Abstract
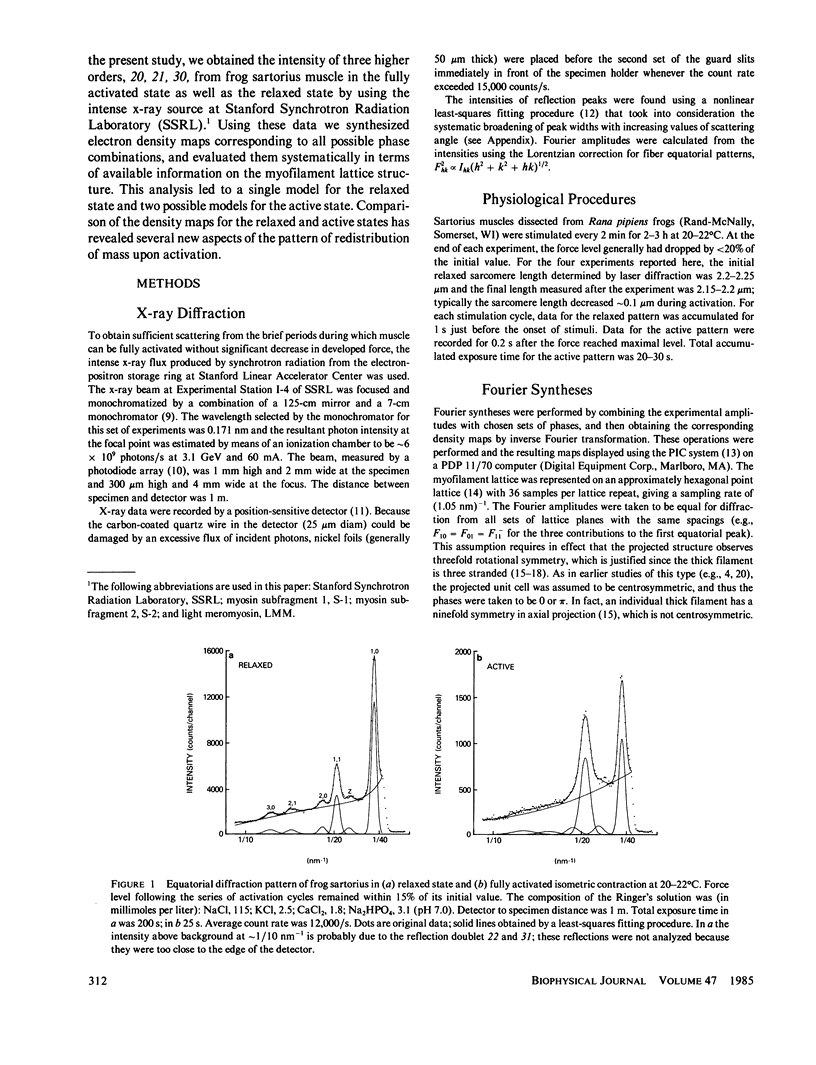
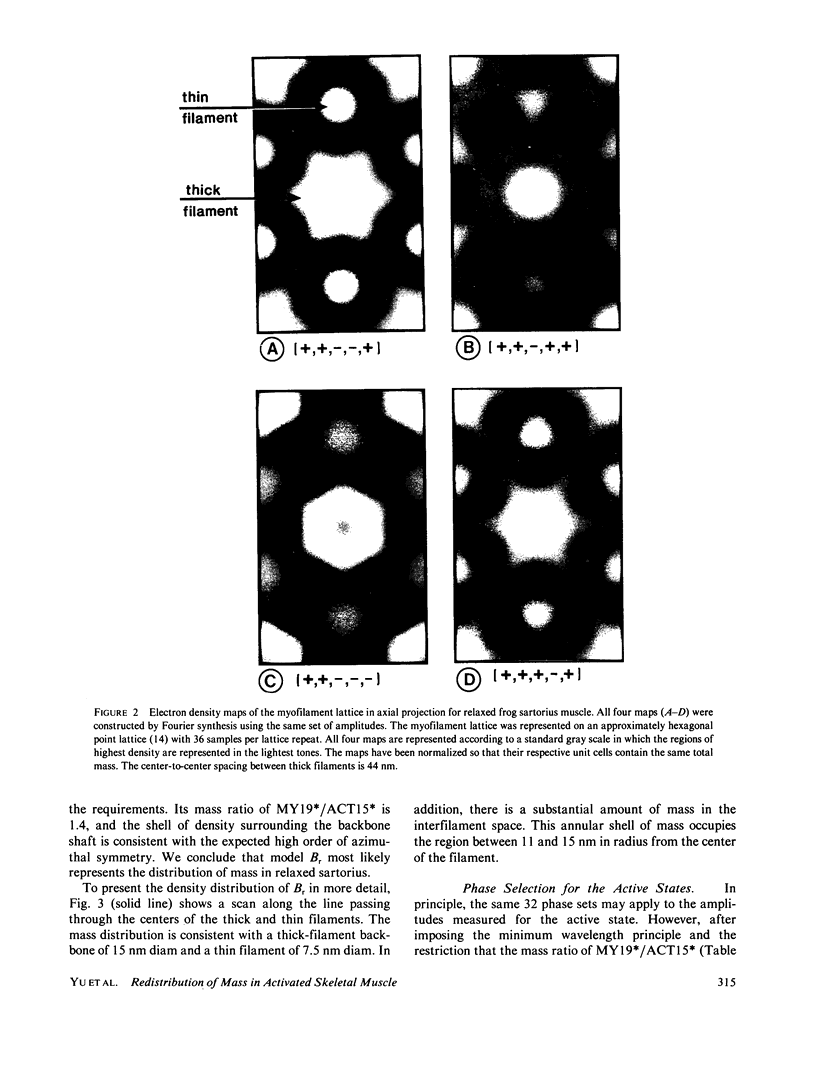
Five orders of equatorial reflection were recorded from both relaxed and fully activated intact frog sartorius muscle using synchrotron x-ray radiation. Electron density maps of the myofilament lattice in axial projection were calculated from the integrated intensities by Fourier synthesis, using all possible phase combinations. These maps were evaluated systematically in terms of their compatibility with electron microscopically and biochemically derived properties of the lattice structure and with the minimum wavelength principle. For the relaxed state, one phase combination emerged as most consistent with these constraints: it shows a thick filament with a compact core surrounded by an annular shell of density. The distribution of mass suggests that the S-2 moiety of the myosin molecule is an integral part of the thick-filament backbone and the S-1 moiety makes up the shell and is tilted or slewed around the backbone. For the active state, there are two feasible maps, which differ according to whether or not the activation process is associated with phase inversion in two of the reflections. Both maps represent patterns of redistribution of mass upon activation in which the thick-filament backbone is practically unaffected and there is movement of density from the annular shell towards the thin filaments. In addition to this outward radial flux of density from the thick-filament periphery, the pattern of net mass transfer involves a pronounced azimuthal component in both cases. The total net mass transfer is equivalent to approximately 20% (no phase change) or approximately 40% (with phase change) of the S-1 mass. From the observed systematic increase in peak widths of the higher orders, the size of the crystalline domain in the myofilament lattice in the relaxed sartorius is estimated to be greater than 650 nm and the variations in myofilament lattice spacing among different myofibrils to be about +/- 3%. Furthermore, in the activated state, the equilibrium positions of the myofilaments are no longer well ordered, but are distributed statistically about the lattice points with a standard deviation of approximately 3 nm.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Bijlenga R. K., ten Heggeler B., Kistler J., Steven A. C., Smith P. R. Comparison of the structural and chemical composition of giant T-even phage heads. J Supramol Struct. 1976;5(4):475–495. doi: 10.1002/jss.400050406. [DOI] [PubMed] [Google Scholar]
- BARANY M., BARANY K., RECKARD T., VOLPE A. MYOSIN OF FAST AND SLOW MUSCLES OF THE RABBIT. Arch Biochem Biophys. 1965 Jan;109:185–191. doi: 10.1016/0003-9861(65)90304-8. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Lowy J., Millman B. M. Low-angle x-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967 Apr 14;25(1):31–45. doi: 10.1016/0022-2836(67)90277-x. [DOI] [PubMed] [Google Scholar]
- Harrington W. F. On the origin of the contractile force in skeletal muscle. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5066–5070. doi: 10.1073/pnas.76.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C. A model of myosin crossbridge structure consistent with the low-angle x-ray diffraction pattern of vertebrate muscle. J Muscle Res Cell Motil. 1980 Jun;1(2):177–191. doi: 10.1007/BF00711798. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Stewart M., Huxley H. E. Cross-bridge movement during muscle contraction. Nature. 1976 Jun 17;261(5561):606–608. doi: 10.1038/261606a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E. Structure of the myosin crossbridge lattice in insect flight muscle. J Mol Biol. 1983 Sep 5;169(1):123–154. doi: 10.1016/s0022-2836(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Ip W., Heuser J. Direct visualization of the myosin crossbridge helices on relaxed rabbit psoas thick filaments. J Mol Biol. 1983 Nov 25;171(1):105–109. doi: 10.1016/s0022-2836(83)80317-9. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. Frog skeletal muscle thick filaments are three-stranded. J Cell Biol. 1983 Jun;96(6):1797–1802. doi: 10.1083/jcb.96.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G. D. Mlab--a mathematical modeling tool. Comput Programs Biomed. 1979 Dec;10(3):271–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Equatorial X-ray reflections and cross arm movement in skeletal muscle. Nature. 1975 Dec 25;258(5537):770–772. doi: 10.1038/258770a0. [DOI] [PubMed] [Google Scholar]
- Maw M. C., Rowe A. J. Fraying of A-filaments into three subfilaments. Nature. 1980 Jul 24;286(5771):412–414. doi: 10.1038/286412a0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Kretzschmar K. M. Structure of myosin subfragment 1 from low-angle X-ray scattering. Biochemistry. 1980 Aug 19;19(17):4103–4108. doi: 10.1021/bi00558a031. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Naylor G. R., Arata T. Cross-bridge properties in the rigor state. Soc Gen Physiol Ser. 1982;37:79–89. [PubMed] [Google Scholar]
- Podolsky R. J., St Onge H., Yu L., Lymn R. W. X-ray diffraction of actively shortening muscle. Proc Natl Acad Sci U S A. 1976 Mar;73(3):813–817. doi: 10.1073/pnas.73.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Cain J. E., Dratz E. A., Blasie J. K. An analysis of lamellar x-ray diffraction from disordered membrane multilayers with application to data from retinal rod outer segments. Biophys J. 1975 Dec;15(12):1201–1233. doi: 10.1016/S0006-3495(75)85895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J. M. General model of myosin filament structure. II. Myosin filaments and cross-bridge interactions in vertebrate striated and insect flight muscles. J Mol Biol. 1972 Dec 14;72(1):125–138. doi: 10.1016/0022-2836(72)90074-5. [DOI] [PubMed] [Google Scholar]
- Squire J. M. Symmetry and three-dimensional arrangement of filaments in vertebrate striated muscle. J Mol Biol. 1974 Nov 25;90(1):153–160. doi: 10.1016/0022-2836(74)90263-0. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- Yu L. C., Lymn R. W., Podolsky R. J. Characterization of a non-indexible equatorial x-ray reflection from frog sartorius muscle. J Mol Biol. 1977 Sep 25;115(3):455–464. doi: 10.1016/0022-2836(77)90165-6. [DOI] [PubMed] [Google Scholar]
- Yu L. P., Hartt J. E., Podolsky R. J. Equatorial x-ray intensities and isometric force levels in frog sartorius muscle. J Mol Biol. 1979 Jul 25;132(1):53–67. doi: 10.1016/0022-2836(79)90495-9. [DOI] [PubMed] [Google Scholar]