Abstract
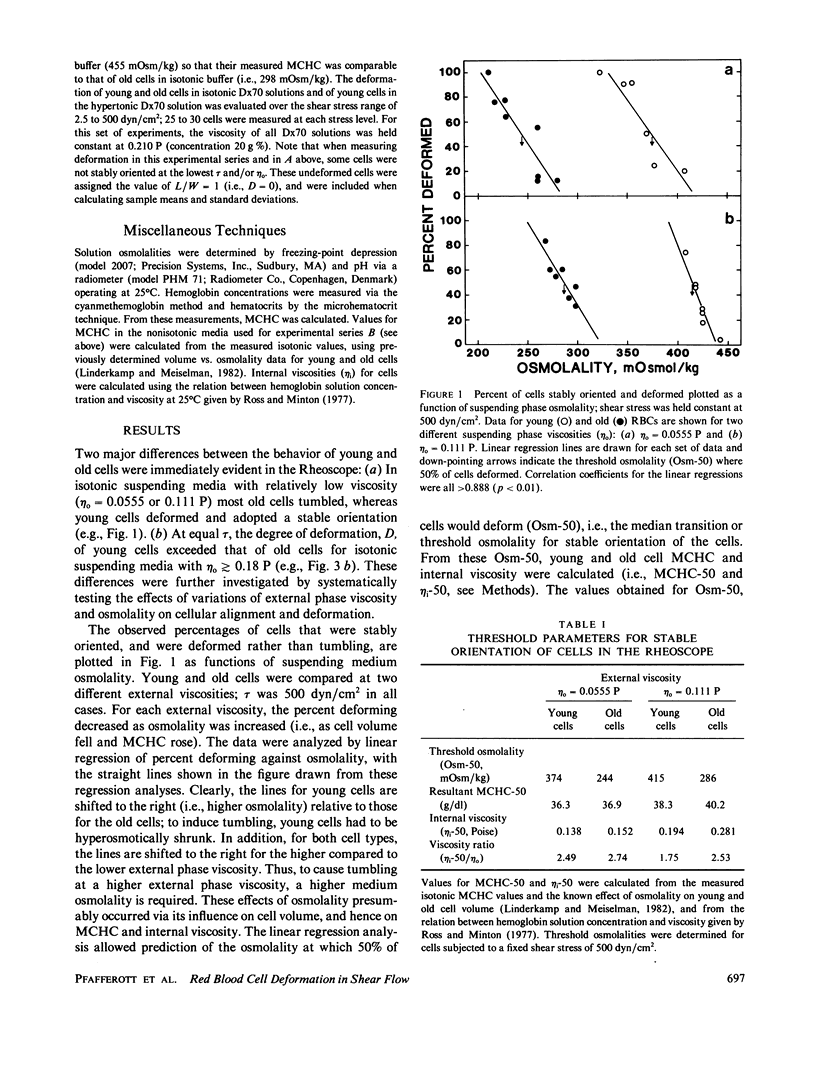
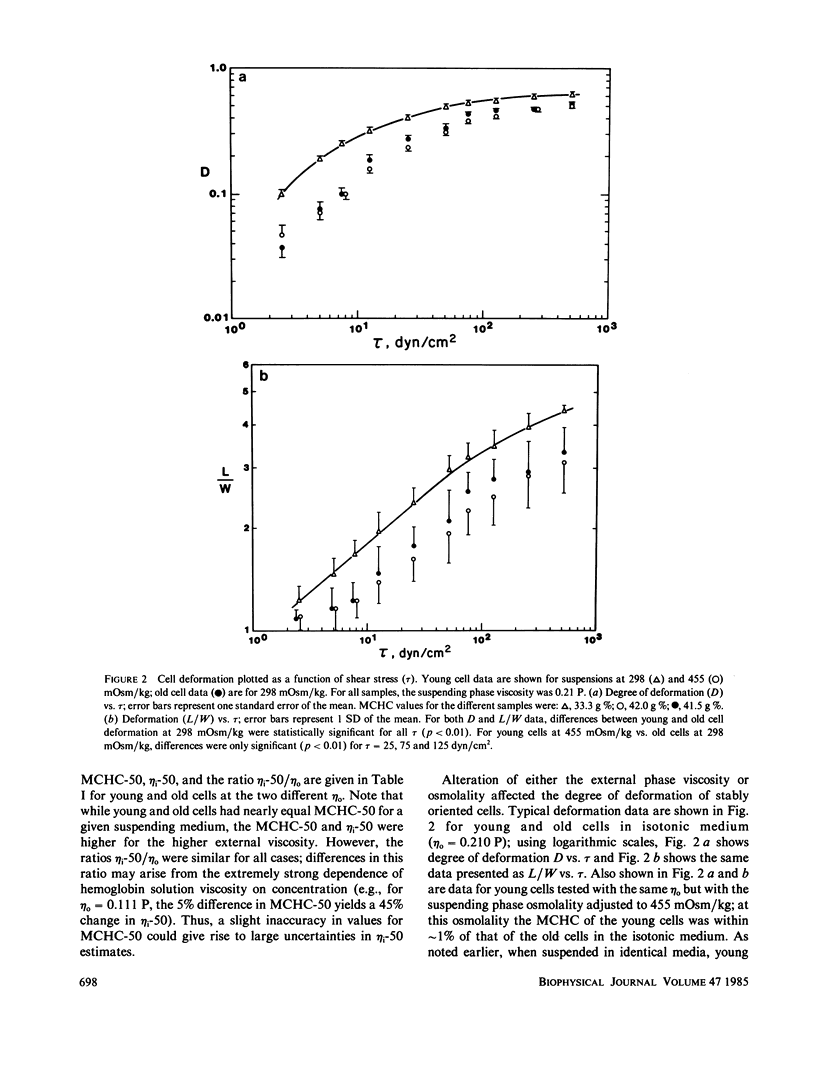
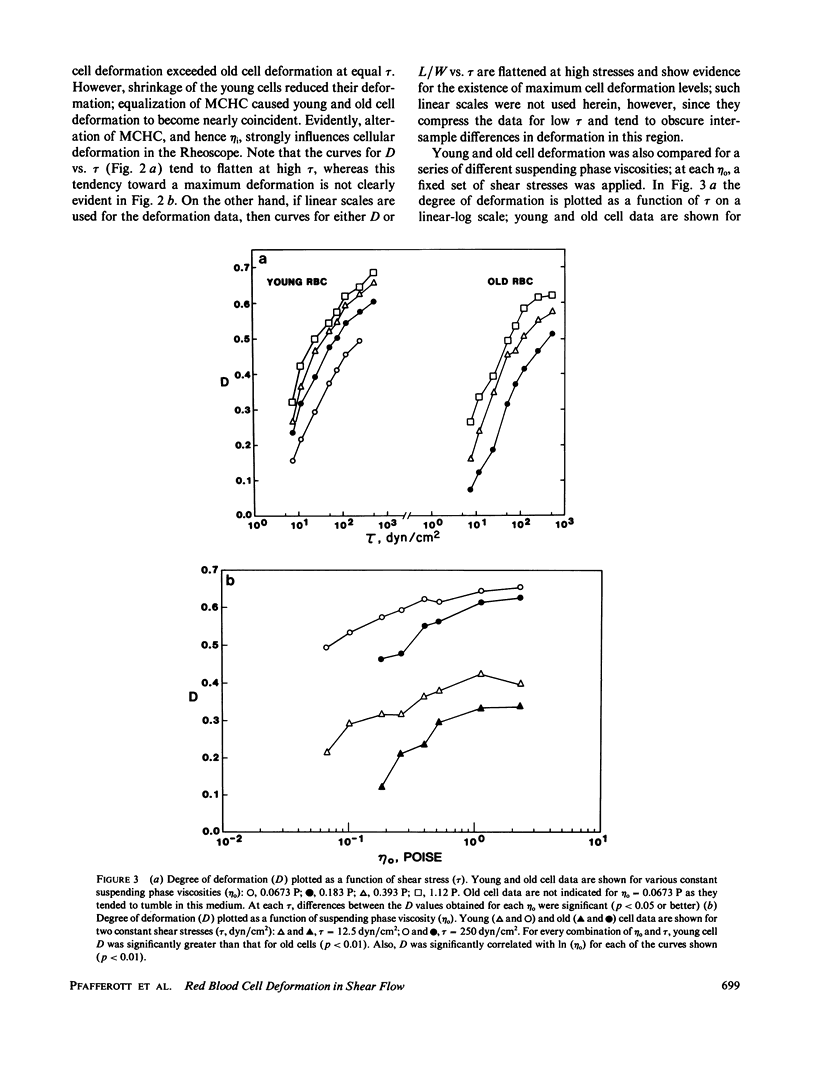
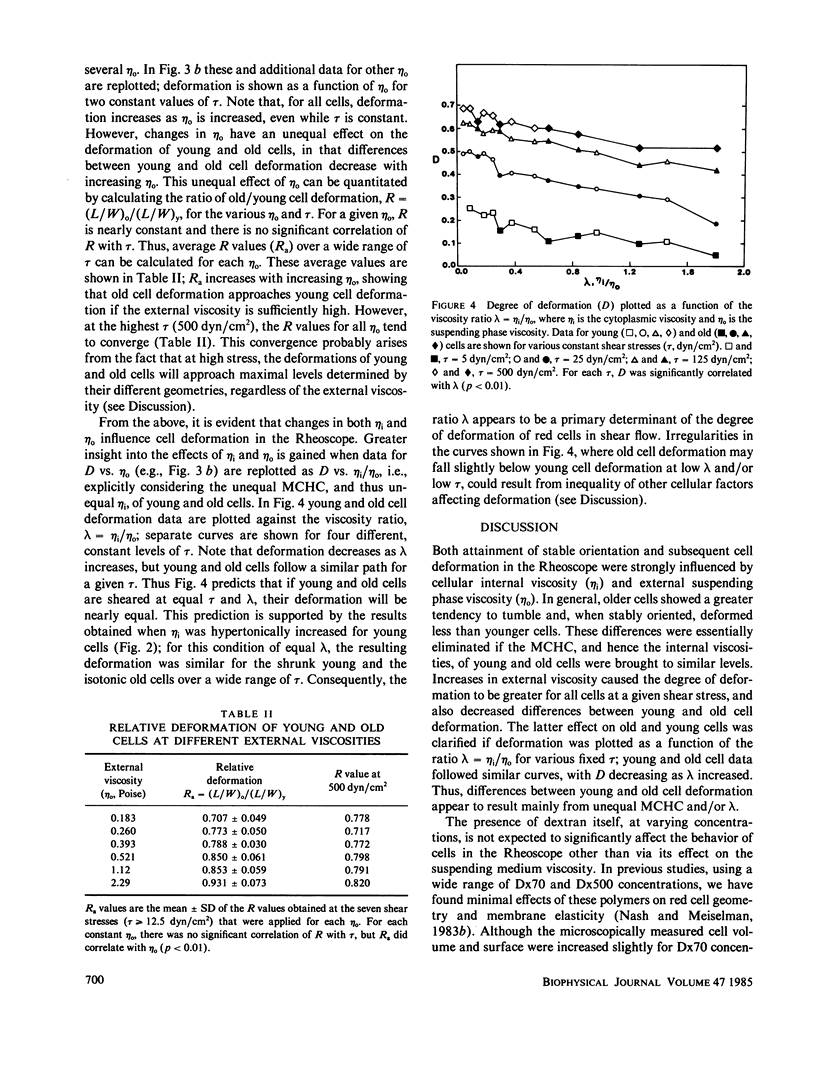
Shear deformation of young and old human red blood cells was examined over a range of shear stresses and suspending phase viscosities (eta o) using a cone-plate Rheoscope. The internal viscosities (eta i) of these cell types differ, and further changes in internal viscosity were induced by alteration of suspension osmolality and hence cell volume. For low suspending viscosities (0.0555 or 0.111 P) old cells tended to tumble in shear flow, whereas young cells achieved stable orientation and deformed. Changes in osmolality, at these external viscosities, altered the percentage of cells deforming, and for each cell type threshold osmolalities (Osm-50) were determined where 50% of cells deformed. The threshold osmolalities were higher for younger cells than for older cells, but the internal viscosities of the two cell types were similar at their respective Osm-50. Threshold osmolalities were also higher for the higher external viscosity, but the ratio of internal to external viscosities (i.e., eta i/eta o) was nearly constant for both external viscosities. Deformation of stably oriented cells increased with increasing shear stress and approached a value limited by cell surface area and volume. For isotonic media, over a wide range of external viscosities and shear stresses, deformation was greater for younger cells than for older cells. However, deformation vs. shear stress data for the two cell types became nearly coincident if young cells were osmotically shrunk to have their internal viscosity close to that for old cells. Increases in external viscosity, at constant shear stress, caused greater deformation for all cells. This effect of external viscosity was not equal for young and old cells; the ratio of old/young cell deformation increased with increasing eta o. However, if deformation was plotted as a function of the ratio lambda = eta i/eta o, at constant shear stress, young and old cell data followed similar paths. Thus the ratio lambda is a major determinant of cell deformation as well as a critical factor affecting stable orientation in shear flow.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien S., Sung K. L., Skalak R., Usami S., Tözeren A. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978 Nov;24(2):463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Dellenback R. J., Gregersen M. I. Blood viscosity: influence of erythrocyte deformation. Science. 1967 Aug 18;157(3790):827–829. doi: 10.1126/science.157.3790.827. [DOI] [PubMed] [Google Scholar]
- Corry W. D., Meiselman H. J. Modification of erythrocyte physicochemical properties by millimolar concentrations of glutaraldehyde. Blood Cells. 1978;4(3):465–483. [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoelasticity. Biophys J. 1976 Jan;16(1):1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. M. On the energy dissipation in a tank-treading human red blood cell. Biophys J. 1980 Nov;32(2):863–868. doi: 10.1016/S0006-3495(80)85022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. M., Stöhr-Lissen M., Schmid-Schönbein H. The red cell as a fluid droplet: tank tread-like motion of the human erythrocyte membrane in shear flow. Science. 1978 Nov 24;202(4370):894–896. doi: 10.1126/science.715448. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Worthy P. R., Evans E. A. Red cell extensional recovery and the determination of membrane viscosity. Biophys J. 1979 Apr;26(1):101–114. doi: 10.1016/S0006-3495(79)85238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderkamp O., Meiselman H. J. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982 Jun;59(6):1121–1127. [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A., Shohet S. B. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983 Jul;20(3):225–242. [PubMed] [Google Scholar]
- Morris D. R., Williams A. R. The effects of suspending medium viscosity on erythrocyte deformation and haemolysis in vitro. Biochim Biophys Acta. 1979 Jan 19;550(2):288–296. doi: 10.1016/0005-2736(79)90215-3. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Effects of dextran and polyvinylpyrrolidone on red cell geometry and membrane elasticity. Ann N Y Acad Sci. 1983;416:255–262. doi: 10.1111/j.1749-6632.1983.tb35192.x. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys J. 1983 Jul;43(1):63–73. doi: 10.1016/S0006-3495(83)84324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash G. B., Wyard S. J. Erythrocyte membrane elasticity during in vivo ageing. Biochim Biophys Acta. 1981 May 6;643(2):269–275. doi: 10.1016/0005-2736(81)90072-9. [DOI] [PubMed] [Google Scholar]
- Pfafferott C., Wenby R., Meiselman H. J. Morphologic and internal viscosity aspects of RBC rheologic behavior. Blood Cells. 1982;8(1):65–78. [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Hard quasispherical model for the viscosity of hemoglobin solutions. Biochem Biophys Res Commun. 1977 Jun 20;76(4):971–976. doi: 10.1016/0006-291x(77)90950-0. [DOI] [PubMed] [Google Scholar]
- Tran-Son-Tay R., Sutera S. P., Rao P. R. Determination of red blood cell membrane viscosity from rheoscopic observations of tank-treading motion. Biophys J. 1984 Jul;46(1):65–72. doi: 10.1016/S0006-3495(84)83999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


