Abstract
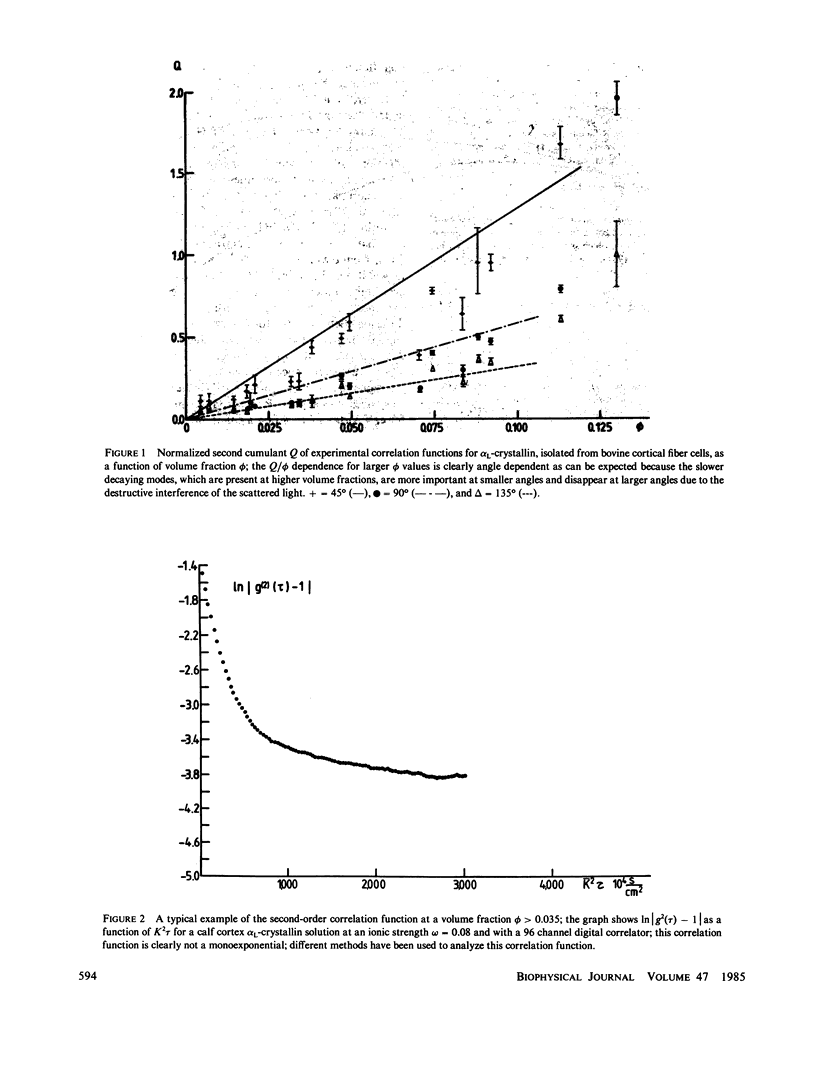
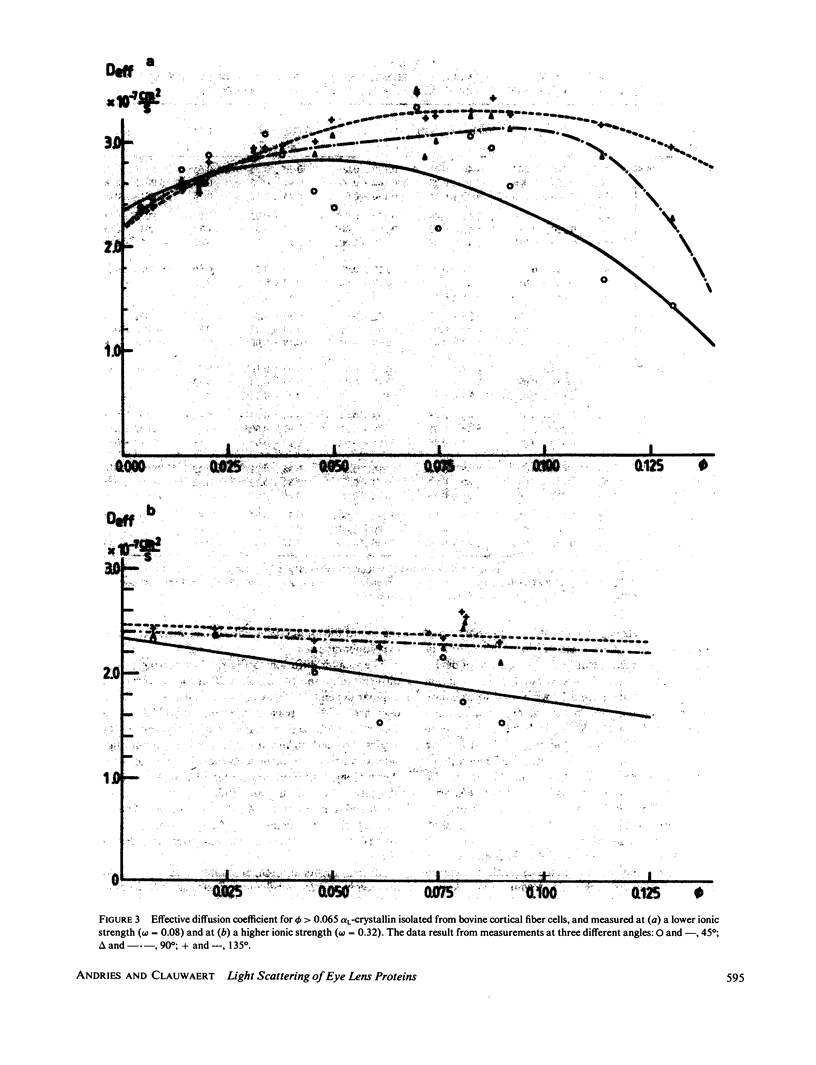
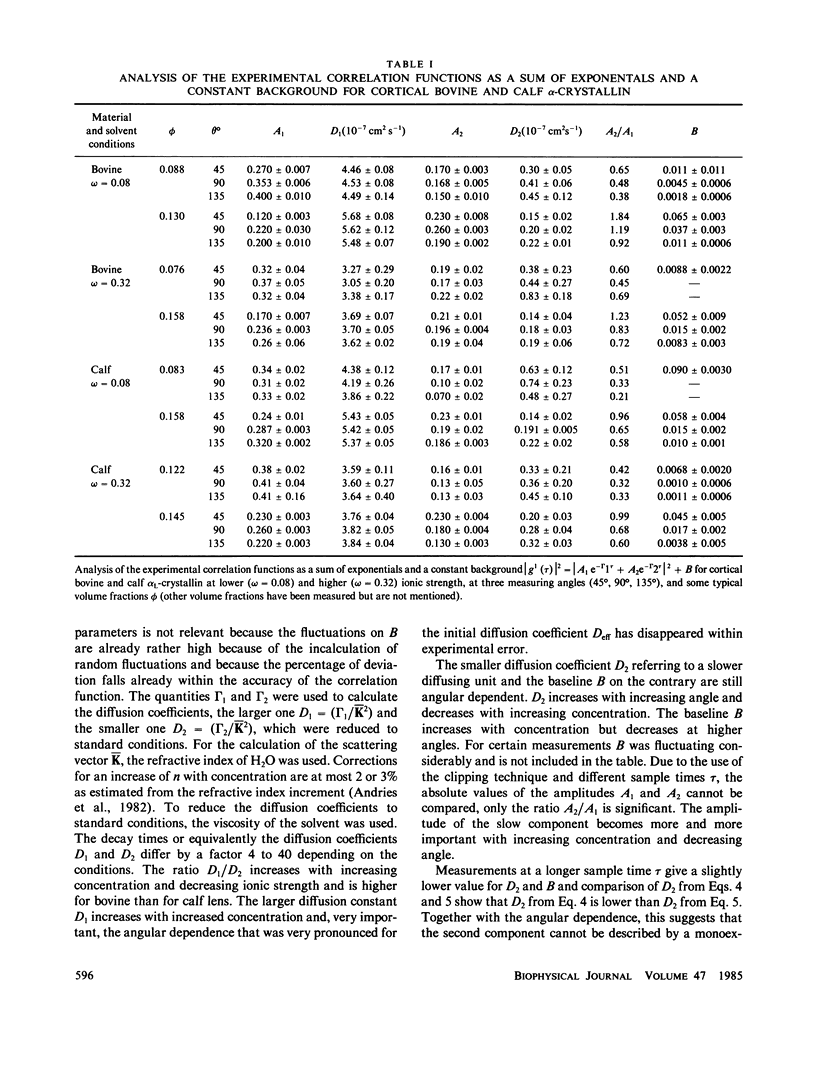
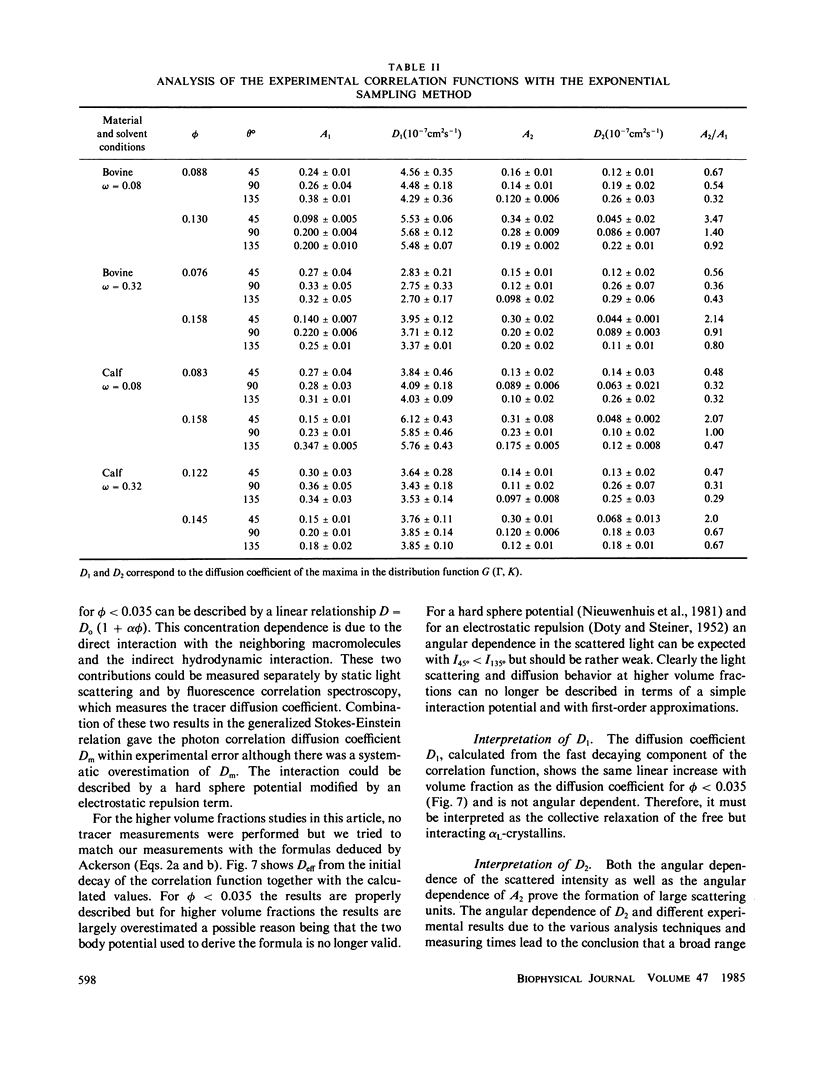
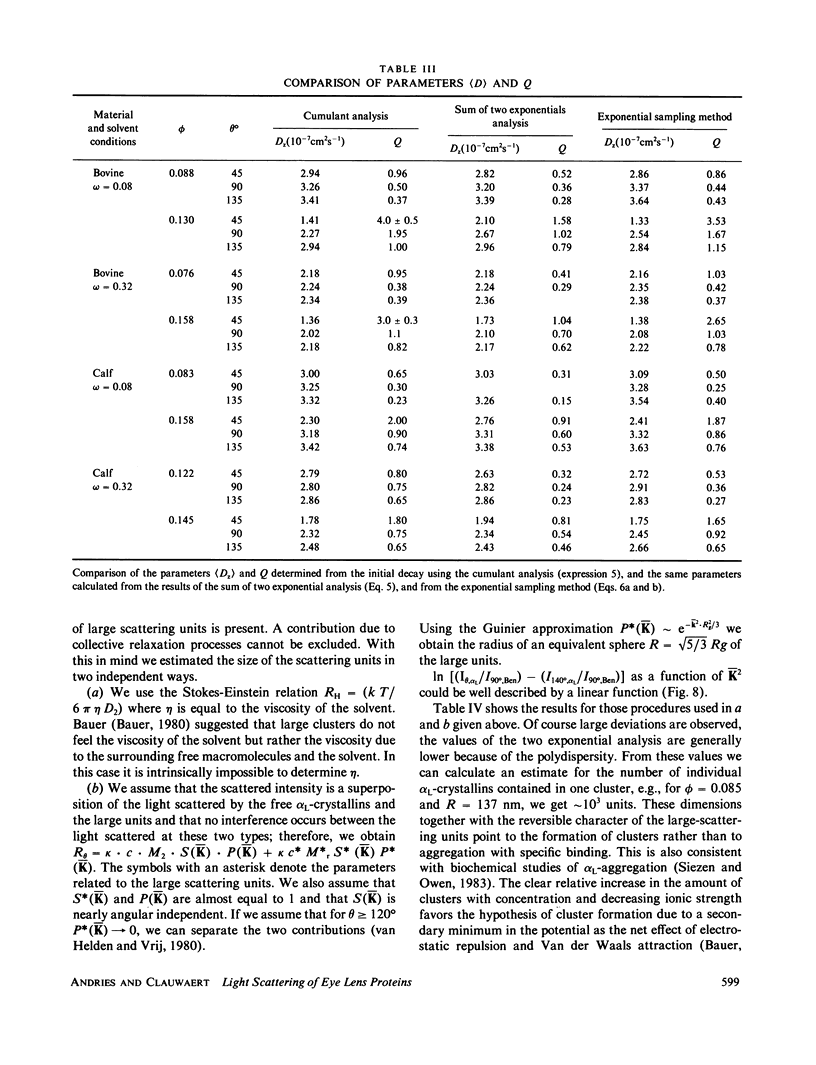
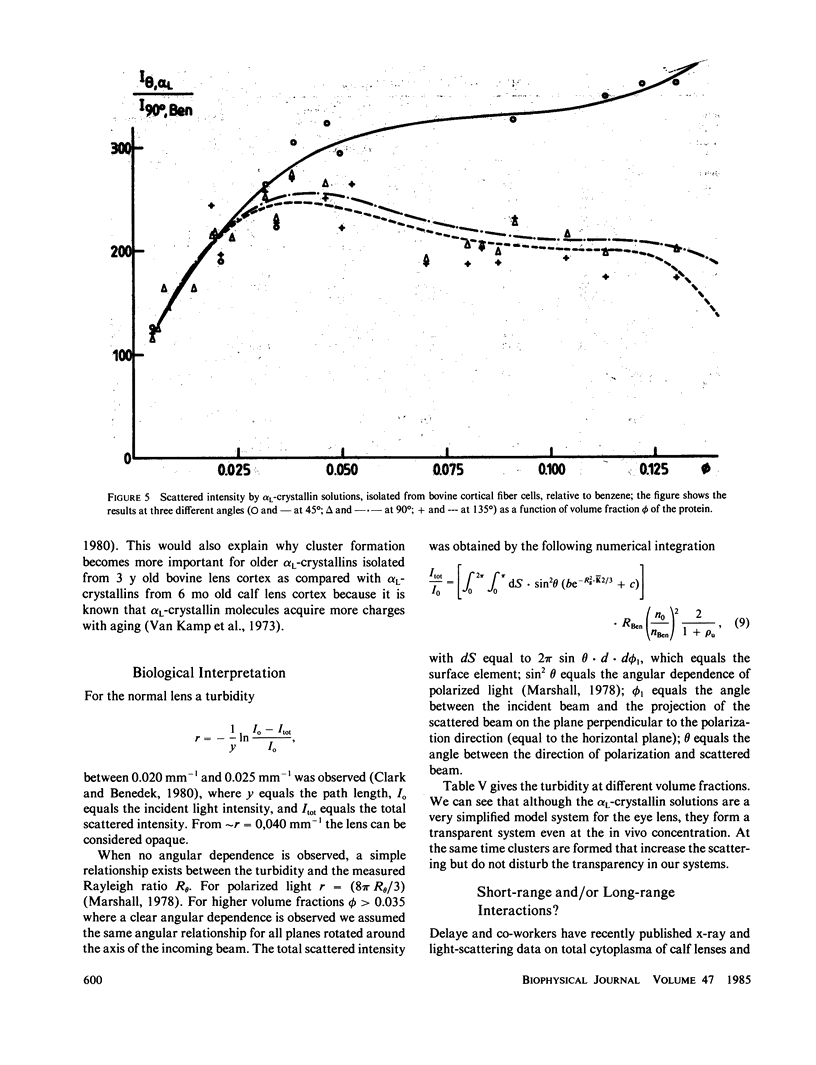
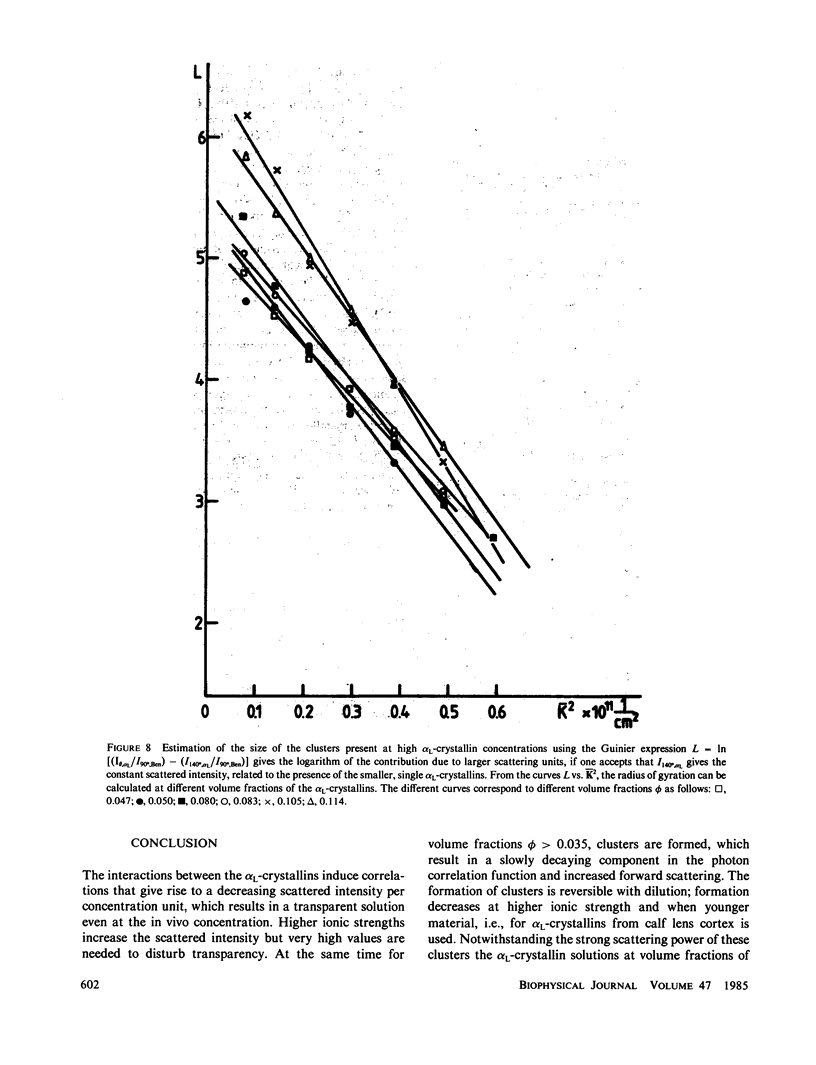
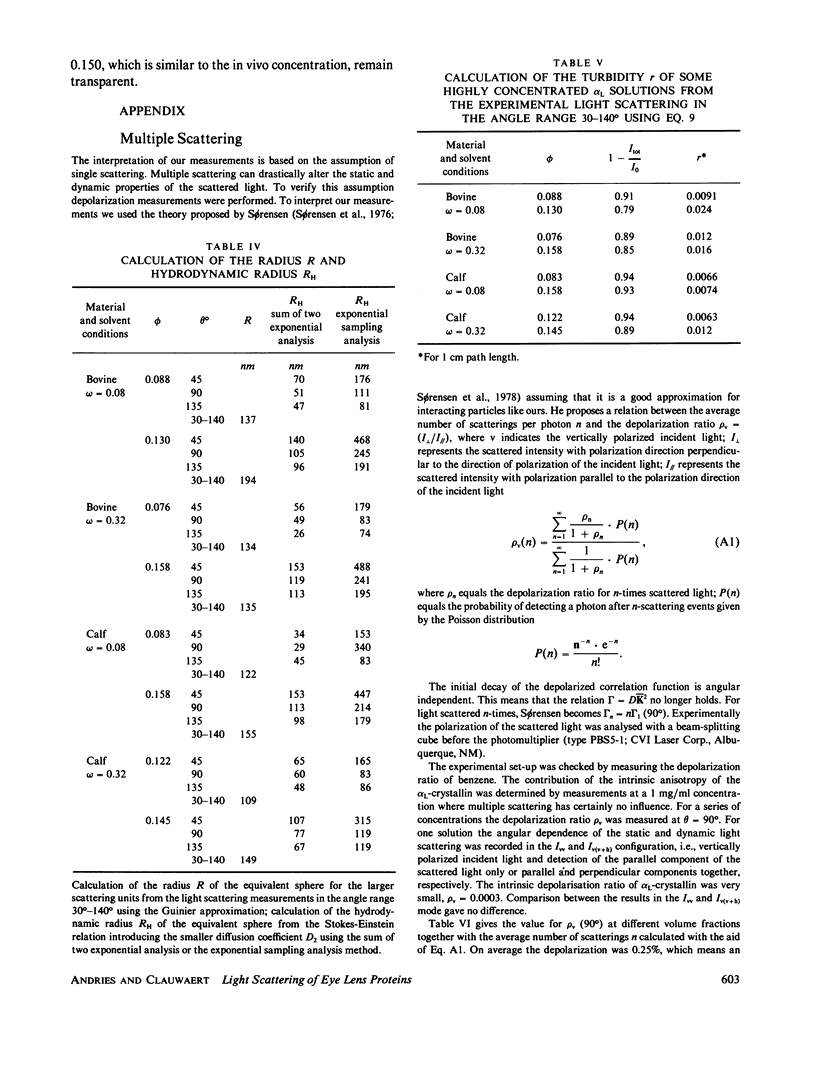
The bovine eye lens protein, alpha L crystallin, has been studied with photon correlation spectroscopy and statical light scattering in the concentration range up to 200 g/l in different solvent conditions. At higher concentration (c greater than 70 g/l) the scattering behavior is quite complicated, which results in nonexponential correlation functions. Three methods have been used for the analysis of these correlation functions, namely, cumulant analysis, sum of two exponentials analysis, and exponential sampling method. These methods resulted in very similar results. The highly concentrated solutions contain two scattering entities: the single alpha L crystallin and a rather heterogeneous population of large clusters. The statical light-scattering experiments can be interpreted in the same way and gave consistent results for the dimensions of the large scattering units. The formation of these clusters, which are strong light scatterers, is superimposed on an increasing degree of correlation between the bulk of the alpha L-crystallins, resulting in a net decrease of light scattering as a function of concentration.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andries C., Backhovens H., Clauwaert J., De Block J., De Voeght F., Dhont C. Physical-chemical studies on bovine eye lens proteins. I. Light-scattering and viscosity studies of low-molecular weight alpha-crystallin isolated from adult and embryonic bovine lenses. Exp Eye Res. 1982 Feb;34(2):239–255. doi: 10.1016/0014-4835(82)90058-6. [DOI] [PubMed] [Google Scholar]
- Andries C., Guedens W., Clauwaert J., Geerts H. Photon and fluorescence correlation spectroscopy and light scattering of eye-lens proteins at moderate concentrations. Biophys J. 1983 Sep;43(3):345–354. doi: 10.1016/S0006-3495(83)84358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim F. A., Bettelheim A. A. Small-angle light scattering studies on xylose cataract formation in bovine lenses. Invest Ophthalmol Vis Sci. 1978 Sep;17(9):896–902. [PubMed] [Google Scholar]
- Bettelheim F. A. On the optical anisotropy of lens fiber cells. Exp Eye Res. 1975 Sep;21(3):231–234. doi: 10.1016/0014-4835(75)90093-7. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Paunovic M. Light scattering of normal human lens I. Application of random density and orientation fluctuation theory. Biophys J. 1979 Apr;26(1):85–99. doi: 10.1016/S0006-3495(79)85237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. Lens proteins. CRC Crit Rev Biochem. 1982;12(1):1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- Clark J. I., Benedek G. B. The effects of glycols, aldehydes, and acrylamide on phase separation and opacification in the calf lens. Invest Ophthalmol Vis Sci. 1980 Jul;19(7):771–776. [PubMed] [Google Scholar]
- Delaye M., Clark J. I., Benedek G. B. Identification of the scattering elements responsible for lens opacification in cold cataracts. Biophys J. 1982 Mar;37(3):647–656. [PMC free article] [PubMed] [Google Scholar]
- Delaye M., Gromiec A. Mutual diffusion of crystallin proteins at finite concentrations: a light-scattering study. Biopolymers. 1983 Apr;22(4):1203–1221. doi: 10.1002/bip.360220413. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Nicoli D. F., Baram H., Benedek G. B. Quantitative verification of the existence of high molecular weight protein aggregates in the intact normal human lens by light-scattering spectroscopy. Invest Ophthalmol Vis Sci. 1978 Jan;17(1):51–57. [PubMed] [Google Scholar]
- Laiken S. L., Printz M. P. Kinetic class analysis of hydrogen-exchange data. Biochemistry. 1970 Mar 31;9(7):1547–1553. doi: 10.1021/bi00809a011. [DOI] [PubMed] [Google Scholar]
- Mathiez P., Weisbuch G., Mouttet C. Inelastic light-scattering study of polyadenilic acid. Biopolymers. 1979 Jun;18(6):1465–1478. doi: 10.1002/bip.1979.360180610. [DOI] [PubMed] [Google Scholar]
- Patkowski A., Chu B. Intensity fluctuation spectroscopy and transfer RNA conformation. III. Influence of NaCl concentration on the size and shape of the initially salt-free tRNA in solution. Biopolymers. 1979 Aug;18(8):2051–2072. doi: 10.1002/bip.1979.360180816. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Bindels J. G., Hoenders H. J. The quaternary structure of bovine alpha-crystallin. Effects of variation in alkaline pH, ionic strength, temperature and calcium ion concentration. Eur J Biochem. 1980 Oct;111(2):435–444. doi: 10.1111/j.1432-1033.1980.tb04958.x. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Owen E. A. Interactions of lens proteins. Self-association and mixed-association studies of bovine alpha-crystallin and gamma-crystallin. Biophys Chem. 1983 Oct;18(3):181–194. doi: 10.1016/0301-4622(83)80030-1. [DOI] [PubMed] [Google Scholar]
- Spector A., Li L. K., Augusteyn R. C., Schneider A., Freund T. -Crystallin. The isolation and characterization of distinct macromolecular fractions. Biochem J. 1971 Sep;124(2):337–343. doi: 10.1042/bj1240337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer J., Rothschild C., Wandel T., Spector A. Transformation of alpha-crystallin polypeptide chains with aging. Invest Ophthalmol. 1974 Feb;13(2):135–146. [PubMed] [Google Scholar]
- van Kamp G. J., Schats L. H., Hoenders H. J. Characteristics of -crystallin related to fiber cell development in calf eye lenses. Biochim Biophys Acta. 1973 Jan 25;295(1):166–173. doi: 10.1016/0005-2795(73)90084-6. [DOI] [PubMed] [Google Scholar]


