Abstract
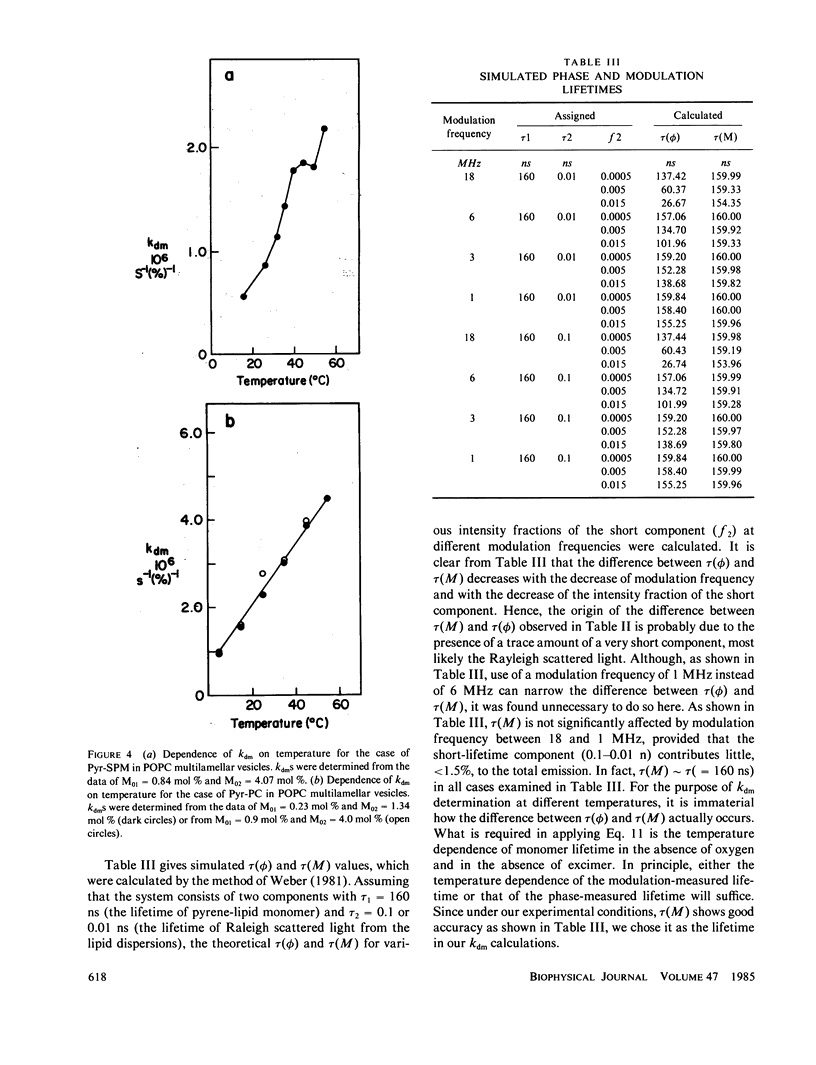
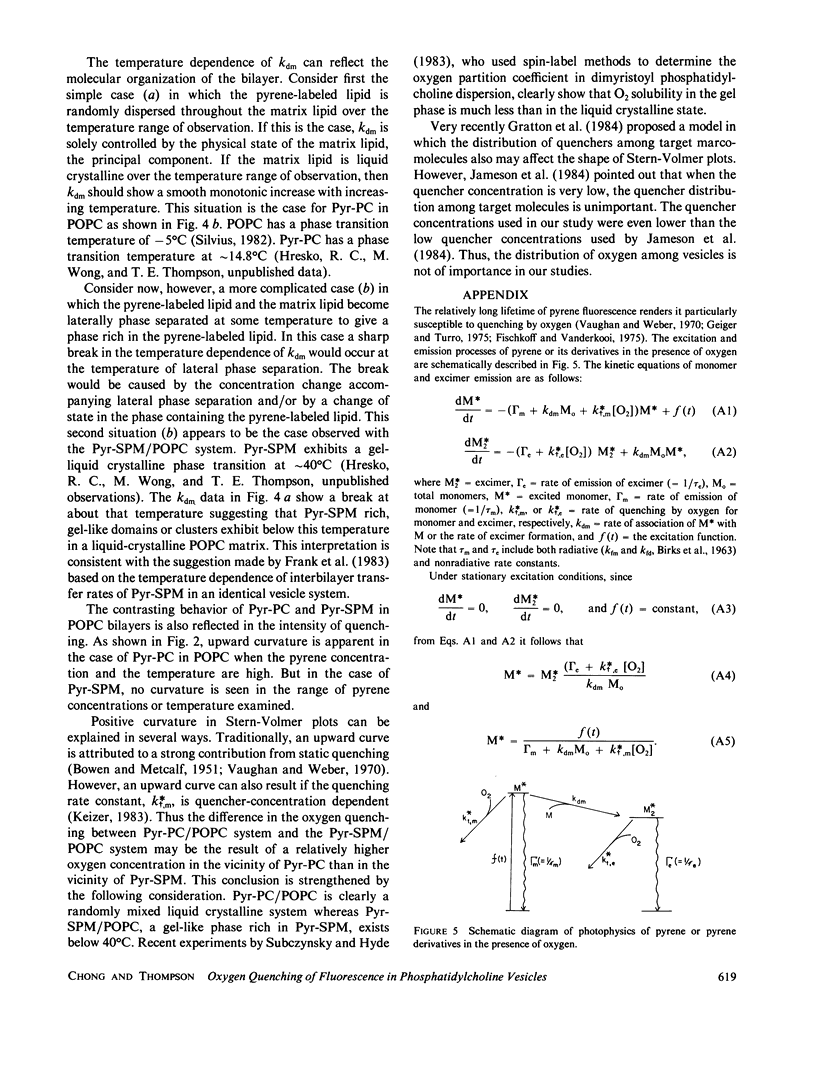
Oxygen quenching has been used as an alternative method to study the temperature dependence of the apparent excimer formation constant, kdm, of N-(10-[1-pyrene]-decanoyl)-sphingomyelin (Pyr-SPM) in 1-palmitoyl-2-oleoyl-L-alpha-phosphatidylcholine (POPC) multilamellar vesicles. In conjunction with the lifetime of Pyr-SPM monomer in the absence of excimer and oxygen, kdm can be determined from the measurements of the monomer intensity as a function of oxygen concentration. The advantage of this method is that kdm can be determined without knowledge of the excimer lifetime and intensity, and without knowledge of the true concentration of oxygen in lipid bilayers. Our results show that kdm increases monotonically with temperature from 16 to 40 degrees C, becomes insensitive to temperature from 40 to 50 degrees C and increases again at 54 degrees C. The temperature-insensitive region corresponds to the temperature range of the phase transition of Pyr-SPM determined by differential scanning calorimetry. This result suggests the existence of Pyr-SPM-enriched domains in POPC vesicles. In contrast, no abrupt change in kdm with temperature occurs in the case of 1-palmitoyl-2-[10-(1-pyrenyl) decanoyl] phosphatidylcholine (Pyr-PC).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Correa-Freire M. C., Freire E., Barenholz Y., Biltonen R. L., Thompson T. E. Thermotropic behavior of monoglucocerebroside--dipalmitoylphosphatidylcholine multilamellar liposomes. Biochemistry. 1979 Feb 6;18(3):442–445. doi: 10.1021/bi00570a008. [DOI] [PubMed] [Google Scholar]
- Donner M., Andre J. C., Bouchy M. Kinetics of partly diffusion controlled reactions. VII - Pyrene excimer formation in erythrocyte membranes. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1183–1191. doi: 10.1016/0006-291x(80)91500-4. [DOI] [PubMed] [Google Scholar]
- Fischkoff S., Vanderkooi J. M. Oxygen diffusion in biological and artificial membranes determined by the fluorochrome pyrene. J Gen Physiol. 1975 May;65(5):663–676. doi: 10.1085/jgp.65.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Geiger M. W., Turro N. J. Pyrene fluorescence lifetime as a probe for oxygen penetration of micelles. Photochem Photobiol. 1975 Dec;22(6):273–276. doi: 10.1111/j.1751-1097.1975.tb06749.x. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Weber G., Alpert B. A model of dynamic quenching of fluorescence in globular proteins. Biophys J. 1984 Apr;45(4):789–794. doi: 10.1016/S0006-3495(84)84223-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann W., Galla H. J. Binding of polylysine to charged bilayer membranes: molecular organization of a lipid.peptide complex. Biochim Biophys Acta. 1978 Jun 2;509(3):474–490. doi: 10.1016/0005-2736(78)90241-9. [DOI] [PubMed] [Google Scholar]
- Jameson D. M., Gratton E., Weber G., Alpert B. Oxygen distribution and migration within Mbdes Fe and Hbdes Fe. Multifrequency phase and modulation fluorometry study. Biophys J. 1984 Apr;45(4):795–803. doi: 10.1016/S0006-3495(84)84224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey J. B., Gotto A. M., Jr, Pownall H. J. Kinetics and mechanism of the spontaneous transfer of fluorescent phospholipids between apolipoprotein-phospholipid recombinants. Effect of the polar headgroup. J Biol Chem. 1982 May 25;257(10):5444–5448. [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S. Concentration of oxygen in lipid bilayers using a spin-label method. Biophys J. 1983 Mar;41(3):283–286. doi: 10.1016/S0006-3495(83)84439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J. M., Callis J. B. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry. 1974 Sep 10;13(19):4000–4006. doi: 10.1021/bi00716a028. [DOI] [PubMed] [Google Scholar]
- Vaughan W. M., Weber G. Oxygen quenching of pyrenebutyric acid fluorescence in water. A dynamic probe of the microenvironment. Biochemistry. 1970 Feb 3;9(3):464–473. doi: 10.1021/bi00805a003. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]


