Abstract
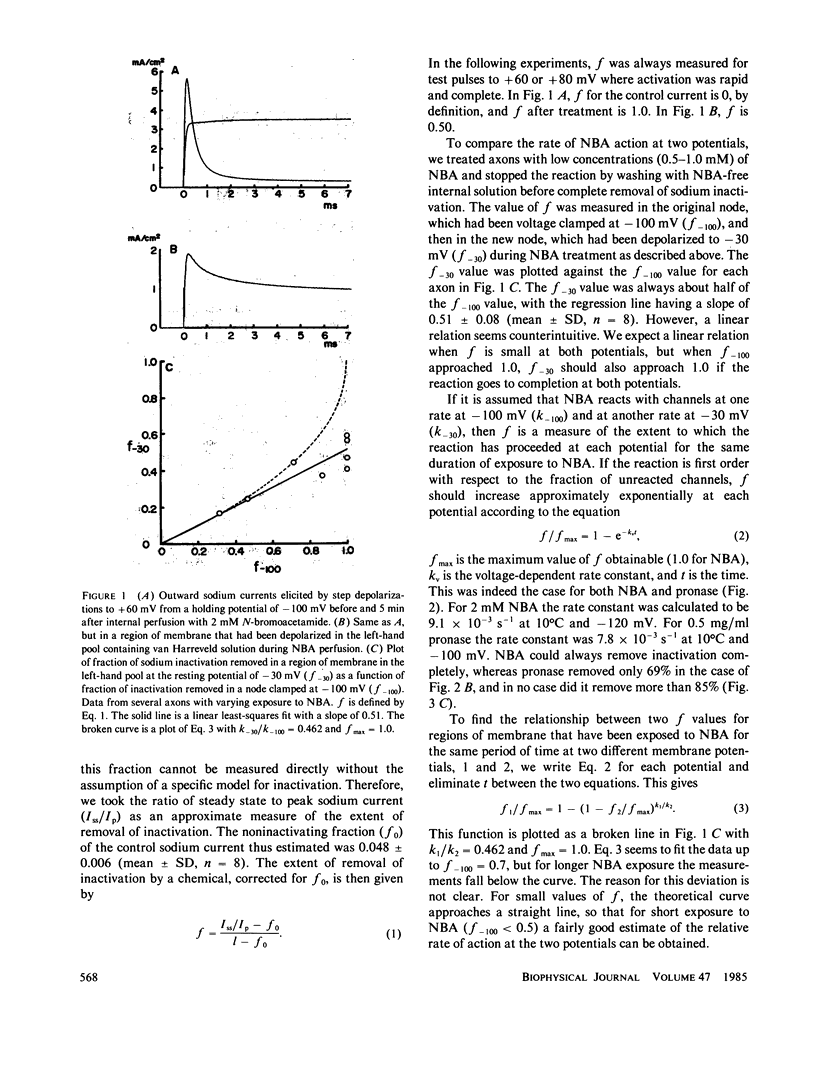
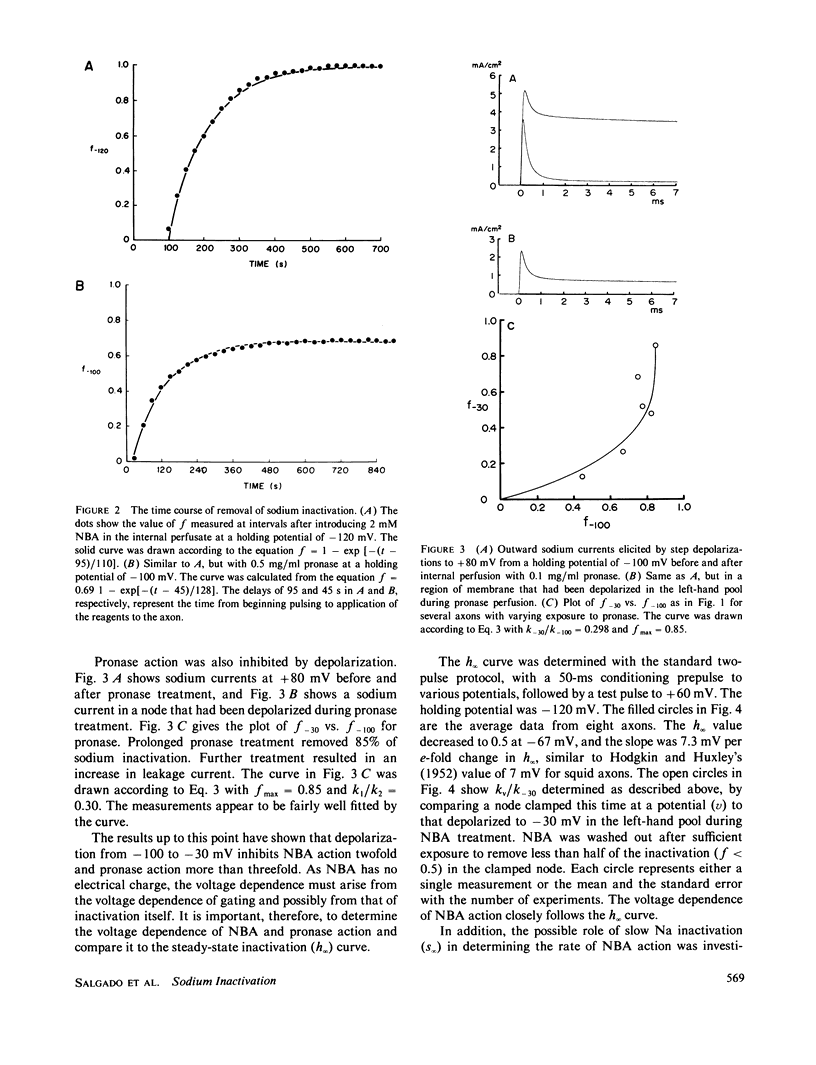
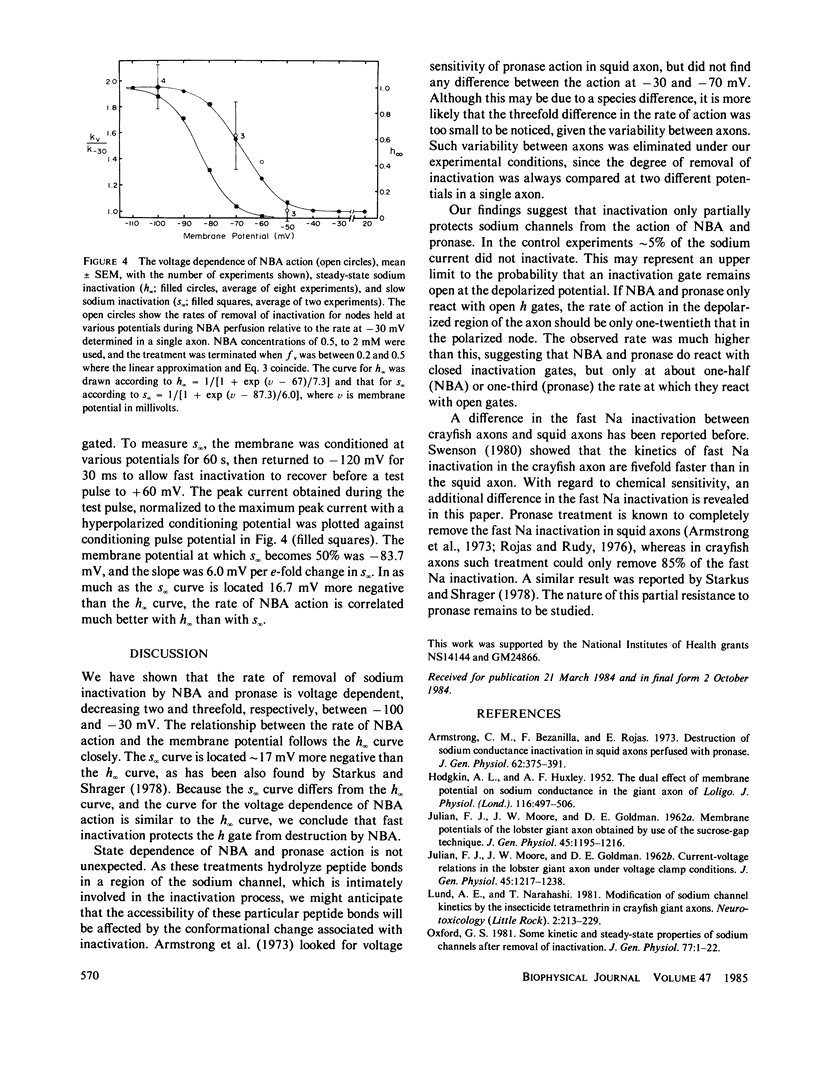
When perfused internally through crayfish giant axons, pronase removed sodium inactivation more than three times as fast at -100 mV as compared with -30 mV. N-bromoacetamide, applied internally, removed sodium inactivation twice as fast at -100 mV as at -30 mV, and the relative rate of removal declined with membrane depolarization in proportion to steady-state sodium inactivation. We conclude that in the closed conformation the sodium inactivation gate is partially protected from destruction by N-bromoacetamide and pronase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Membrane potentials of the lobster giant axon obtained by use of the sucrose-gap technique. J Gen Physiol. 1962 Jul;45:1195–1216. doi: 10.1085/jgp.45.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. E., Narahashi T. Modification of sodium channel kinetics by the insecticide tetramethrin in crayfish giant axons. Neurotoxicology. 1981 Oct;2(2):213–229. [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler J. P., Valenzeno D. P. Reexamination of the double sucrose gap technique for the study of lobster giant axons. Theory and experiments. Biophys J. 1983 Nov;44(2):261–269. doi: 10.1016/S0006-3495(83)84298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Rudy B. Destruction of the sodium conductance inactivation by a specific protease in perfused nerve fibres from Loligo. J Physiol. 1976 Nov;262(2):501–531. doi: 10.1113/jphysiol.1976.sp011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Shrager P. Modification of slow sodium inactivation in nerve after internal perfusion with trypsin. Am J Physiol. 1978 Nov;235(5):C238–C244. doi: 10.1152/ajpcell.1978.235.5.C238. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Jr Gating charge immobilization and sodium current inactivation in internally perfused crayfish axons. Nature. 1980 Oct 16;287(5783):644–645. doi: 10.1038/287644a0. [DOI] [PubMed] [Google Scholar]


