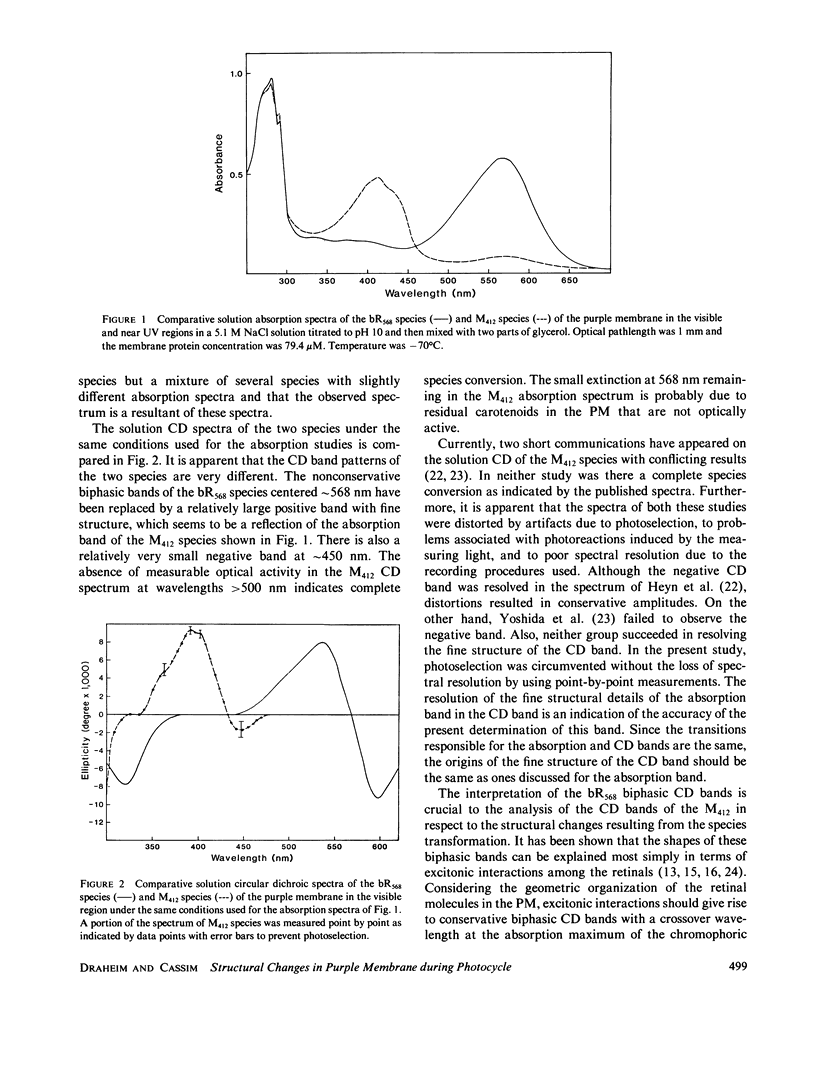
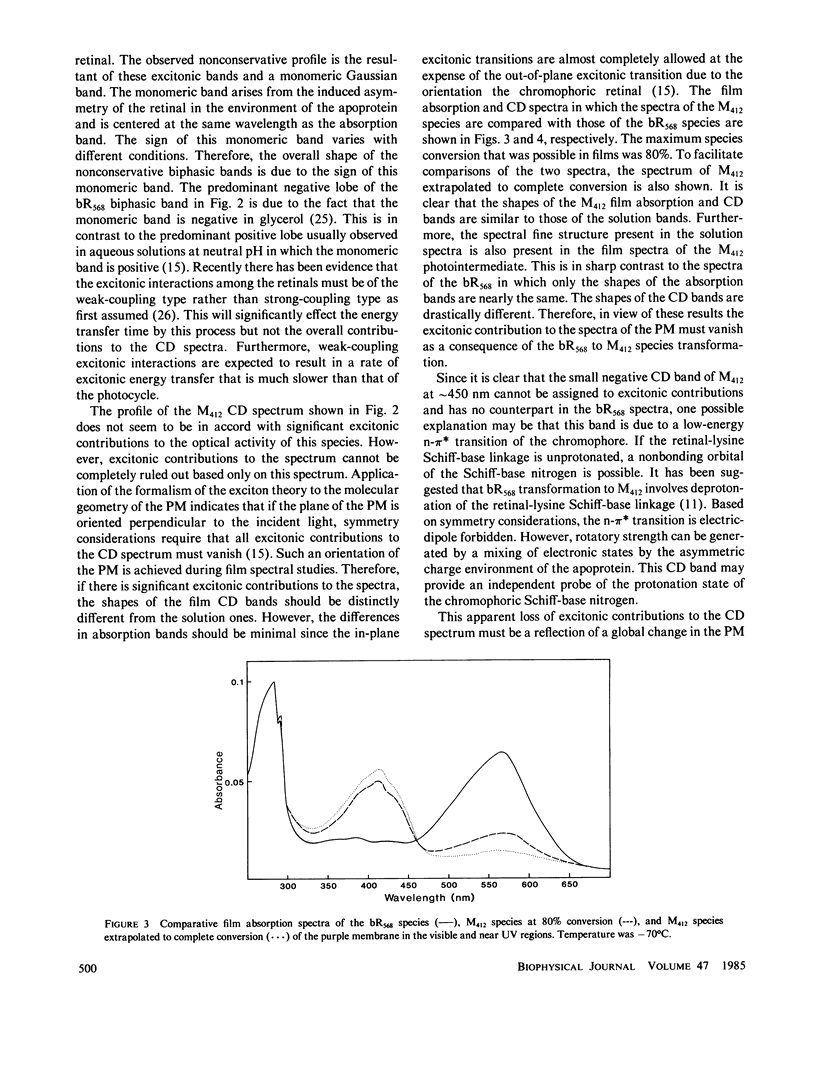
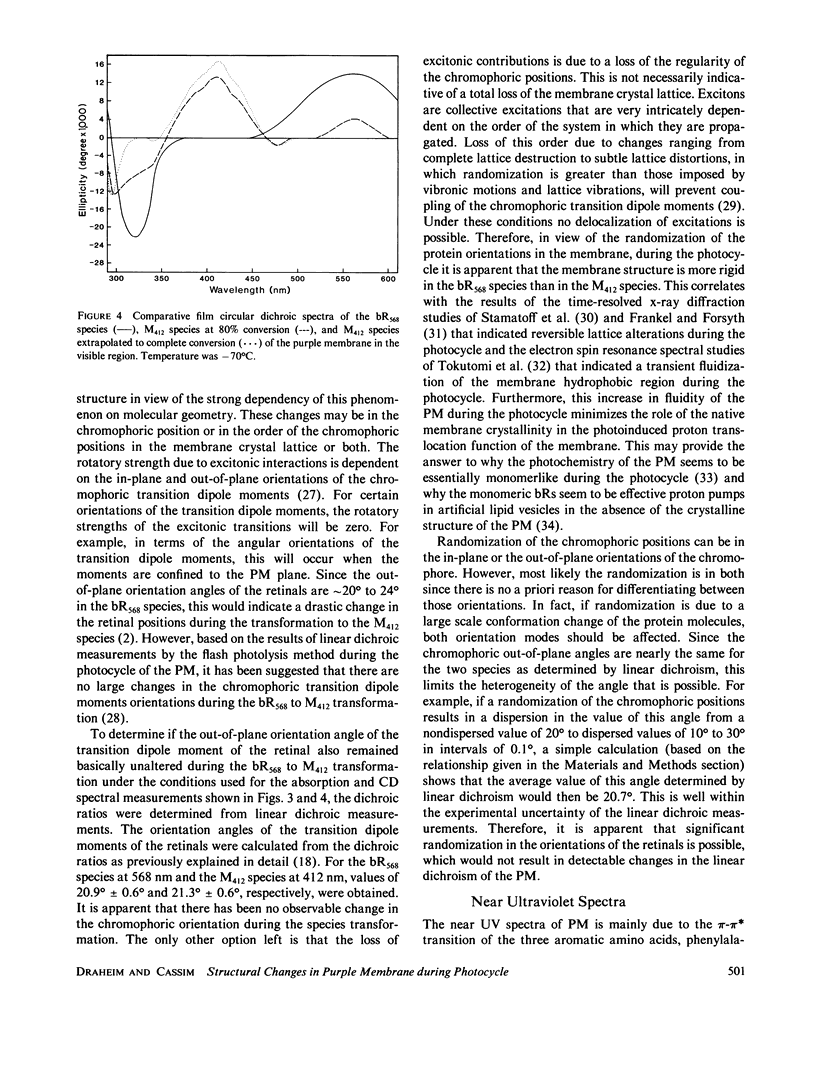
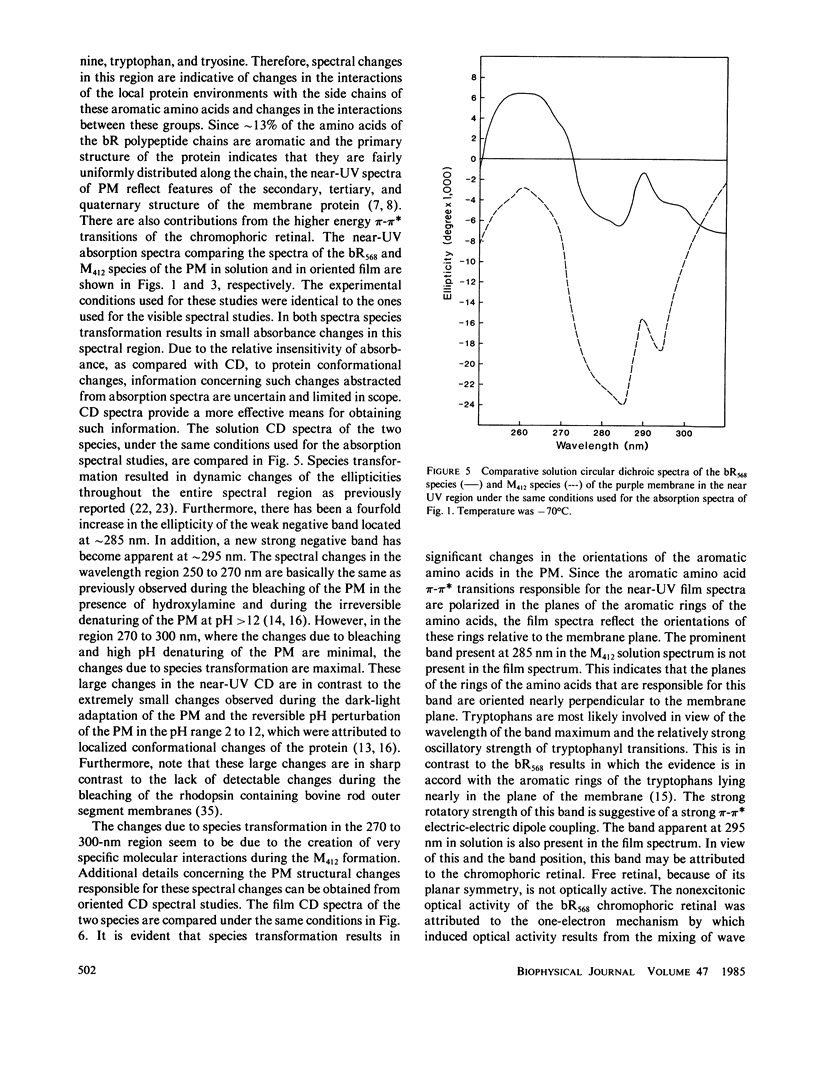
Abstract
Both the solution and the oriented film absorption and circular dichroic spectra of the bacteriorhodopsin (bR568) and M412 intermediate of the purple membrane photocycle were compared over the wavelength region 800-183 nm to assess structural changes during this photocycle. The main findings are (a) loss of the excitonic interaction among the chromophoric retinal transitions indicating disordering of the retinal orientations in the membrane and distortions of the membrane hexagonal crystal lattice, (b) structural change of the chromophoric retinal, (c) changes in the key interactions between the retinal and specific groups in the local environment of the apoprotein, (d) significant changes of the tertiary structure of the bR with negligible secondary structure involvement, and (e) a net tilting of the rodlike segments of the bR polypeptides away from the membrane normal. These findings are in accord with large scale global structural changes of the membrane during the photocycle and with structural metastability of the bR molecules. An important implication of these changes is the possibility of transmembrane retinal-regulated pulsating channels during the photocycle. The significance of this possibility in respect to models for the proton translocation function of this membrane is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K., Dollinger G., Eisenstein L., Singh A. K., Zimányi L. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4972–4976. doi: 10.1073/pnas.79.16.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of bleaching and regeneration on the purple membrane structure of Halobaterium halobium. Biophys J. 1977 Sep;19(3):285–297. doi: 10.1016/s0006-3495(77)85588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czégé J., Dér A., Zimányi L., Keszthelyi L. Restriction of motion of protein side chains during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7273–7277. doi: 10.1073/pnas.79.23.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Bacteriorhodopsin monomers pump protons. FEBS Lett. 1979 Dec 15;108(2):307–310. doi: 10.1016/0014-5793(79)80552-9. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- El-Sayed M. A., Karvaly B., Fukumoto J. M. Primary step in the bacteriorhodopsin photocycle: photochemistry or excitation transfer? Proc Natl Acad Sci U S A. 1981 Dec;78(12):7512–7516. doi: 10.1073/pnas.78.12.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretation of the absorption and circular dichroic spectra of oriented purple membrane films. Biophys J. 1979 Jun;26(3):427–440. doi: 10.1016/S0006-3495(79)85263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretations of the effects of pH on the spectra of purple membrane. J Mol Biol. 1979 Dec 15;135(3):595–609. doi: 10.1016/0022-2836(79)90166-9. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G. K., Cassim J. Y. Orientations of the retinyl and the heme chromophores in the brown membrane of Halobacterium halobium. J Mol Biol. 1981 Oct 15;152(1):35–47. doi: 10.1016/0022-2836(81)90094-2. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., TenEyck L. F. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:295–310. doi: 10.1101/sqb.1972.036.01.040. [DOI] [PubMed] [Google Scholar]
- Rafferty C. N., Cassim J. Y., McConnell D. G. Circular dichroism, optical rotatory dispersion, and absorption studies on the conformation of bovine rhodopsin iw situ and solubilized with detergent. Biophys Struct Mech. 1977 Mar 2;2(4):227–320. [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H. Light energy conversion in Halobacterium halobium. J Supramol Struct. 1974;2(5-6):769–774. doi: 10.1002/jss.400020519. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ohno K., Takeuchi Y., Kagawa Y. Prolonged lifetime of the 410-nm intermediate of bacteriorhodopsin in the presence of guanidine hydrochloride. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1111–1116. doi: 10.1016/0006-291x(77)91497-8. [DOI] [PubMed] [Google Scholar]


