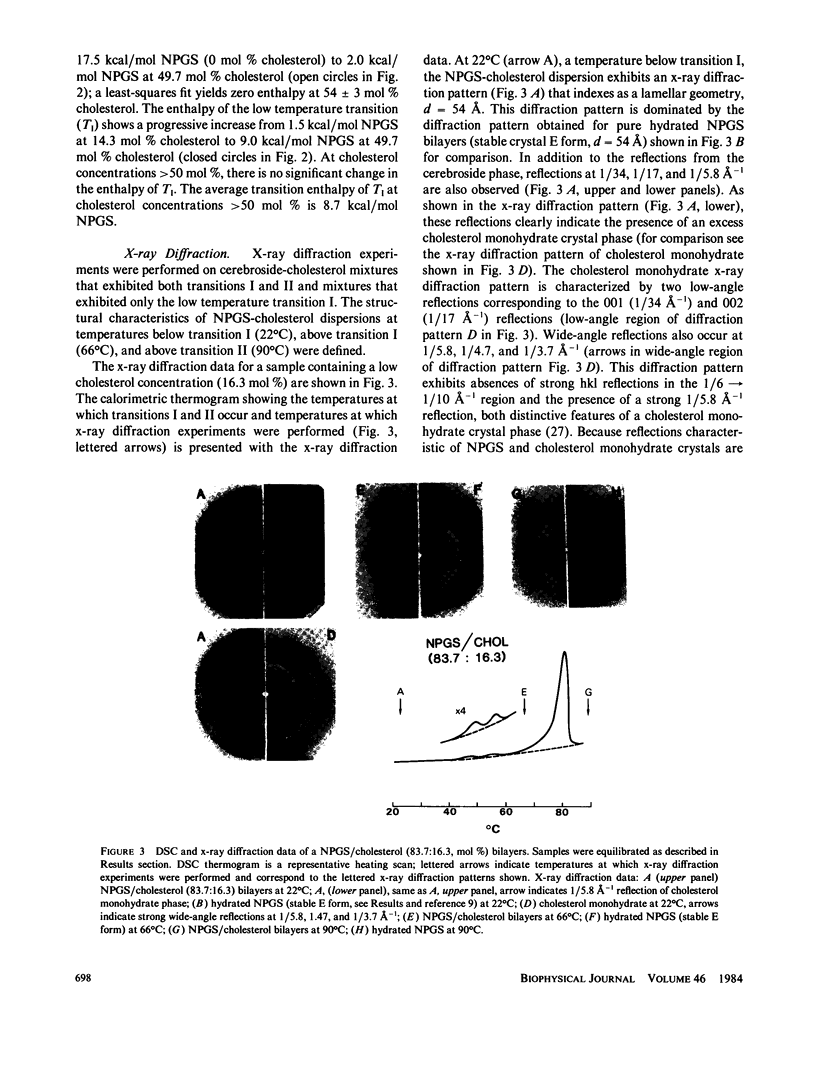
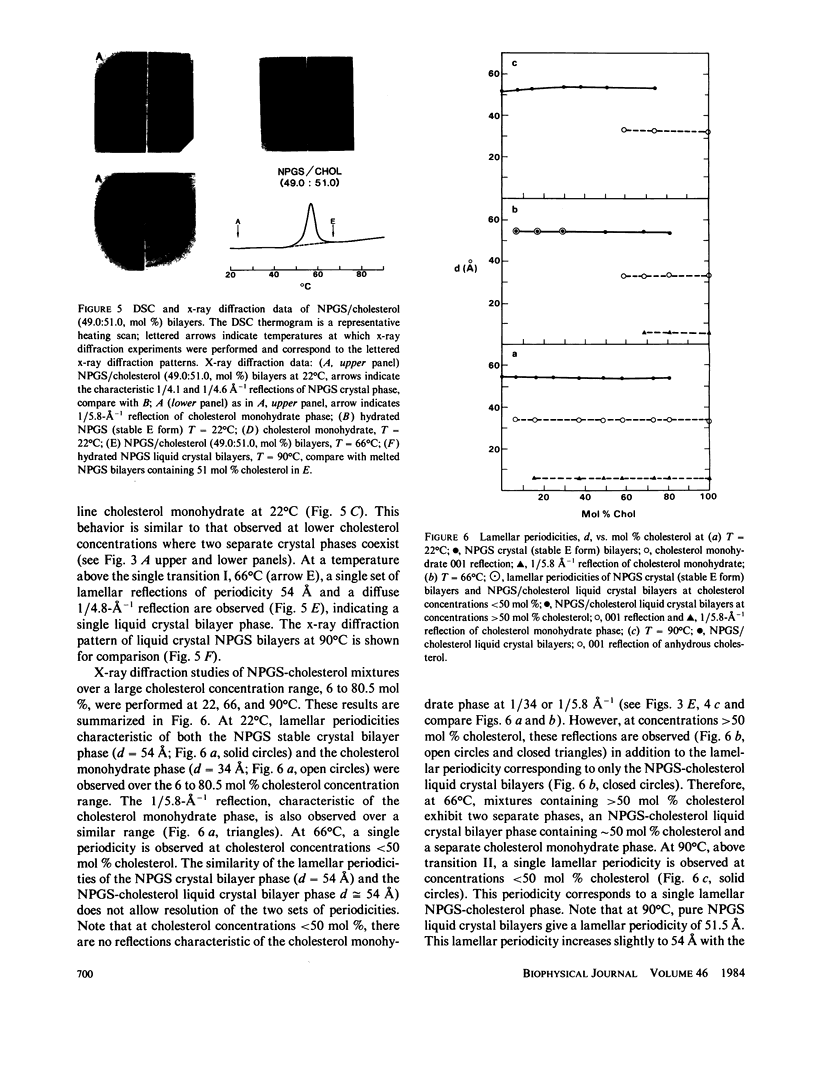
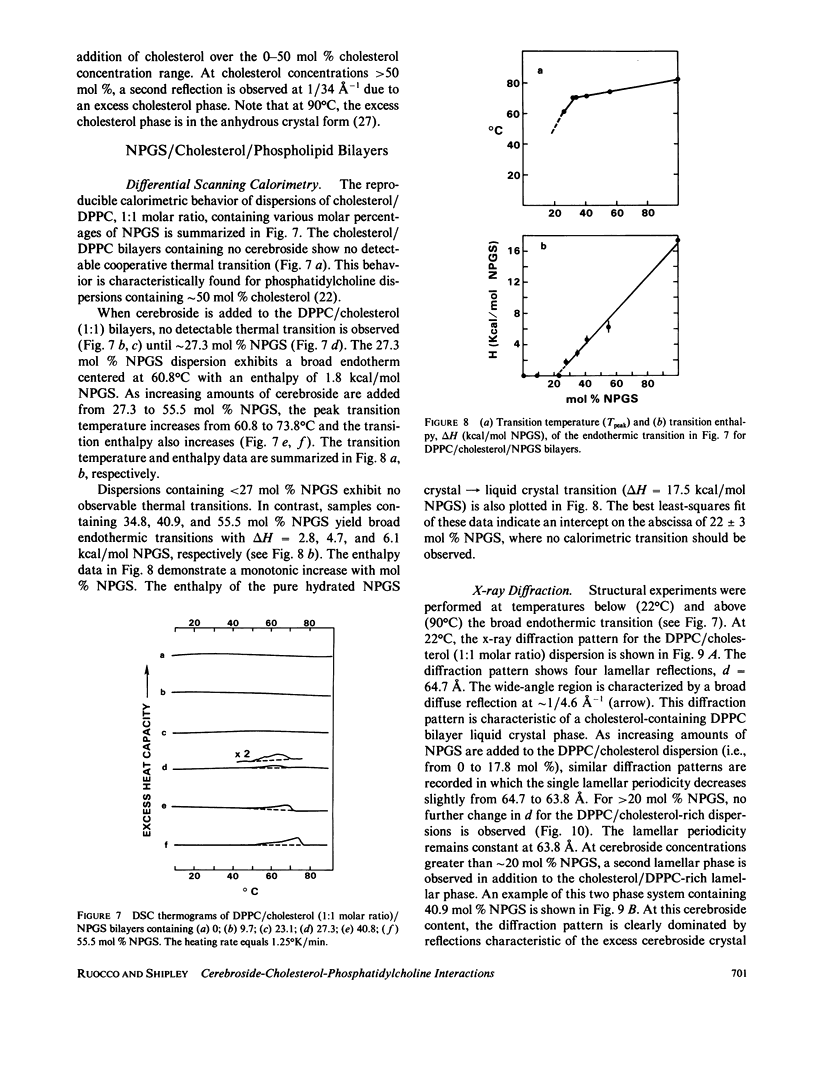
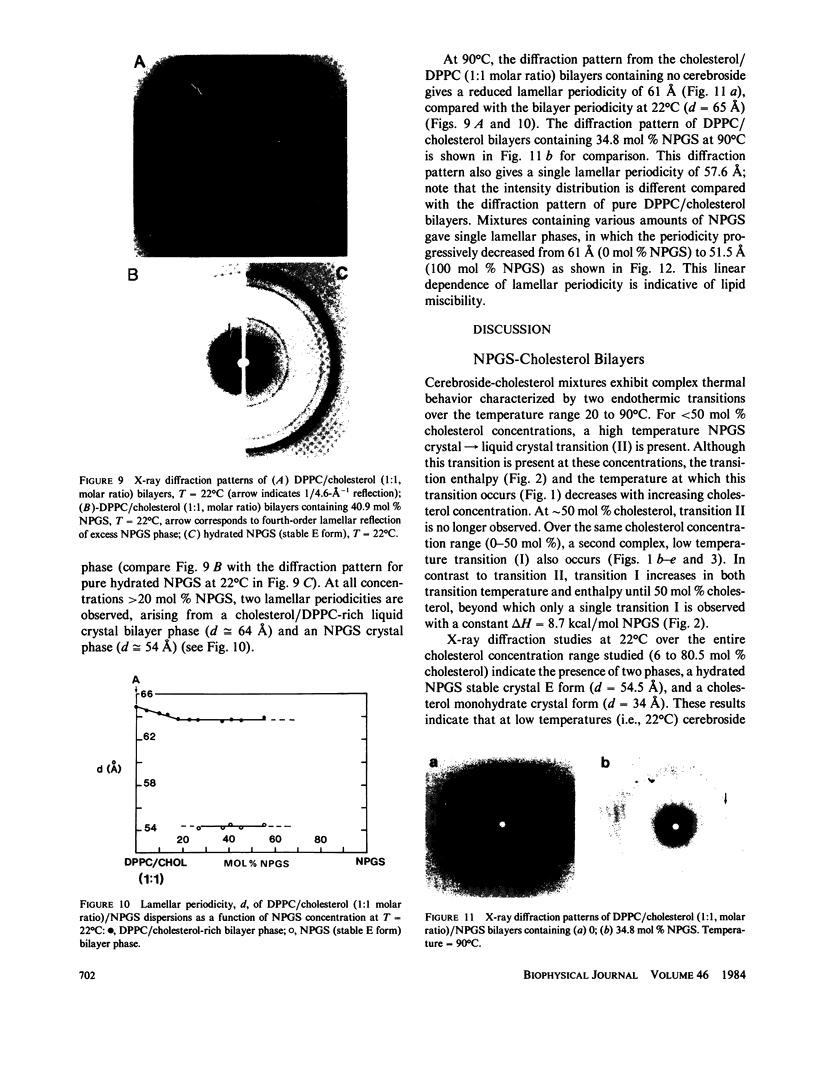
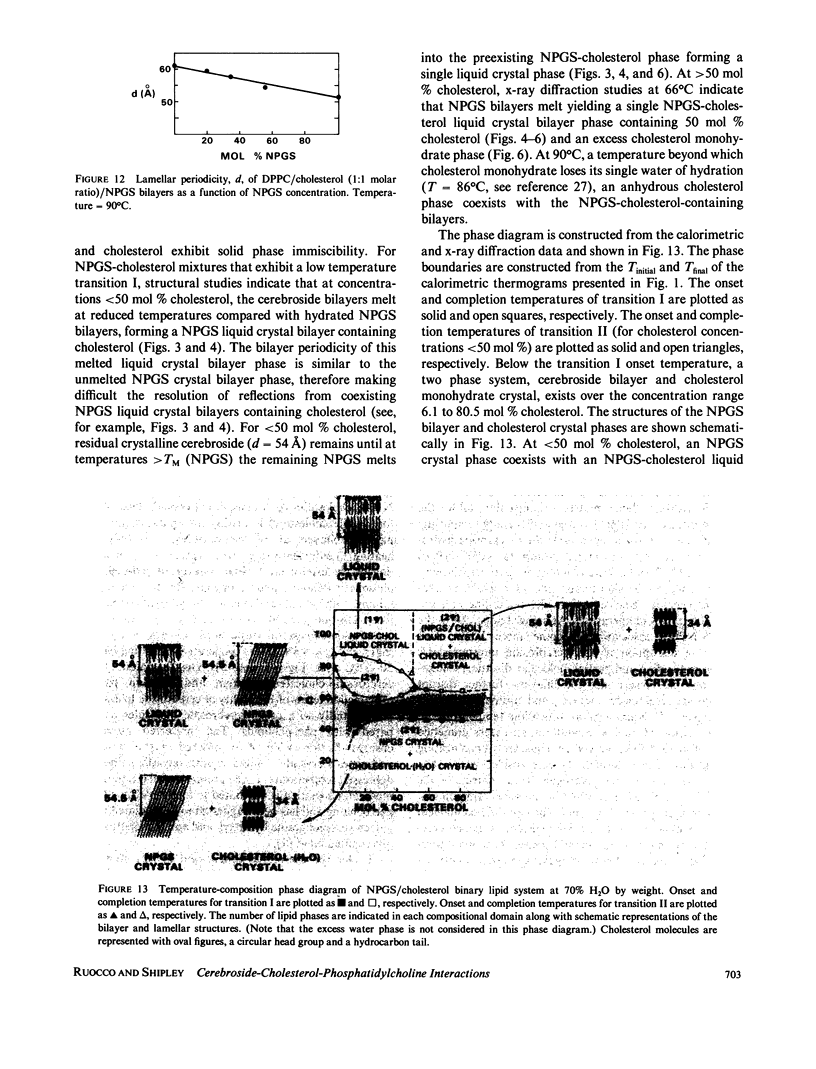
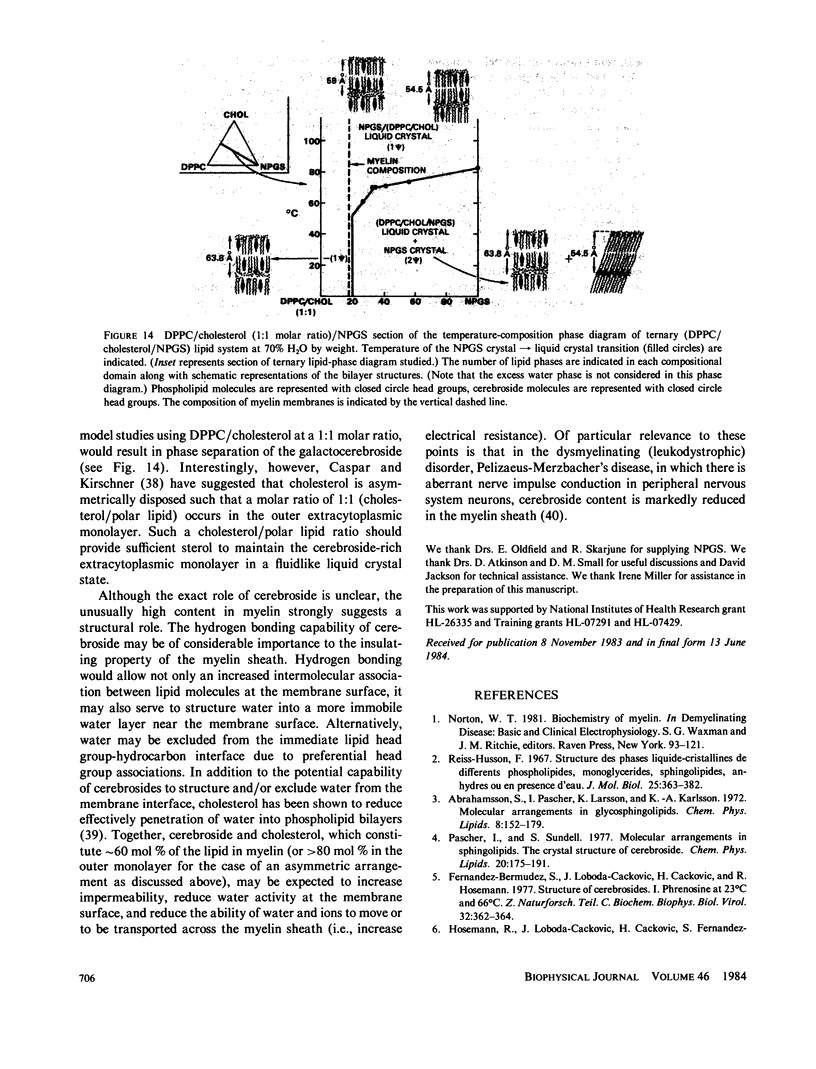
The interaction of the galactocerebroside, N-palmitoylgalactosylsphingosine (NPGS), with cholesterol has been studied by differential scanning calorimetry (DSC) and x-ray diffraction. Thermal and structural studies demonstrate complex behavior characterized by two endothermic transitions: transition I (TI approximately equal to 50-60 degrees C) corresponding to an NPGS-cholesterol bilayer gel----bilayer liquid crystal transition II (TII where TI less than TII less than TNPGS) corresponding to an NPGS bilayer crystal (stable E form)----bilayer liquid crystal transition. For mixtures containing from 6 to 80 mol % cholesterol, x-ray diffraction studies at 22 degrees C (T less than TI) indicate two separate lamellar phases; an NPGS crystal bilayer phase and a cholesterol monohydrate phase. For cholesterol concentrations less than 50 mol % at TI less than T less than TII, NPGS-cholesterol liquid crystal bilayer and excess NPGS crystal bilayer phases are observed. For greater than 50 mol % cholesterol concentrations at these temperatures, an excess cholesterol monohydrate phase coexists with the NPGS-cholesterol liquid crystal bilayers. At T greater than TII, complete NPGS-cholesterol miscibility is only observed for less than 50 mol % cholesterol concentrations, whereas at greater than 50 mol % cholesterol an excess cholesterol phase is present. The solid phase immiscibility of cerebroside and cholesterol at low temperatures is suggested to result from preferential NPGS-NPGS associations via hydrogen bonding. The unique thermal and structural behavior of NPGS-cholesterol dispersions is contrasted with the behavior of cholesterol-phosphatidycholine and cholesterol-sphingomyelin bilayers. Thermal and structural studies of NPGS in dipalmitoylphosphatidylcholine (DPPC)/cholesterol (1:1, molar ratio) bilayers have been performed. For dispersions containing less than 20 mol % NPGS at 22 degrees C there are no observable calorimetric transitions and x-ray diffraction studies indicate complete lipid miscibility. At greater than 20 mol % NPGS, a high temperature transition is observed that is shown by x-ray diffraction studies to be due to an excess NPGS crystal bilayer----liquid crystal bilayer transition. Complete miscibility of NPGS in DPPC/cholesterol bilayers is observed at T greater than TNPGS. The properties of NPGS/DPPC/cholesterol bilayers are discussed in terms of the lipid composition of the myelin sheath.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach D., Sela B., Miller I. R. Compositional aspects of lipid hydration. Chem Phys Lipids. 1982 Dec;31(4):381–394. doi: 10.1016/0009-3084(82)90073-1. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Levin I. W. Molecular conformations of cerebrosides in bilayers determined by Raman spectroscopy. Biophys J. 1980 Dec;32(3):1007–1021. doi: 10.1016/S0006-3495(80)85032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunow M. R. Two gel states of cerebrosides. Calorimetric and Raman spectroscopic evidence. Biochim Biophys Acta. 1979 Sep 28;574(3):542–546. doi: 10.1016/0005-2760(79)90250-9. [DOI] [PubMed] [Google Scholar]
- Calhoun W. I., Shipley G. G. Sphingomyelin--lecithin bilayers and their interaction with cholesterol. Biochemistry. 1979 May 1;18(9):1717–1722. doi: 10.1021/bi00576a013. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Kirschner D. A. Myelin membrane structure at 10 A resolution. Nat New Biol. 1971 May 12;231(19):46–52. doi: 10.1038/newbio231046a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Cherry R. J., Chapman D. Physical properties of lecithin-cerebroside bilayers. Biochim Biophys Acta. 1971 Oct 12;249(1):301–317. doi: 10.1016/0005-2736(71)90108-8. [DOI] [PubMed] [Google Scholar]
- Correa-Freire M. C., Freire E., Barenholz Y., Biltonen R. L., Thompson T. E. Thermotropic behavior of monoglucocerebroside--dipalmitoylphosphatidylcholine multilamellar liposomes. Biochemistry. 1979 Feb 6;18(3):442–445. doi: 10.1021/bi00570a008. [DOI] [PubMed] [Google Scholar]
- Curatolo W. Thermal behavior of fractionated and unfractionated bovine brain cerebrosides. Biochemistry. 1982 Apr 13;21(8):1761–1764. doi: 10.1021/bi00537a010. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Freire E., Anthony F., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of stearoylsphingomyelin-cholesterol dispersions. Biochemistry. 1981 Dec 8;20(25):7115–7118. doi: 10.1021/bi00528a010. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 1979 May 15;18(10):2112–2117. doi: 10.1021/bi00577a042. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bermudez S., Loboda-Cacković J., Cacković H., Hosemann R. Structure of cerebrosides I. Phrenosine at 23 degrees C and 66 degrees C. Z Naturforsch C. 1977 May-Jun;32(5-6):362–374. doi: 10.1515/znc-1977-5-608. [DOI] [PubMed] [Google Scholar]
- Freire E., Bach D., Correa-Freire M., Miller I., Barenholz Y. Calorimetric investigation of the complex phase behavior of glucocerebroside dispersions. Biochemistry. 1980 Aug 5;19(16):3662–3665. doi: 10.1021/bi00557a004. [DOI] [PubMed] [Google Scholar]
- Hosemann R., Loboda-Cacković J., Cacković H., Fernandez-Bermúdez S., Baltá-Calleja F. J. Structure of cerebrosides. II. Small angle X-ray diffraction study of cerasine. Z Naturforsch C. 1979 Dec;34(12):1121–1124. doi: 10.1515/znc-1979-1206. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Jenkinson T. J., Kamat V. B., Chapman D. Physical studies of myelin. I. Thermal analysis. Biochim Biophys Acta. 1968 Sep 2;164(1):101–109. doi: 10.1016/0005-2760(68)90076-3. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Larsson D., Karlsson D. A. Molecular arrangements in glycosphingolipids. Chem Phys Lipids. 1972 Mar;8(2):152–179. doi: 10.1016/0009-3084(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Lecuyer H., Dervichian D. G. Structure of aqueous mixtures of lecithin and cholesterol. J Mol Biol. 1969 Oct 14;45(1):39–57. doi: 10.1016/0022-2836(69)90208-3. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. II. Mictures involving lipids. Biochim Biophys Acta. 1977 Nov 14;472(3-4):285–344. doi: 10.1016/0304-4157(77)90001-6. [DOI] [PubMed] [Google Scholar]
- Linington C., Rumsby M. G. Accessibility of galactosyl ceramides to probe reagents in central nervous system myelin. J Neurochem. 1980 Oct;35(4):983–992. doi: 10.1111/j.1471-4159.1980.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Linington C., Rumsby M. G. On the accessibility and localisation of cerebrosides in central nervous system myelin. Adv Exp Med Biol. 1978;100:263–273. doi: 10.1007/978-1-4684-2514-7_19. [DOI] [PubMed] [Google Scholar]
- Loomis C. R., Shipley G. G., Small D. M. The phase behavior of hydrated cholesterol. J Lipid Res. 1979 May;20(4):525–535. [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Neuringer L. J., Sears B., Jungalwala F. B. Deuterium NMR studies of cerebroside-phospholipid bilayers. Biochim Biophys Acta. 1979 Dec 12;558(3):325–329. doi: 10.1016/0005-2736(79)90268-2. [DOI] [PubMed] [Google Scholar]
- Presti F. T., Pace R. J., Chan S. I. Cholesterol-phospholipid interaction in membranes. 2. Stoichiometry and molecular packing of cholesterol-rich domains. Biochemistry. 1982 Aug 3;21(16):3831–3835. doi: 10.1021/bi00259a017. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 1981 Oct 13;20(21):5957–5966. doi: 10.1021/bi00524a006. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Shipley G. G. Hydration of N-palmitoylgalactosylsphingosine compared to monosaccharide hydration. Biochim Biophys Acta. 1983 Nov 9;735(2):305–308. doi: 10.1016/0005-2736(83)90307-3. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Shipley G. G., Oldfield E. Galactocerebroside-phospholipid interactions in bilayer membranes. Biophys J. 1983 Jul;43(1):91–101. doi: 10.1016/S0006-3495(83)84327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J., Latorre R. Influence of cholesterol on water penetration into bilayers. Science. 1982 Apr 2;216(4541):65–67. doi: 10.1126/science.7063872. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Deuterium nuclear magnetic resonance investigation of deuterium-labelled N-hexadeconoylgalactosylceramides (cerebrosides). Biochim Biophys Acta. 1979 Sep 21;556(2):208–218. doi: 10.1016/0005-2736(79)90043-9. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Deuterium nuclear magnetic resonance studies of N-palmitoylglucosylceramide (cerebroside) head group structure. Biochemistry. 1982 Jun 22;21(13):3154–3160. doi: 10.1021/bi00256a019. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Stoffel W., Sorgo W. Asymmetry of the lipid-bilayer of Sindbis virus. Chem Phys Lipids. 1976 Oct;17(2-3):324–335. doi: 10.1016/0009-3084(76)90077-3. [DOI] [PubMed] [Google Scholar]
- Witter B., Debuch H., Klein H. Lipid investigation of central and peripheral nervous system in connatal Pelizaeus-Merzbacher's disease. J Neurochem. 1980 Apr;34(4):957–962. doi: 10.1111/j.1471-4159.1980.tb09671.x. [DOI] [PubMed] [Google Scholar]