Abstract
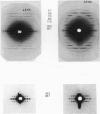
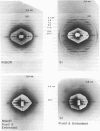
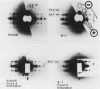
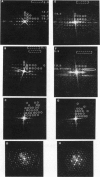
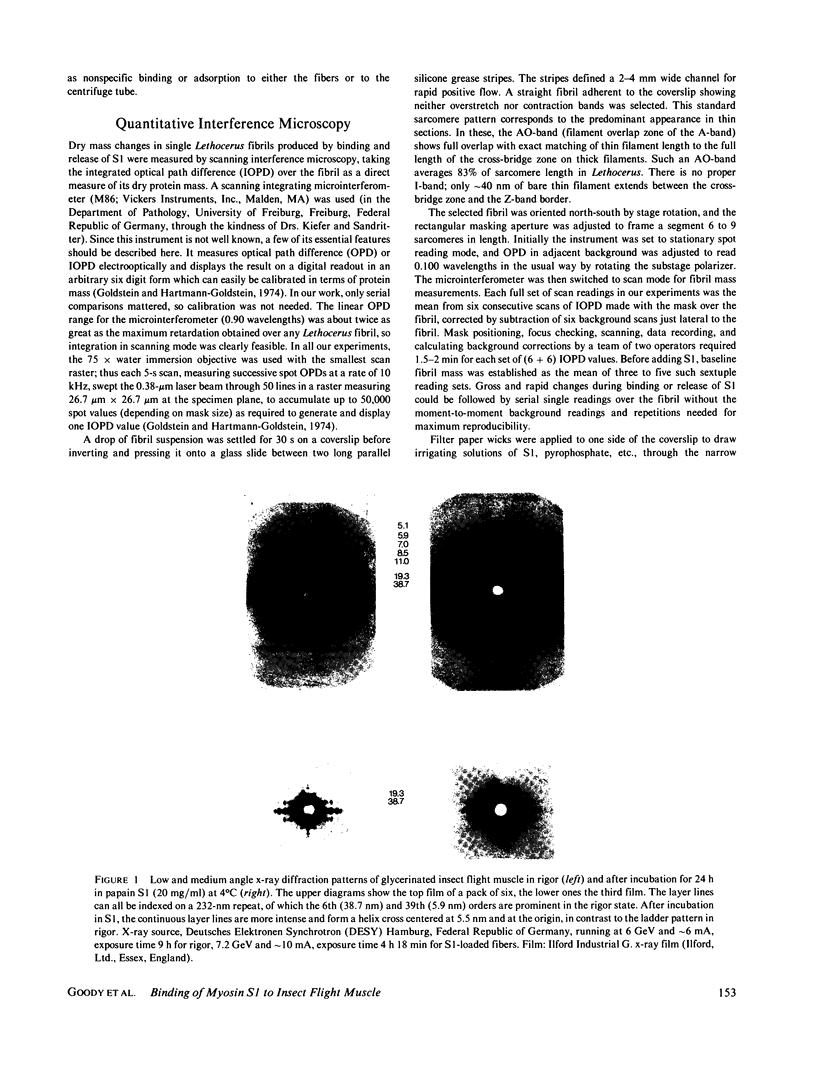
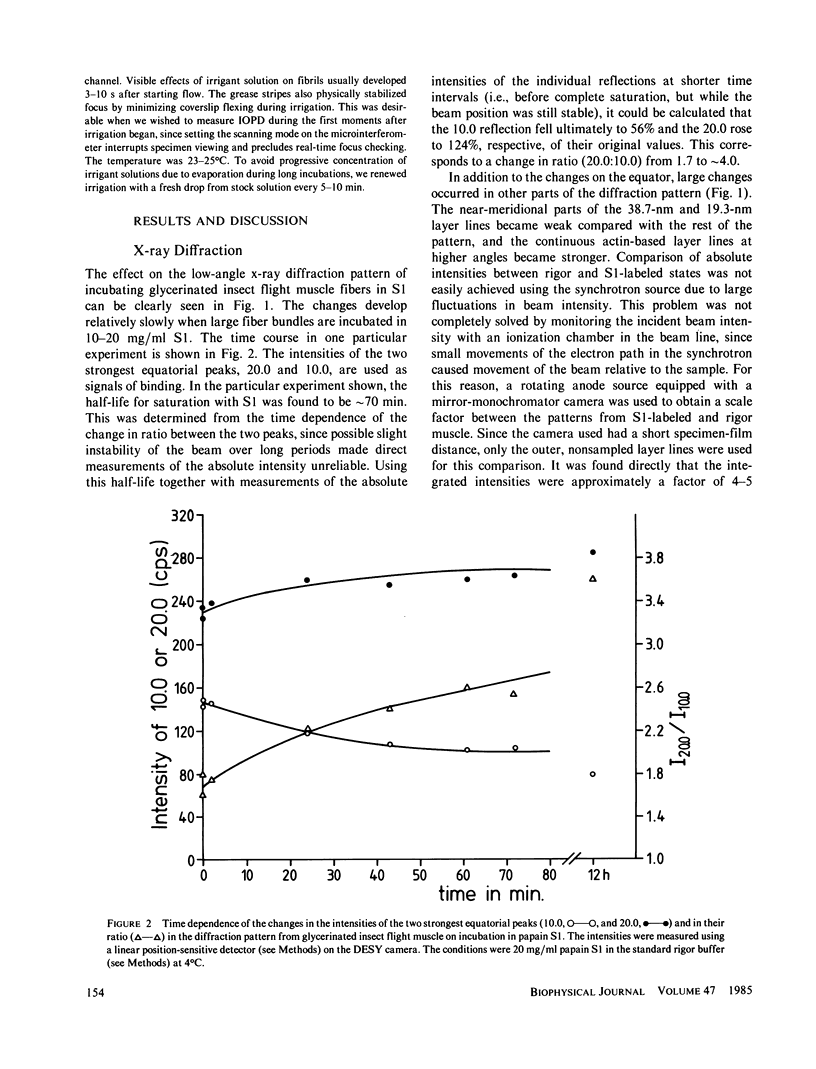
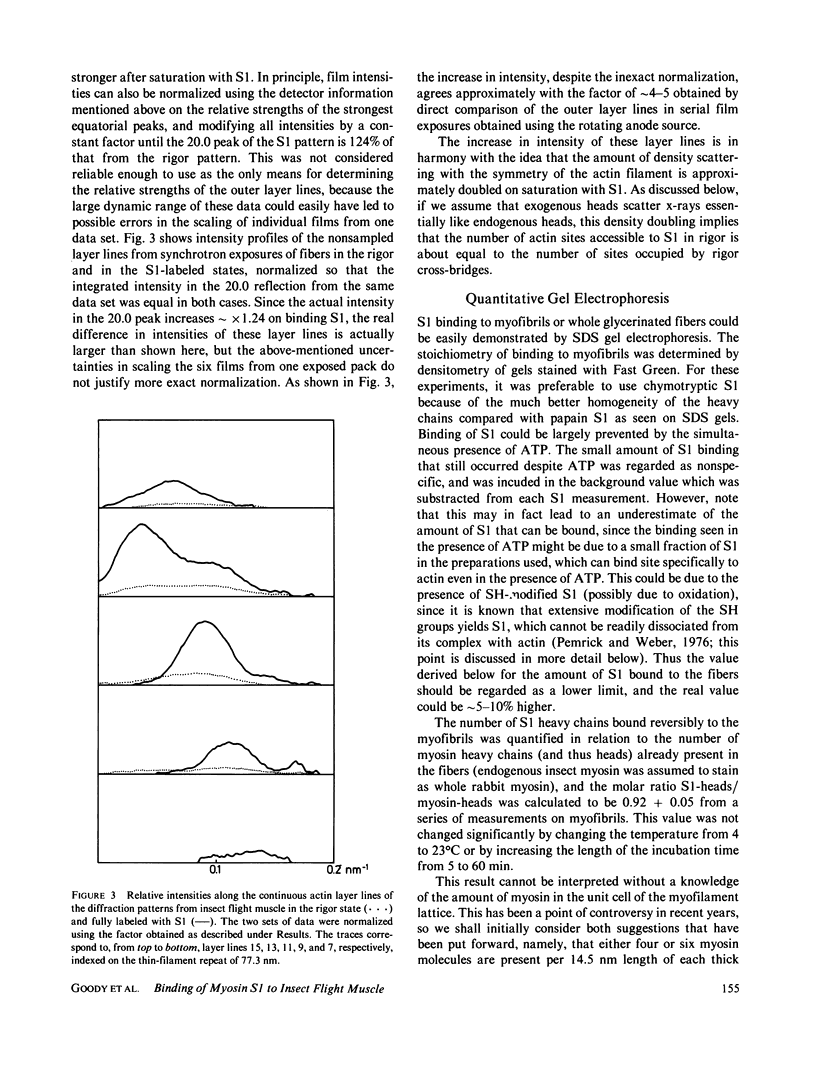
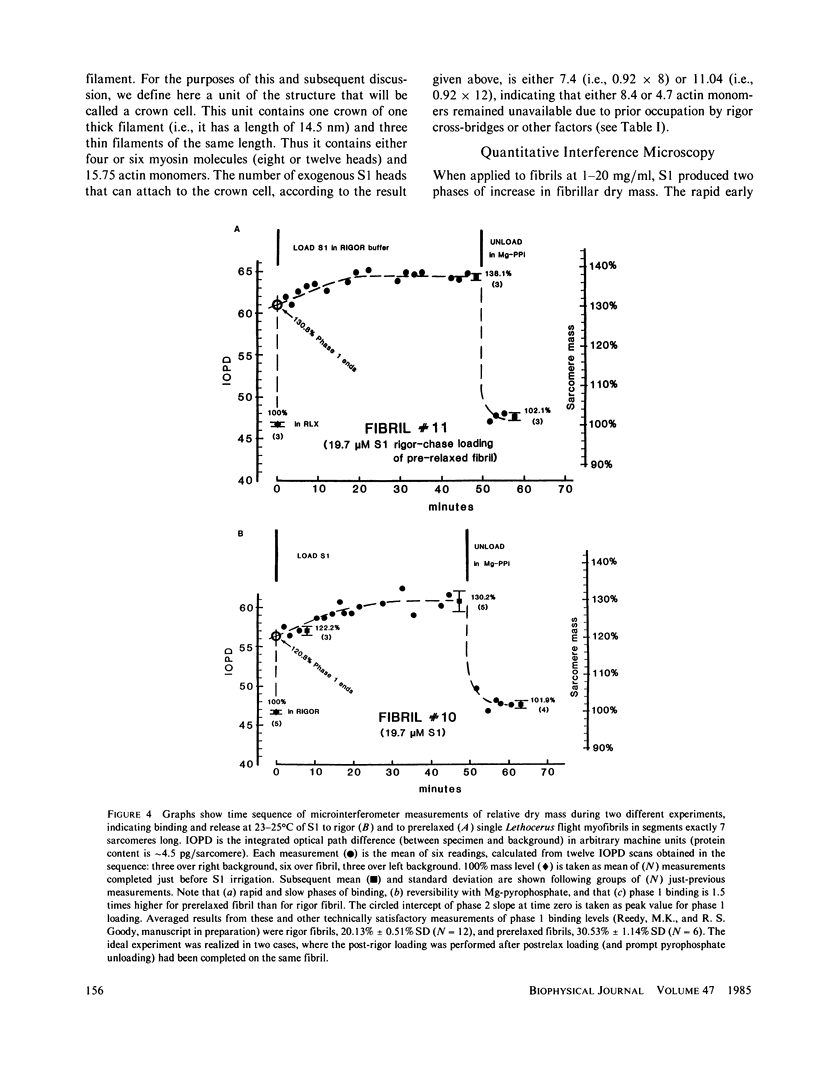
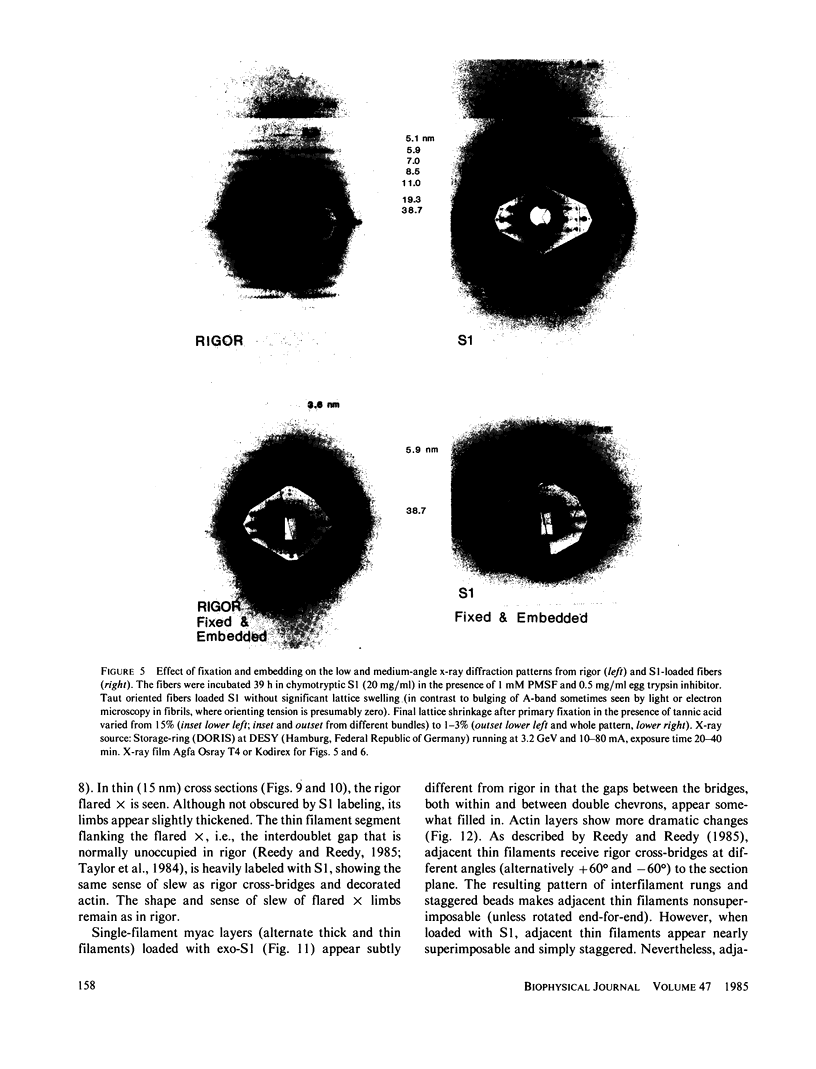
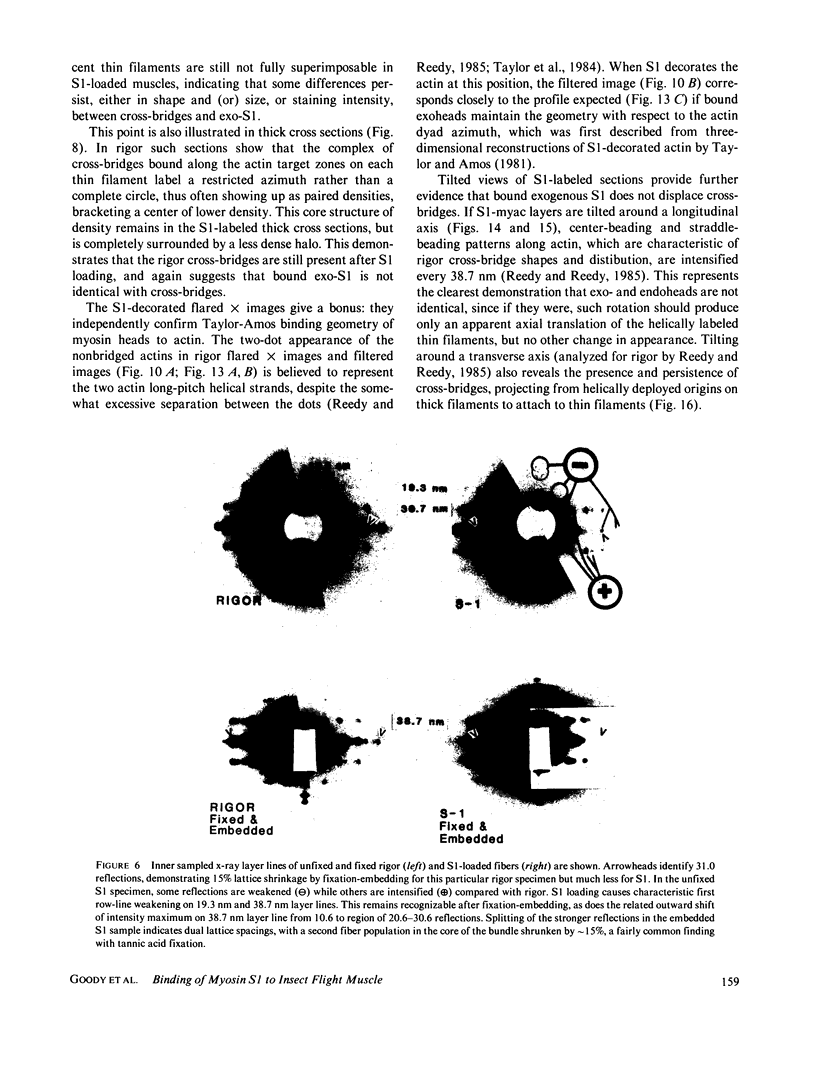
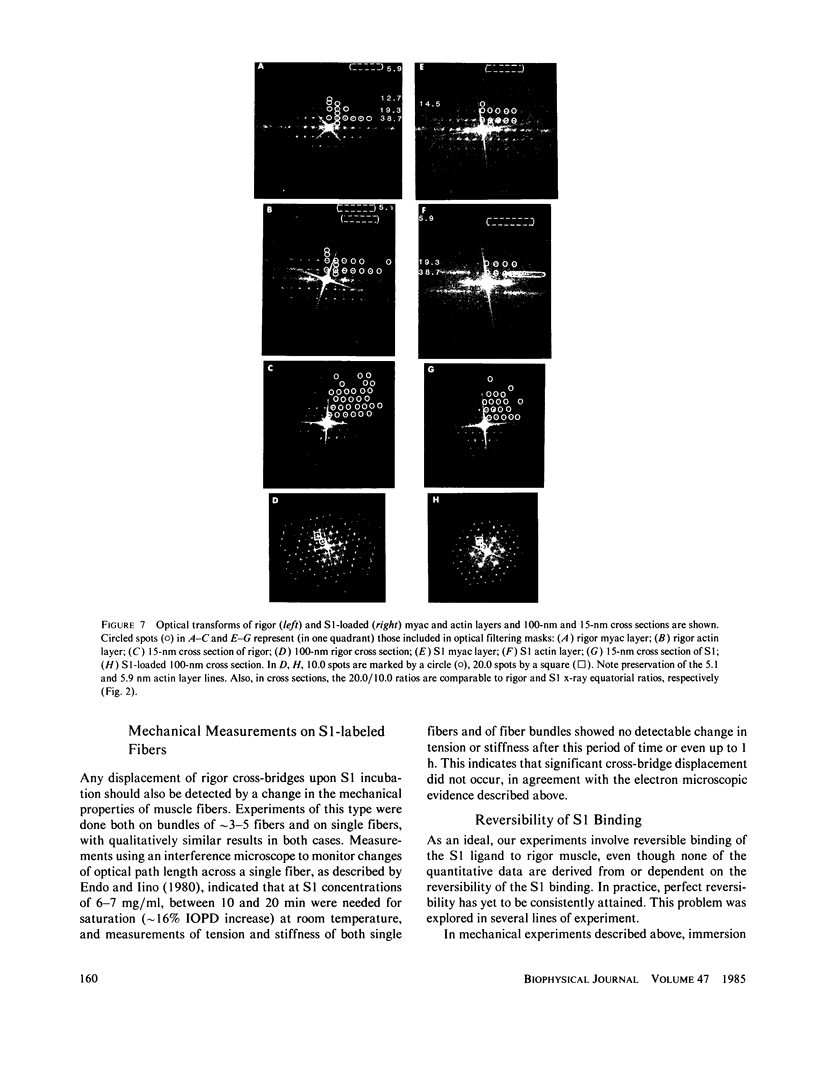
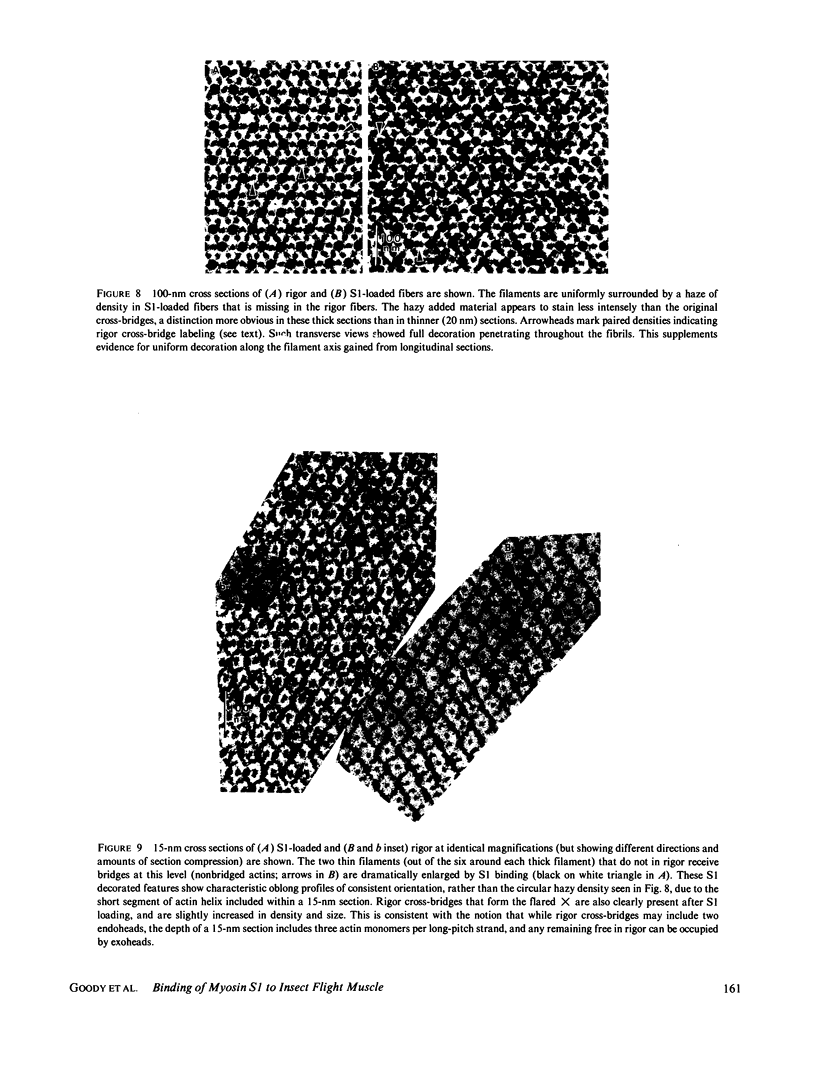
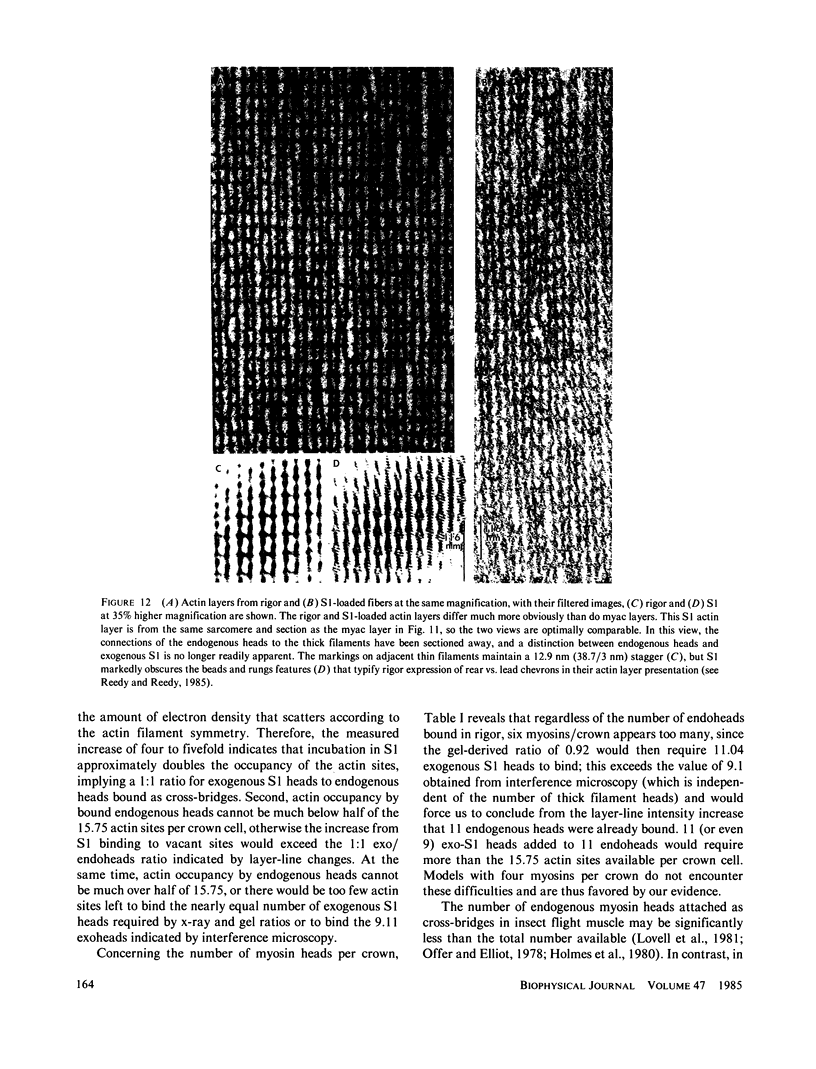
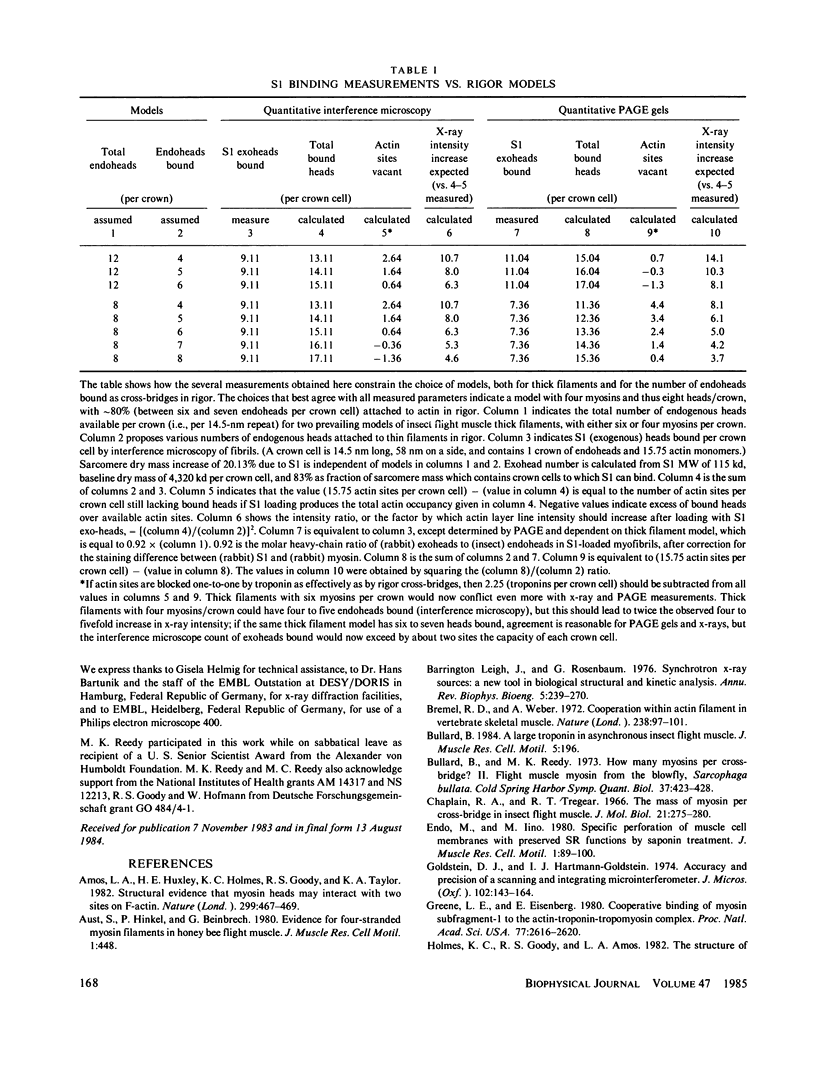
The binding of rabbit muscle myosin subfragment 1 (S1) to glycerinated insect flight muscle fibers has been studied by low-angle x-ray diffraction, quantitative sodium dodecyl sulfate gel electrophoresis, quantitative interference microscopy, and electron microscopy. Changes induced in the rigor x-ray diffraction pattern are consistent with the idea that vacant myosin-binding sites on thin filaments are filled by exogenous S1. Electron microscopy indicates that S1 permeates and labels fibers and fibrils completely. Electron micrographs also show that cross-bridges are not displaced by exogenous S1 under the conditions used, and this is supported by the unchanged mechanical stiffness of the S1-labeled fibers. The amount of bound S1, as measured by gel electrophoresis and interference microscopy, together with the magnitude of the intensity changes in the x-ray diffraction pattern, is consistent with a thick filament structure that contains four molecules of endogenous myosin per 14.5 nm of its length, but does not agree well with earlier estimates of six myosins per crown. Lack of information on possible inhibition of S1-binding by factors other than the presence of cross-bridges, e.g., troponin, render uncertain calculations of the number of attached cross-bridges in the rigor state. However, it appears that at least 75% of the endogenous myosin heads are attached. Occupancy of binding sites on thin filaments after incubation with S1 is high, probably greater than 85%, so that x-ray scattering from those parts of the structure that adhere to the symmetry of the thin filaments can be treated as diffraction from S1-decorated thin filaments. In addition, we show in thin flared X cross sections that exo-S1 heads bind to actin with the geometry described in decorated actin by Taylor, K.A., and L.A. Amos.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Huxley H. E., Holmes K. C., Goody R. S., Taylor K. A. Structural evidence that myosin heads may interact with two sites on F-actin. Nature. 1982 Sep 30;299(5882):467–469. doi: 10.1038/299467a0. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Chaplain R. A., Tregear R. T. The mass of myosin per cross-bridge in insect fibrillar flight muscle. J Mol Biol. 1966 Nov 14;21(2):275–280. doi: 10.1016/0022-2836(66)90098-2. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. C., Goody R. S., Amos L. A. The structure of S1-decorated actin filaments calculated from x-ray diffraction data with phases derived from electron micrographs. Ultramicroscopy. 1982;9(1-2):37–44. doi: 10.1016/0304-3991(82)90227-3. [DOI] [PubMed] [Google Scholar]
- Konrad M., Goody R. S. Kinetic and thermodynamic properties of the ternary complex between F-actin, myosin subfragment 1 and adenosine 5'-[beta, gamma-imido]triphosphate. Eur J Biochem. 1982 Nov 15;128(2-3):547–555. doi: 10.1111/j.1432-1033.1982.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Leigh J. B. Synchrotron x-ray sources: a new tool in biological structural and kinetic analysis. Annu Rev Biophys Bioeng. 1976;5:239–270. doi: 10.1146/annurev.bb.05.060176.001323. [DOI] [PubMed] [Google Scholar]
- Lovell S. J., Knight P. J., Harrington W. F. Fraction of myosin heads bound to thin filaments in rigor fibrils from insect flight and vertebrate muscles. Nature. 1981 Oct 22;293(5834):664–666. doi: 10.1038/293664a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Offer G., Elliott A. Can a myosin molecule bind to two actin filaments? Nature. 1978 Jan 26;271(5643):325–329. doi: 10.1038/271325a0. [DOI] [PubMed] [Google Scholar]
- Pemrick S., Weber A. Mechanism of inhibition of relaxation by N-ethylmaleimide treatment of myosin. Biochemistry. 1976 Nov 16;15(23):5193–5198. doi: 10.1021/bi00668a038. [DOI] [PubMed] [Google Scholar]
- Potter J. D. The content of troponin, tropomyosin, actin, and myosin in rabbit skeletal muscle myofibrils. Arch Biochem Biophys. 1974 Jun;162(2):436–441. doi: 10.1016/0003-9861(74)90202-1. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Goody R. S., Hofmann W., Rosenbaum G. Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. I. X-ray diffraction monitoring during preparation for electron microscopy of muscle fibres fixed in rigor, in ATP and in AMPPNP. J Muscle Res Cell Motil. 1983 Feb;4(1):25–53. doi: 10.1007/BF00711957. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Leonard K. R., Freeman R., Arad T. Thick myofilament mass determination by electron scattering measurements with the scanning transmission electron microscope. J Muscle Res Cell Motil. 1981 Mar;2(1):45–64. doi: 10.1007/BF00712061. [DOI] [PubMed] [Google Scholar]
- Reedy M. K. Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice. J Mol Biol. 1968 Jan 28;31(2):155–176. doi: 10.1016/0022-2836(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Squire J. M. General model of myosin filament structure. II. Myosin filaments and cross-bridge interactions in vertebrate striated and insect flight muscles. J Mol Biol. 1972 Dec 14;72(1):125–138. doi: 10.1016/0022-2836(72)90074-5. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Amos L. A. A new model for the geometry of the binding of myosin crossbridges to muscle thin filaments. J Mol Biol. 1981 Apr 5;147(2):297–324. doi: 10.1016/0022-2836(81)90442-3. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Reedy M. C., Córdova L., Reedy M. K. Three-dimensional reconstruction of rigor insect flight muscle from tilted thin sections. 1984 Jul 26-Aug 1Nature. 310(5975):285–291. doi: 10.1038/310285a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R., Barnett V. A. Orientation and rotational mobility of spin-labelled myosin heads in insect flight muscle in rigor. J Muscle Res Cell Motil. 1983 Jun;4(3):367–378. doi: 10.1007/BF00712002. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear R. T., Squire J. M. Myosin content and filament structure in smooth and striated muscle. J Mol Biol. 1973 Jun 25;77(2):279–290. doi: 10.1016/0022-2836(73)90336-7. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- White D. C. Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol. 1970 Jul;208(3):583–605. doi: 10.1113/jphysiol.1970.sp009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J. S. Structure of the backbone in myosin filaments of muscle. Nature. 1979 Jan 4;277(5691):37–40. doi: 10.1038/277037a0. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Harrison S. C., Leberman R. The minor proteins in tomato bushy stunt and turnip crinkle viruses. Virology. 1974 Jun;59(2):509–515. doi: 10.1016/0042-6822(74)90460-7. [DOI] [PubMed] [Google Scholar]