Abstract
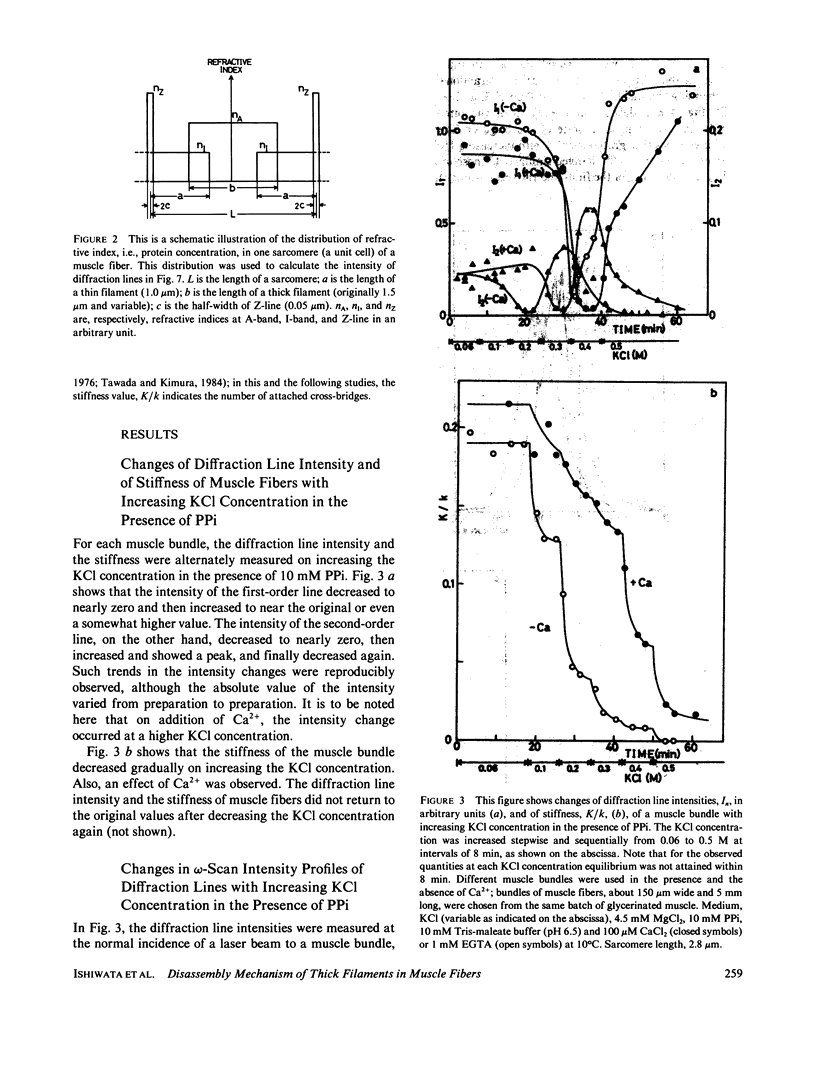
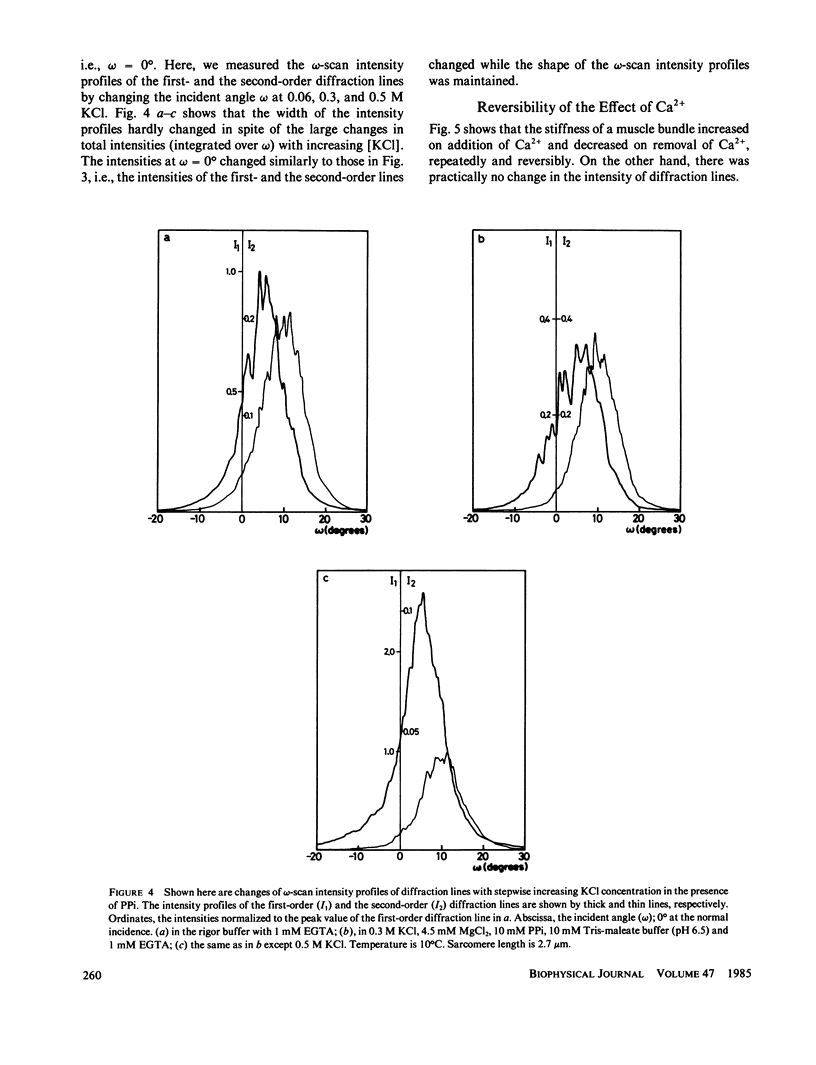
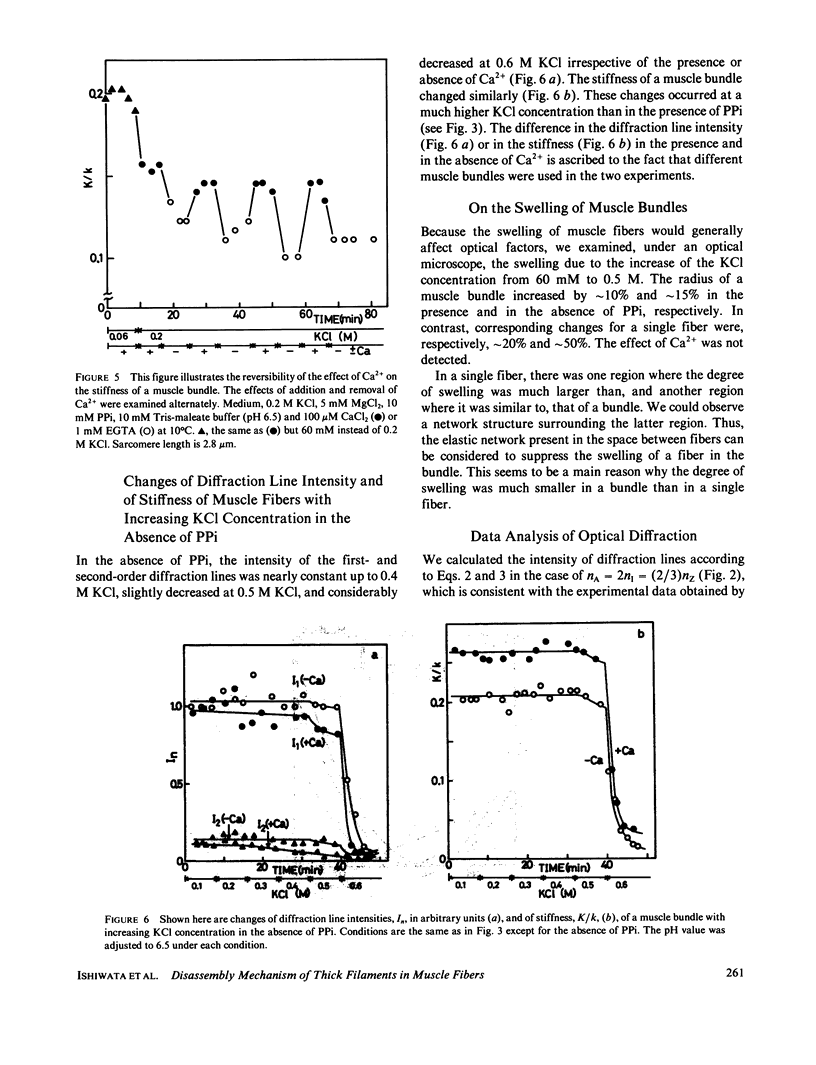
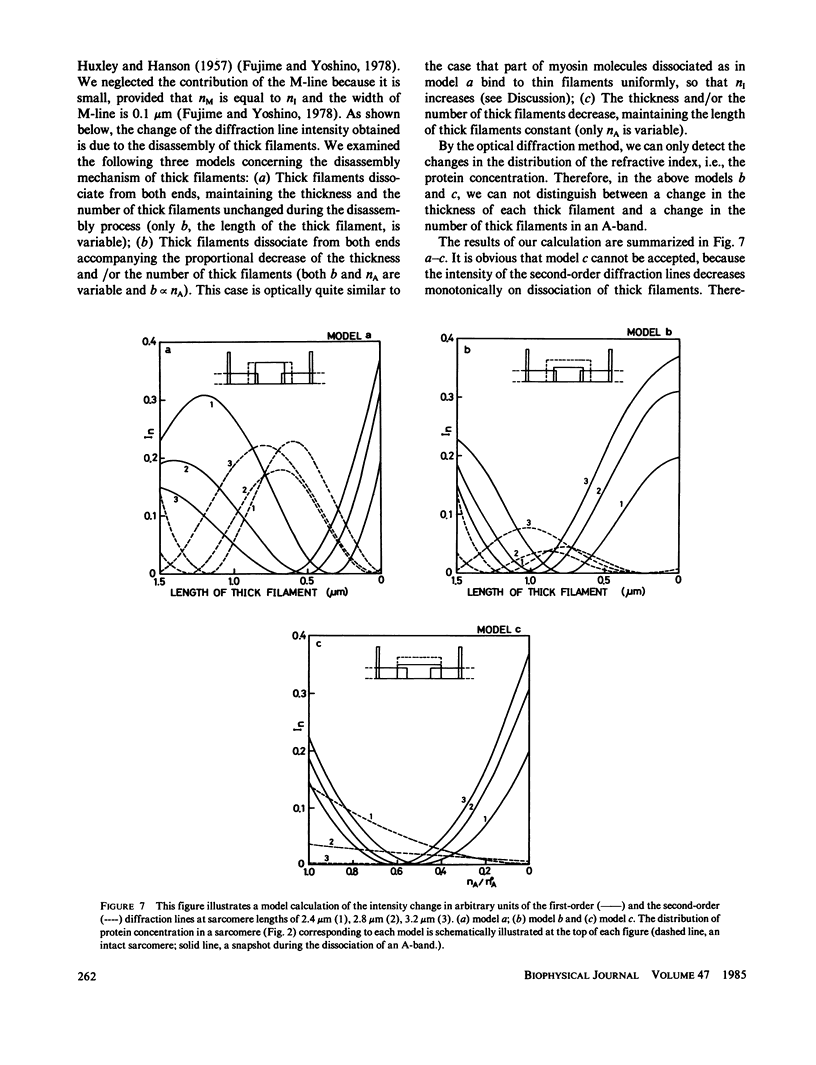
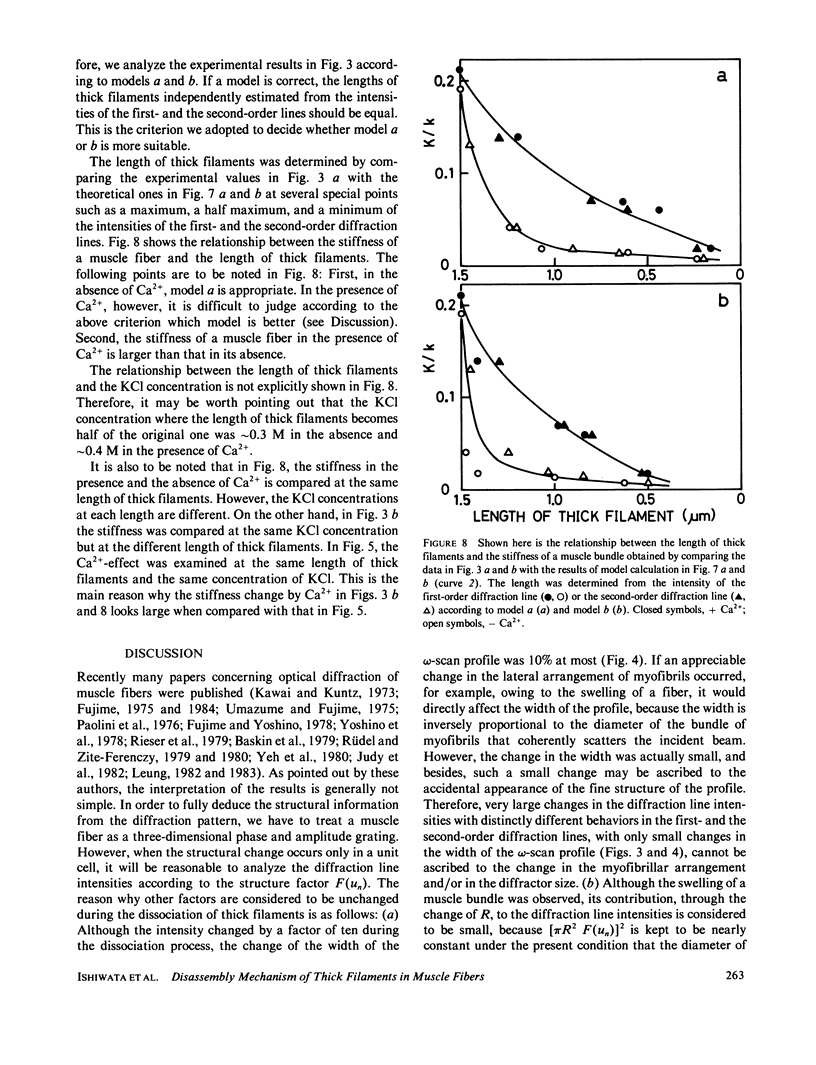
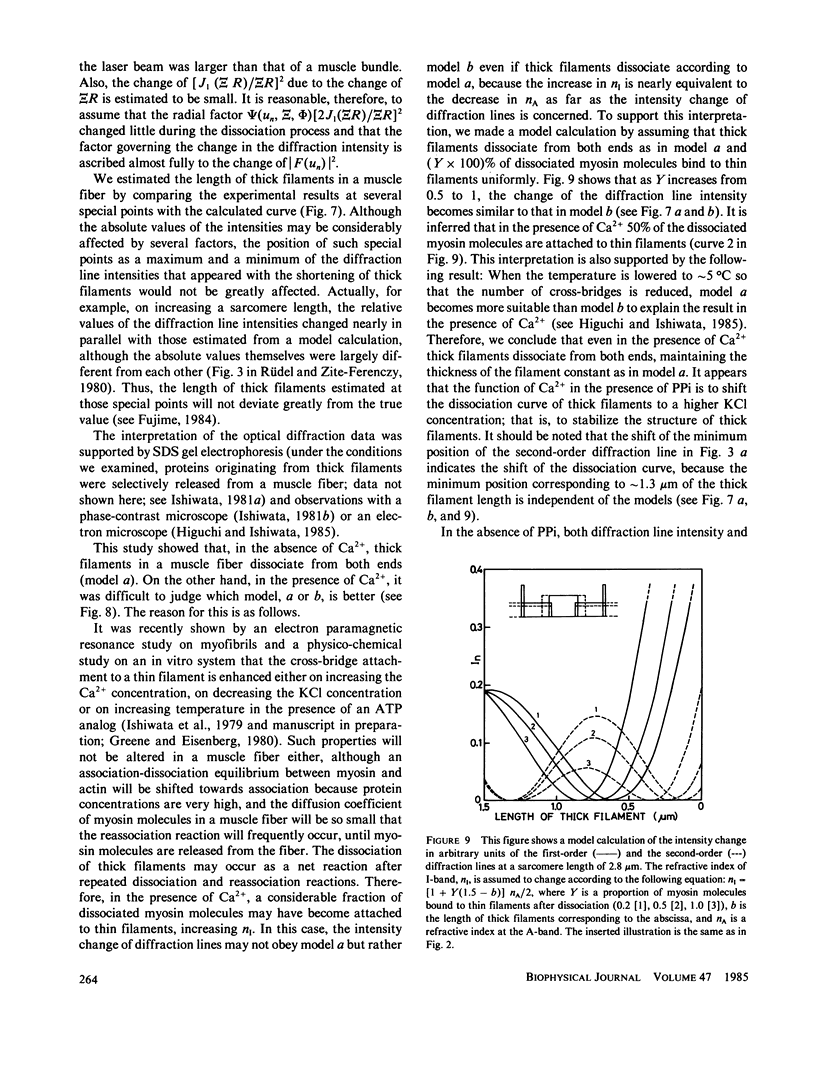
We show in this paper that the change of the internal structure of a sarcomere in a rabbit glycerinated psoas muscle fiber can be examined by analyzing the intensity change of the first- and the second-order optical diffraction lines. A unit-cell (sarcomere)-structure model has been applied to the estimation of the length of thick filaments in a muscle fiber while they undergo dissociation. The optical factors, except for the unit-cell-structure factor, hardly changed during the dissociation of the filaments. Our results show that thick filaments dissociate from both ends on increasing the KCl concentration in the presence of 10 mM pyrophosphate and 5 mM MgCl2. Micromolar concentrations of Ca2+ suppressed to some extent the dissociation of thick filaments. The disassembly of thick filaments occurred at higher KCl concentrations in the absence of pyrophosphate. There was a correlation between the stability of the thick filament structure and cross-bridge formation, which was induced either by the addition of micromolar concentrations of Ca2+ in the presence of Mg-pyrophosphate or by removal of Mg-pyrophosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskin R. J., Roos K. P., Yeh Y. Light diffraction study of single skeletal muscle fibres. Biophys J. 1979 Oct;28(1):45–64. doi: 10.1016/S0006-3495(79)85158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujime S. An intensity expression of optical diffraction from striated muscle fibres. J Muscle Res Cell Motil. 1984 Oct;5(5):577–587. doi: 10.1007/BF00713262. [DOI] [PubMed] [Google Scholar]
- Fujime S. Optical diffraction study of muscle fibers. Biochim Biophys Acta. 1975 Jan 30;379(1):227–238. doi: 10.1016/0005-2795(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Fujime S., Yoshino S. Optical diffraction study of muscle fibers. I. A theoretical basis. Biophys Chem. 1978 Sep;8(4):305–315. doi: 10.1016/0301-4622(78)80013-1. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E., HANSON J. Quantitative studies on the structure of cross-striated myofibrils. I. Investigations by interference microscopy. Biochim Biophys Acta. 1957 Feb;23(2):229–249. doi: 10.1016/0006-3002(57)90325-6. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Himmelfarb S. Effect of adenosine di- and triphosphates on the stability of synthetic myosin filaments. Biochemistry. 1972 Aug 1;11(16):2945–2952. doi: 10.1021/bi00766a004. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Ishiwata S. Disassembly kinetics of thick filaments in rabbit skeletal muscle fibers. Effects of ionic strength, Ca2+ concentration, pH, temperature, and cross-bridges on the stability of thick filament structure. Biophys J. 1985 Mar;47(3):267–275. doi: 10.1016/S0006-3495(85)83916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S. Melting from both ends of an A-band in a myofibril. Observation with a phase-contrast microscope. J Biochem. 1981 May;89(5):1647–1650. doi: 10.1093/oxfordjournals.jbchem.a133361. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Judy M. M., Summerour V., LeConey T., Roa R. L., Templeton G. H. Muscle diffraction theory. Relationship between diffraction subpeaks and discrete sarcomere length distributions. Biophys J. 1982 Feb;37(2):475–487. doi: 10.1016/S0006-3495(82)84694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer B., Bell A. L. Myosin filamentogenesis: effects of pH and ionic concentration. J Mol Biol. 1966 Sep;20(2):391–401. doi: 10.1016/0022-2836(66)90070-2. [DOI] [PubMed] [Google Scholar]
- Katsura I., Noda H. Assembly of myosin molecules into the structure of thick filaments of muscle. Adv Biophys. 1973;5(0):177–202. [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976 Sep;68(3):267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Kuntz I. D. Optical diffraction studies of muscle fibers. Biophys J. 1973 Sep;13(9):857–876. doi: 10.1016/S0006-3495(73)86031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A. F. Laser diffraction of single intact cardiac muscle cells at rest. J Muscle Res Cell Motil. 1982 Dec;3(4):399–418. doi: 10.1007/BF00712091. [DOI] [PubMed] [Google Scholar]
- Leung A. F. Light diffraction by striated muscle fibres in the transverse direction. J Muscle Res Cell Motil. 1983 Oct;4(5):557–568. doi: 10.1007/BF00712115. [DOI] [PubMed] [Google Scholar]
- NODA H., EBASHI S. Aggregation of myosin A. Biochim Biophys Acta. 1960 Jul 15;41:386–392. doi: 10.1016/0006-3002(60)90035-4. [DOI] [PubMed] [Google Scholar]
- Niederman R., Peters L. K. Native bare zone assemblage nucleates myosin filament assembly. J Mol Biol. 1982 Nov 15;161(4):505–517. doi: 10.1016/0022-2836(82)90404-1. [DOI] [PubMed] [Google Scholar]
- Paolini P. J., Sabbadini R., Roos K. P., Baskin R. J. Sarcomere length dispersion in single skeletal muscle fibers and fiber bundles. Biophys J. 1976 Aug;16(8):919–930. doi: 10.1016/S0006-3495(76)85742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinset-Härström I., Truffy J. Effect of adenosine triphosphate, inorganic phosphate and divalent cations on the size and structure of synthetic myosin filaments. An electron microscope study. J Mol Biol. 1979 Oct 15;134(1):173–188. doi: 10.1016/0022-2836(79)90419-4. [DOI] [PubMed] [Google Scholar]
- Reisler E., Cheung P., Oriol-Audit C., Lake J. A. Growth of synthetic myosin filaments from myosin minifilaments. Biochemistry. 1982 Feb 16;21(4):701–707. doi: 10.1021/bi00533a018. [DOI] [PubMed] [Google Scholar]
- Reisler E., Smith C., Seegan G. Myosin minifilaments. J Mol Biol. 1980 Oct 15;143(1):129–145. doi: 10.1016/0022-2836(80)90127-8. [DOI] [PubMed] [Google Scholar]
- Rieser G. D., Sabbadini R. A., Paolini P. J. Calcium and pH-induced structural changes in skinned muscle fibers: prevention by N-ethylmaleimide. Biochem Biophys Res Commun. 1979 Sep 12;90(1):179–186. doi: 10.1016/0006-291x(79)91606-1. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Efficiency of light diffraction by cross-striated muscle fibers under stretch and during isometric contraction. Biophys J. 1980 Jun;30(3):507–516. doi: 10.1016/S0006-3495(80)85110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Interpretation of light diffraction by cross-striated muscle as Bragg reflexion of light by the lattice of contractile proteins. J Physiol. 1979 May;290(2):317–330. doi: 10.1113/jphysiol.1979.sp012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Ishikawa H. In situ reconstitution of myosin filaments within the myosin-extracted myofibril in cultured skeletal muscle cells. J Cell Biol. 1982 Feb;92(2):324–332. doi: 10.1083/jcb.92.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada K., Kimura M. Stiffness of glycerinated rabbit psoas fibers in the rigor state. Filament-overlap relation. Biophys J. 1984 Mar;45(3):593–602. doi: 10.1016/S0006-3495(84)84197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada K., Yoshida A., Morita K. Myosin-free ghosts of single fibers and an attempt to re-form myosin filaments in the ghost fibers. J Biochem. 1976 Jul;80(1):121–127. doi: 10.1093/oxfordjournals.jbchem.a131243. [DOI] [PubMed] [Google Scholar]
- Trinick J., Cooper J. Sequential disassembly of vertebrate muscle thick filaments. J Mol Biol. 1980 Aug 15;141(3):315–321. doi: 10.1016/0022-2836(80)90183-7. [DOI] [PubMed] [Google Scholar]
- Umazume Y., Fujime S. Electro-optical property of extremely stretched skinned muscle fibers. Biophys J. 1975 Feb;15(2 Pt 1):163–180. doi: 10.1016/s0006-3495(75)85799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y., Baskin R. J., Lieber R. L., Roos K. P. Theory of light diffraction by single skeletal muscle fibers. Biophys J. 1980 Mar;29(3):509–522. doi: 10.1016/S0006-3495(80)85149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Umazume Y., Natori R., Fujime S., Chiba S. Optical diffraction study of muscle fibers. II. Electro-optical properties of muscle fibers. Biophys Chem. 1978 Sep;8(4):317–326. doi: 10.1016/0301-4622(78)80014-3. [DOI] [PubMed] [Google Scholar]


