Abstract
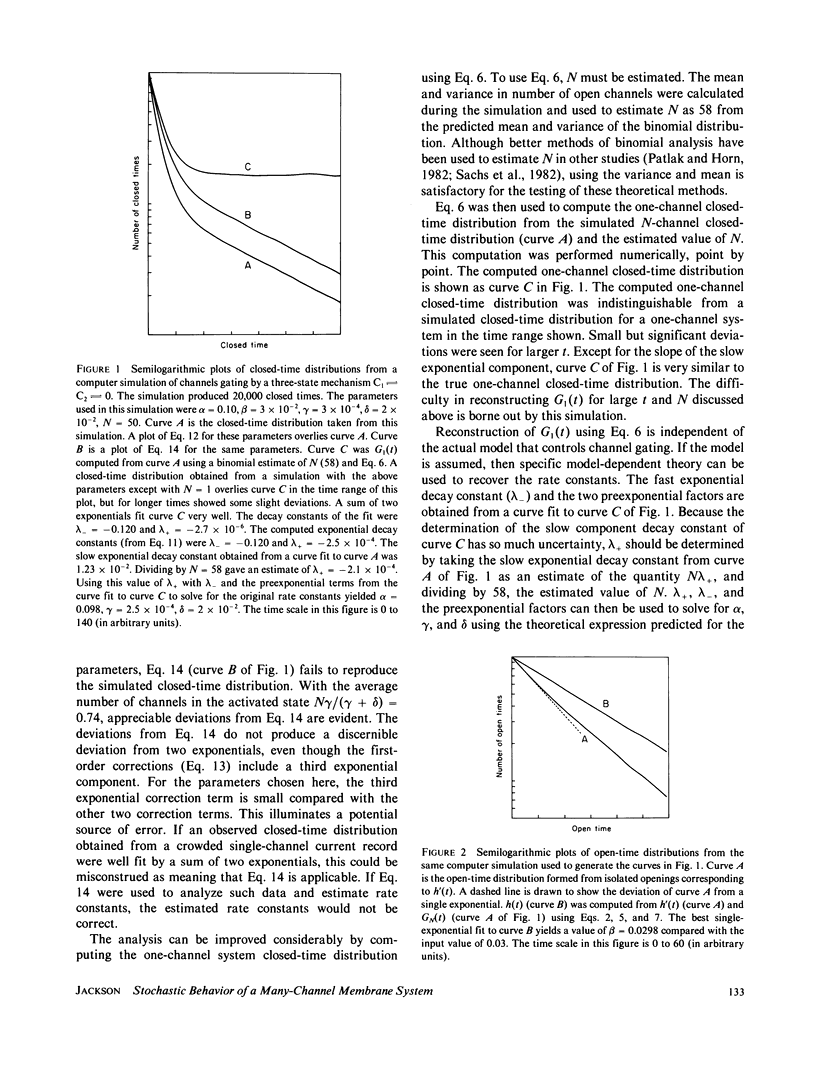
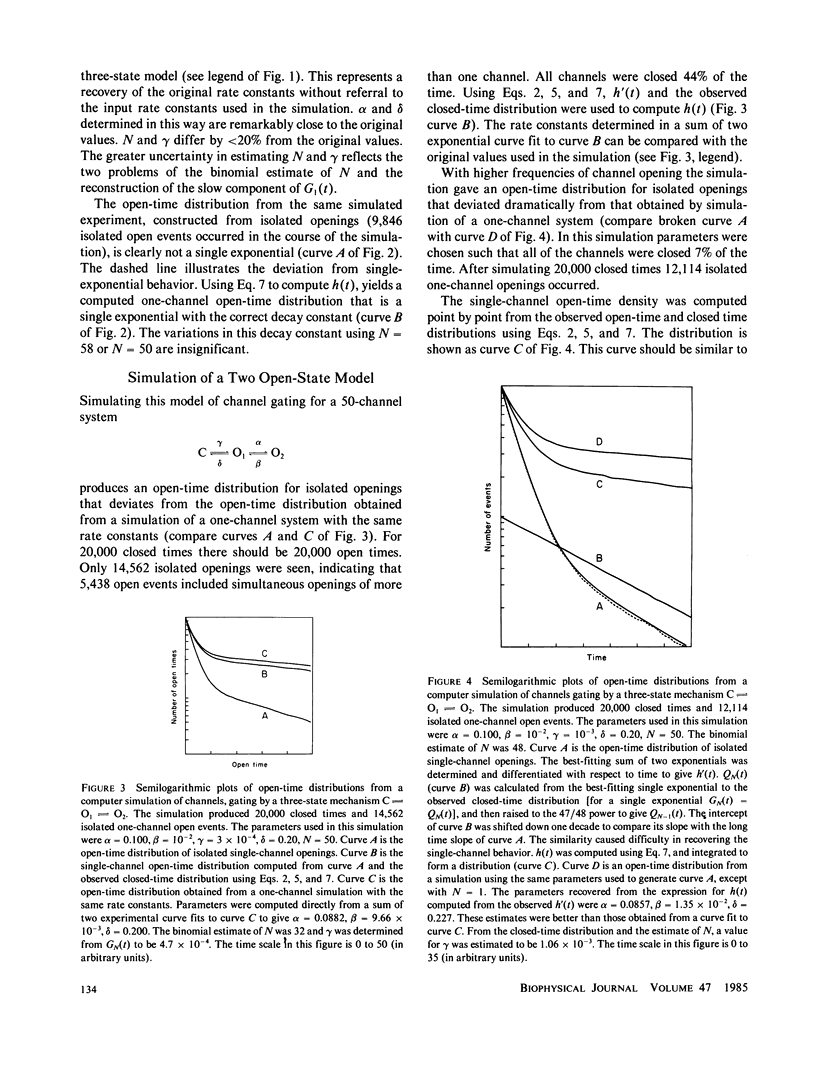
A stochastic theory of channel-gating transitions is developed for a stationary system with many channels, with applications to patch-clamp single-channel experiments. Exact probability density and distribution functions for closed times, open times, and first transit times in an N-channel system are obtained in terms of N and the solutions for a one-channel system. Once N is determined, the expressions derived here can be used to analyze data records that are crowded by many channel openings and where multilevel events are common. The three-state model is treated as a specific example. Computer simulations of three-state models indicate that the equations derived here can be used to recover useful information from crowded single-channel current records. The simulations also revealed some of the limitations to the usefulness of these equations. The probability that a channel that has not opened is in a particular closed state was examined as a function of time. This analysis led to a useful limit where the distribution of unopened channels between various closed states is constant in time. This limit simplifies the mathematical treatment of closed-time probabilities, and provides a general method for the analysis of many-channel systems when channels open infrequently.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Single-channel currents from acetylcholine receptors in embryonic chick muscle. Kinetic and conductance properties of gaps within bursts. Biophys J. 1984 Jan;45(1):187–198. doi: 10.1016/S0006-3495(84)84147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Parker I. Rapid kinetics of single glutamate-receptor channels. Nature. 1982 Feb 4;295(5848):410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Blumenthal R., Latorre R., Lecar H. Kinetics of the opening and closing of individual excitability-inducing material channels in a lipid bilayer. J Gen Physiol. 1974 Jun;63(6):707–721. doi: 10.1085/jgp.63.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gration K. A., Lambert J. J., Ramsey R. L., Rand R. P., Usherwood P. N. Closure of membrane channels gated by glutamate receptors may be a two-step process. Nature. 1982 Feb 18;295(5850):599–603. doi: 10.1038/295599a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Askanas V., Engel W. K. Single cholinergic receptor channel currents in cultured human muscle. J Neurosci. 1982 Oct;2(10):1465–1473. doi: 10.1523/JNEUROSCI.02-10-01465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Mathers D. A., Barker J. L. Single channel currents activated by gamma-aminobutyric acid, muscimol, and (-)-pentobarbital in cultured mouse spinal neurons. J Neurosci. 1982 Jul;2(7):889–894. doi: 10.1523/JNEUROSCI.02-07-00889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. D., Dionne V. E. Single-channel acetylcholine receptor kinetics. Biophys J. 1984 Jan;45(1):153–163. doi: 10.1016/S0006-3495(84)84144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Labarca P., Fredkin D. R., Suarez-Isla B. A. Channel properties of the purified acetylcholine receptor from Torpedo californica reconstituted in planar lipid bilayer membranes. Biophys J. 1984 Jan;45(1):165–174. doi: 10.1016/S0006-3495(84)84145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., Sachs F. Single ionic channels observed in tissue-cultured muscle. Nature. 1979 Dec 20;282(5741):861–863. doi: 10.1038/282861a0. [DOI] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


