Abstract
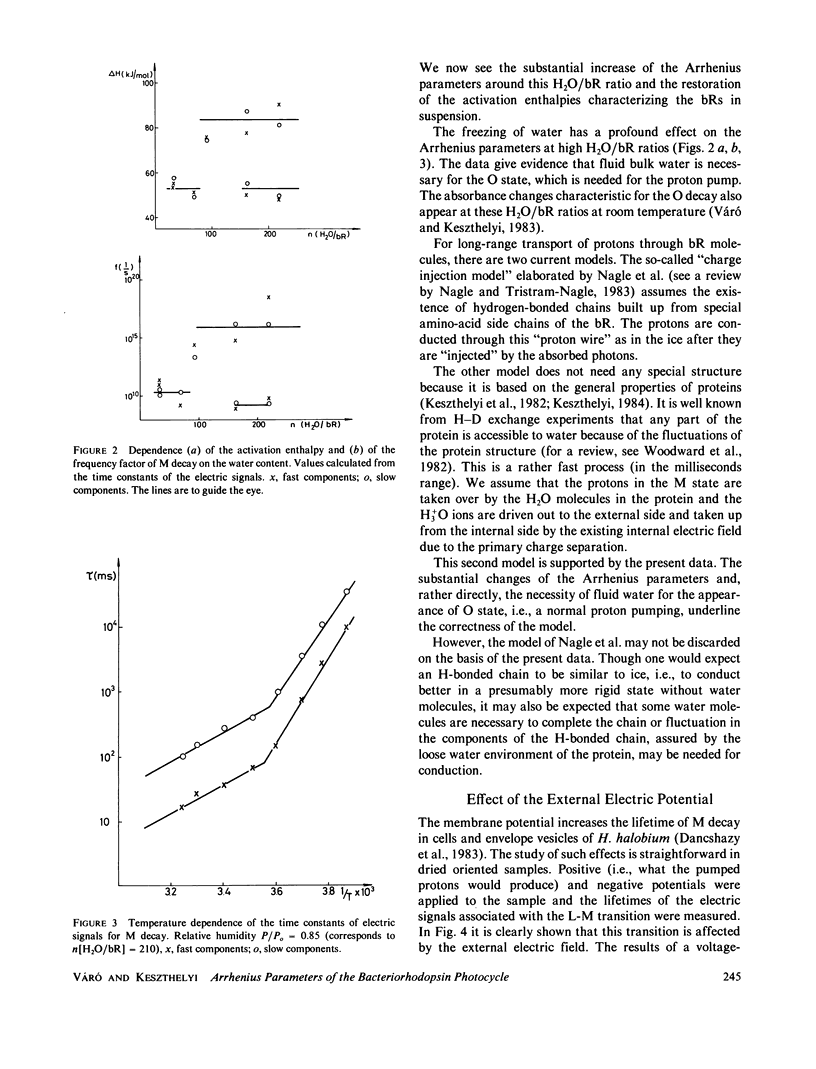
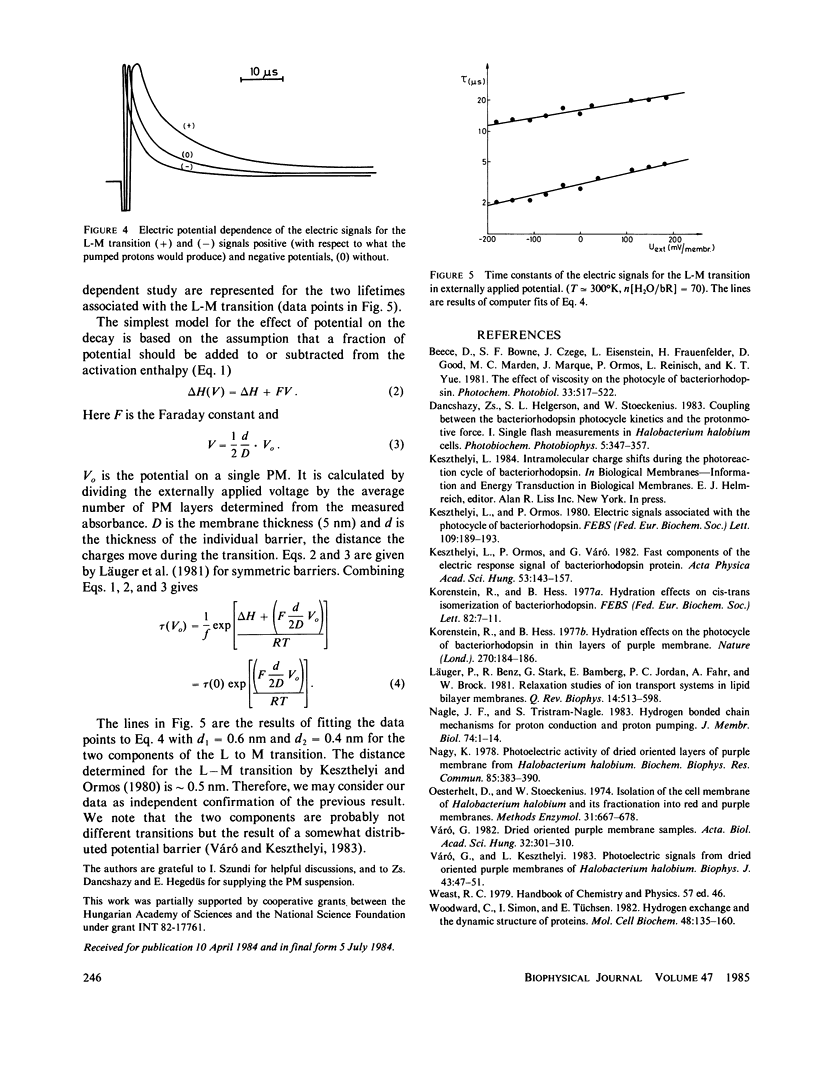
In dried oriented samples of purple membranes isolated from Halobacterium halobium the Arrhenius parameters of the photocycle showed an abrupt change at a water content of approximately 80 H2O molecules per bacteriorhodopsin molecule. This makes probable the existence of a water-dependent conformational change of the protein. This result underlines the importance of water in the proton-conduction mechanism inside the protein. The effect of the external electric potential on the rate constants of the photoelectric signals was also measured. The data demonstrate that the membrane potential affects the steps of the proton transport during the photocycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Korenstein R., Hess B. Hydration effects on cis--trans isomerization of bacteriorhodopsin. FEBS Lett. 1977 Oct 1;82(1):7–11. doi: 10.1016/0014-5793(77)80874-0. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G., Bamberg E., Jordan P. C., Fahr A., Brock W. Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys. 1981 Nov;14(4):513–598. doi: 10.1017/s003358350000247x. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Tristram-Nagle S. Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J Membr Biol. 1983;74(1):1–14. doi: 10.1007/BF01870590. [DOI] [PubMed] [Google Scholar]
- Nagy K. Photoelectric activity of dried, oriented layers of purple membrane from Halobacterium halobium. Biochem Biophys Res Commun. 1978 Nov 14;85(1):383–390. doi: 10.1016/s0006-291x(78)80054-0. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Váró G., Keszthelyi L. Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys J. 1983 Jul;43(1):47–51. doi: 10.1016/S0006-3495(83)84322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C., Simon I., Tüchsen E. Hydrogen exchange and the dynamic structure of proteins. Mol Cell Biochem. 1982 Oct 29;48(3):135–160. doi: 10.1007/BF00421225. [DOI] [PubMed] [Google Scholar]


