Abstract
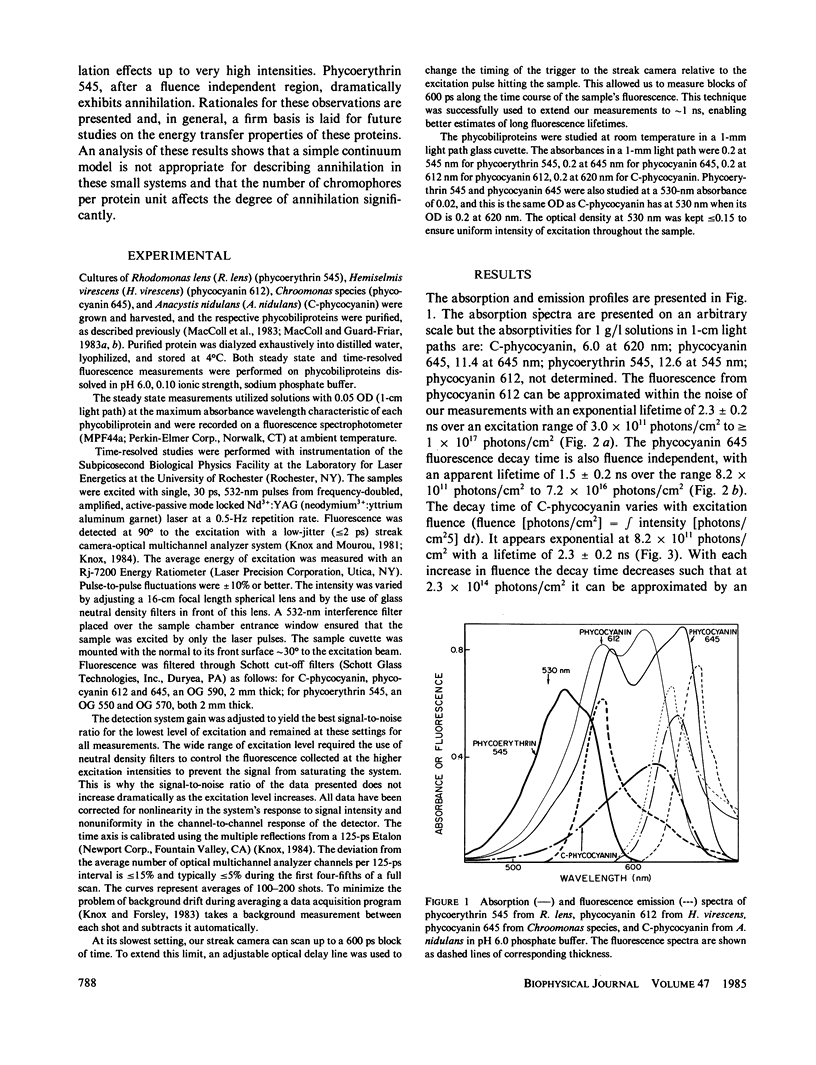
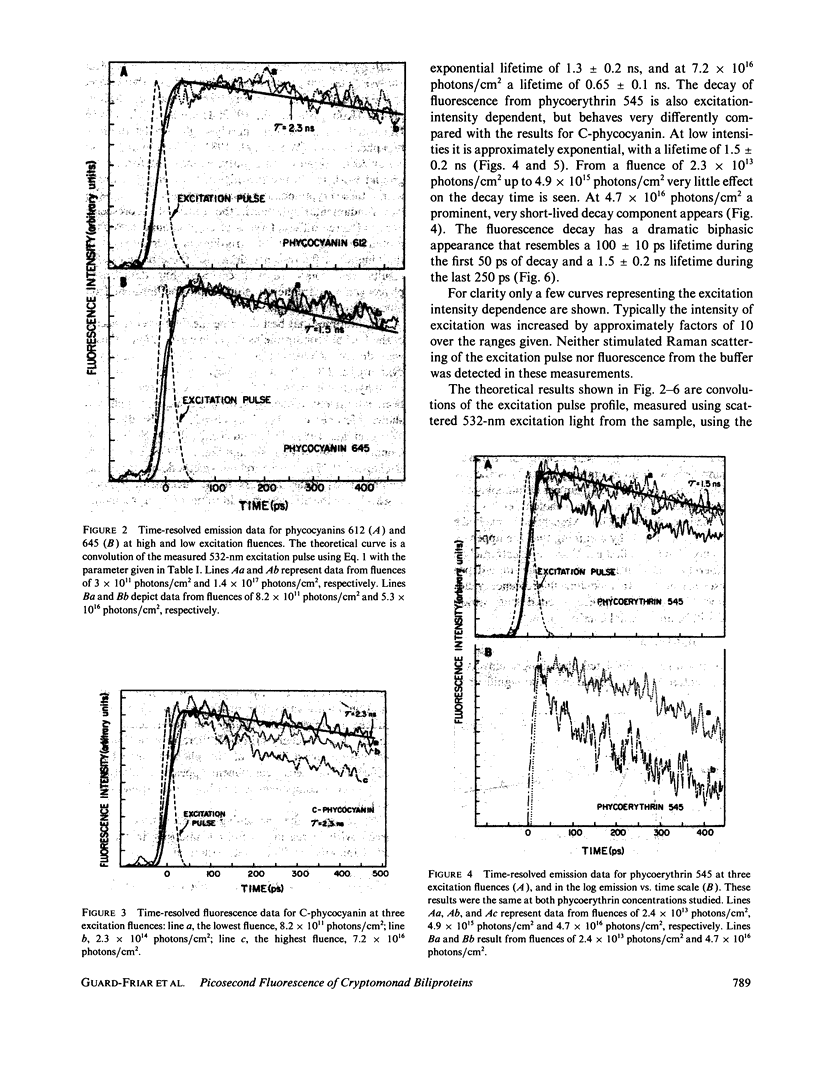
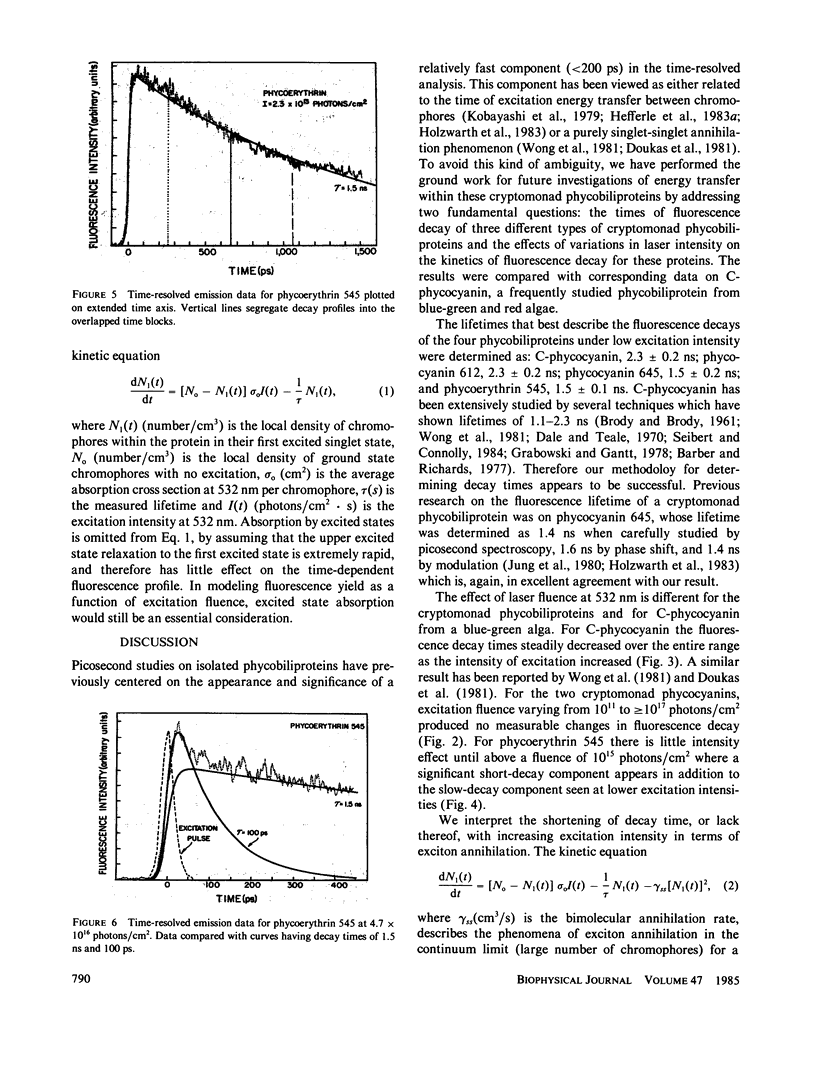
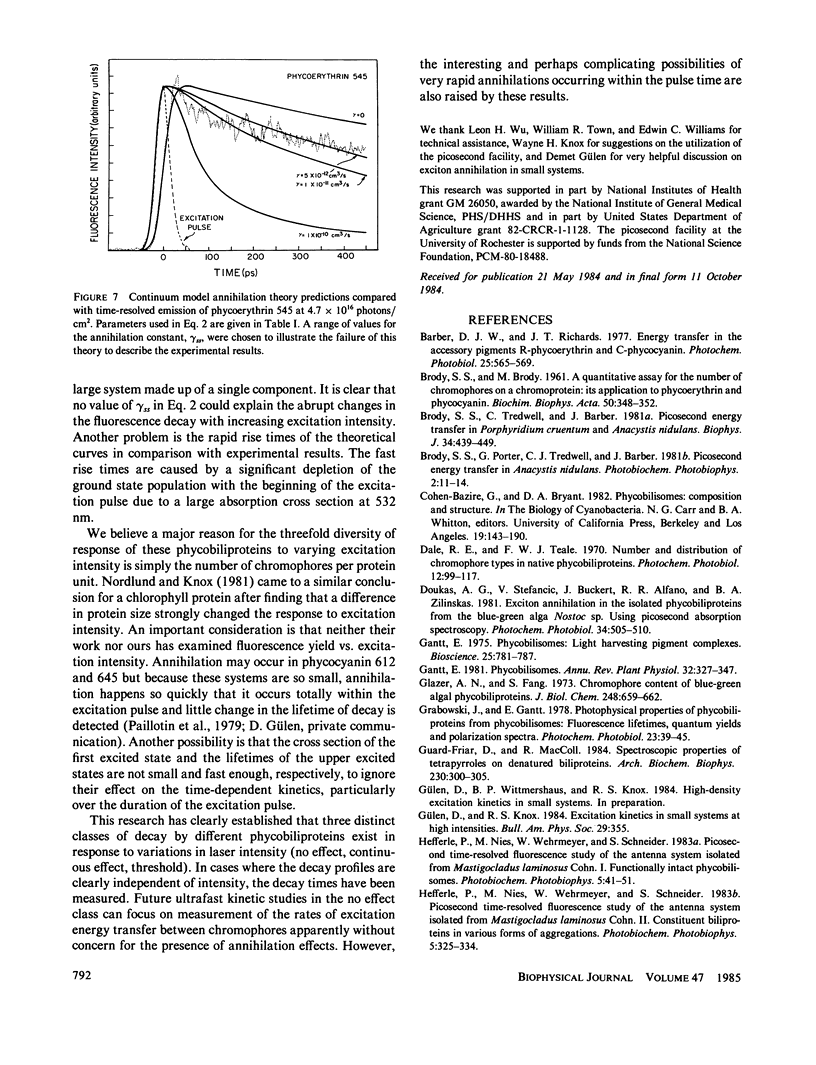
The fluorescence of purified biliproteins (phycocyanin 645, phycocyanin 612, and phycoerythrin 545) from three cryptomonads, Chroomonas species, Hemiselmis virescens, and Rhodomonas lens, and C-phycocyanin from Anacystis nidulans has been time resolved in the picosecond region with a streak camera system having less than or equal to 2-ps jitter. The fluorescence lifetimes of phycocyanins from Chroomonas species and Hemiselmis virescens are 1.5 +/- 0.2 ns and 2.3 +/- 0.2 ns, respectively, regardless of the fluence of the 30 ps, 532-nm excitation pulse. (Fluence [or photons/cm2] = f intensity [photons/cm2s]dt.). In contrast, that of C-phycocyanin is 2.3 +/- 0.2 ns when the excitation fluence is 8.2 X 10(11) photons/cm2 and decreases to a decay approximated by an exponential decay time of 0.65 +/- 0.1 ns at 7.2 X 10(16) photons/cm2. The cryptomonad phycoerythrin fluorescence decay lifetime is also dependent on intensity, having a decay time of 1.5 +/- 0.1 ns at low fluences and becoming clearly biphasic at higher fluences (greater than 10(15) photons/cm2). We interpret the shortening of decay times for C-phycocyanin and phycoerythrin 545 in terms of exciton annihilation, and have discussed the applicability of exciton annihilation theories to the high fluence effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody S. S., Treadwell C., Barber J. Picosecond energy transfer in Porphyridium cruentum and Anacystis nidulans. Biophys J. 1981 Jun;34(3):439–449. doi: 10.1016/S0006-3495(81)84861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. E., Teale F. W. Number and distribution of chromophore types in native phycobiliproteins. Photochem Photobiol. 1970 Aug;12(2):99–117. doi: 10.1111/j.1751-1097.1970.tb06044.x. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Fang S. Chromophore content of blue-green algal phycobiliproteins. J Biol Chem. 1973 Jan 25;248(2):659–662. [PubMed] [Google Scholar]
- Guard-Friar D., MacColl R. Spectroscopic properties of tetrapyrroles on denatured biliproteins. Arch Biochem Biophys. 1984 Apr;230(1):300–305. doi: 10.1016/0003-9861(84)90111-5. [DOI] [PubMed] [Google Scholar]
- Jung J., Song P. S., Paxton R. J., Edelstein M. S., Swanson R., Hazen E. E., Jr Molecular topography of the phycocyanin photoreceptor from Chroomonas species. Biochemistry. 1980 Jan 8;19(1):24–32. doi: 10.1021/bi00542a005. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Degenkolb E. O., Bersohn R., Rentzepis P. M., MacColl R., Berns D. S. Energy transfer among the chromophores in phycocyanins measured by picosecond kinetics. Biochemistry. 1979 Nov 13;18(23):5073–5078. doi: 10.1021/bi00590a008. [DOI] [PubMed] [Google Scholar]
- MacColl R., Guard-Friar D. Phycocyanin 645. The chromophore assay of phycocyanin 645 from the cryptomonad protozoa Chroomonas species. J Biol Chem. 1983 Dec 10;258(23):14327–14329. [PubMed] [Google Scholar]
- MacColl R., Habig W., Berns D. S. Characterization of phycocyanin from Chromonas species. J Biol Chem. 1973 Oct 25;248(20):7080–7086. [PubMed] [Google Scholar]
- MacColl R., Lee J. J., Berns D. S. Protein aggregation in C-phycocyanin. Studies at very low concentrations with the photoelectric scanner of the ultracentrifuge. Biochem J. 1971 May;122(4):421–426. doi: 10.1042/bj1220421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund T. M., Knox W. H. Lifetime of fluorescence from light-harvesting chlorophyll a/b proteins. Excitation intensity dependence. Biophys J. 1981 Oct;36(1):193–201. doi: 10.1016/S0006-3495(81)84723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillotin G., Swenberg C. E., Breton J., Geacintov N. E. Analysis of picosecond laser induced fluorescence phenomena in photosynthetic membranes utilizing a master equation approach. Biophys J. 1979 Mar;25(3):513–533. doi: 10.1016/S0006-3495(79)85320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter G., Tredwell C. J., Searle G. F., Barber J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part I. In the intact alga. Biochim Biophys Acta. 1978 Feb 9;501(2):232–245. doi: 10.1016/0005-2728(78)90029-4. [DOI] [PubMed] [Google Scholar]
- Searle G. F., Barber J., Porter G., Tredwell C. J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part II. In the isolated light harvesting complex (phycobilisomes). Biochim Biophys Acta. 1978 Feb 9;501(2):246–256. doi: 10.1016/0005-2728(78)90030-0. [DOI] [PubMed] [Google Scholar]
- Swenberg C. E., Geacintov N. E., Pope M. Bimolecular quenching of excitons and fluorescence in the photosynthetic unit. Biophys J. 1976 Dec;16(12):1447–1452. doi: 10.1016/S0006-3495(76)85786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


