Abstract
Although fluorescence photobleaching recovery (FPR) experiments are usually interpreted in terms of the translational motions of a fluorescently labeled species, rotational motions can also modulate recovery through the cosine-squared laws for dipolar absorption and emission processes. In a complex interacting system, translational and rotational contributions may both be simultaneously present. We show how these contributions can be separated in solution studies using an FPR setup in which (a) the linear polarization of the low-intensity observation beam and the high-intensity photobleaching pulse can be varied independently, and (b) all emitted fluorescent photons are counted equally. The fluorescence recovery signal obtained with the observation beam polarized at the magic angle, 54.7 degrees, from the bleach polarization direction is independent of label orientation, whereas the anisotropy function formed from a combination of parallel and perpendicular polarizations isolates the orientational recovery. The anisotropy function is identical to that in fluorescence correlation spectroscopy and, for rigid-body rotational diffusion, can be expressed as a sum of five exponential terms.
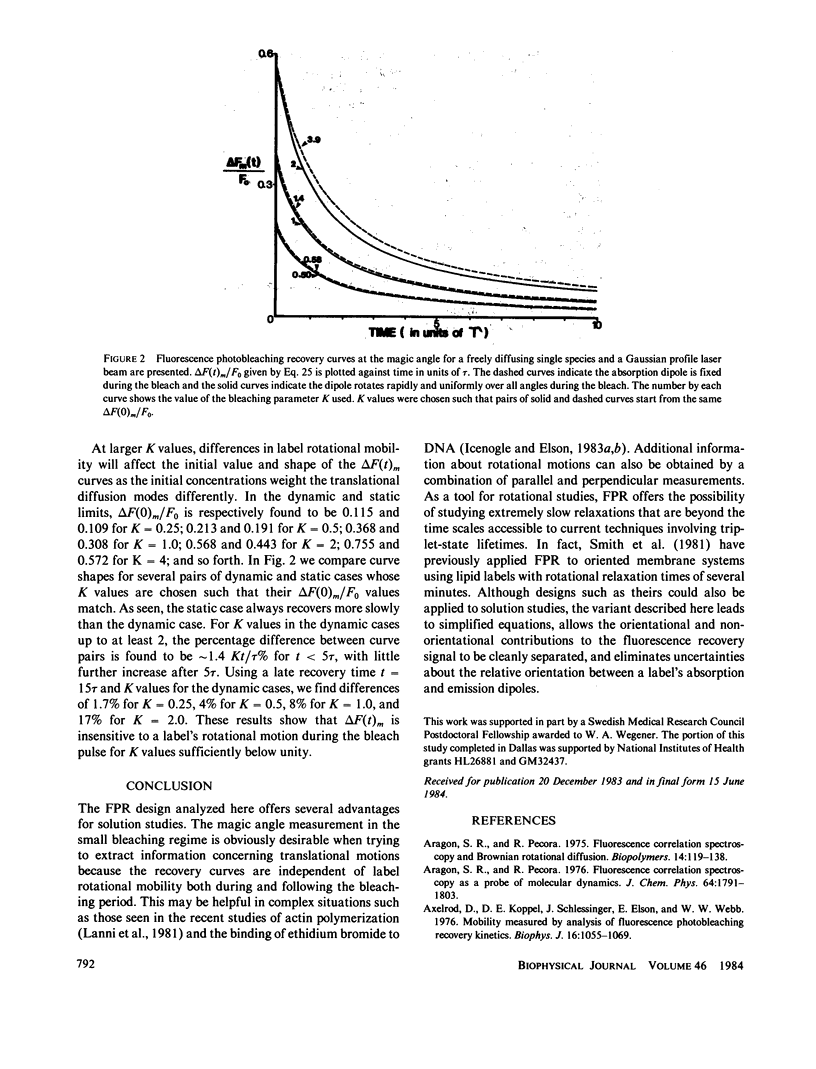
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg M., Rigler R. Fluorescence correlation spectroscopy applied to rotational diffusion of macromolecules. Q Rev Biophys. 1976 Feb;9(1):69–81. doi: 10.1017/s003358350000216x. [DOI] [PubMed] [Google Scholar]
- Icenogle R. D., Elson E. L. Fluorescence correlation spectroscopy and photobleaching recovery of multiple binding reactions. I. Theory and FCS measurements. Biopolymers. 1983 Aug;22(8):1919–1948. doi: 10.1002/bip.360220808. [DOI] [PubMed] [Google Scholar]
- Icenogle R. D., Elson E. L. Fluorescence correlation spectroscopy and photobleaching recovery of multiple binding reactions. II. FPR and FCS measurements at low and high DNA concentrations. Biopolymers. 1983 Aug;22(8):1949–1966. doi: 10.1002/bip.360220809. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni F., Taylor D. L., Ware B. R. Fluorescence photobleaching recovery in solutions of labeled actin. Biophys J. 1981 Aug;35(2):351–364. doi: 10.1016/S0006-3495(81)84794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Weis R. M., McConnell H. M. Measurement of rotational motion in membranes using fluorescence recovery after photobleaching. Biophys J. 1981 Oct;36(1):73–91. doi: 10.1016/S0006-3495(81)84717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. A. Fluorescence recovery spectroscopy as a probe of slow rotational motions. Biophys J. 1984 Dec;46(6):795–803. doi: 10.1016/S0006-3495(84)84078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


