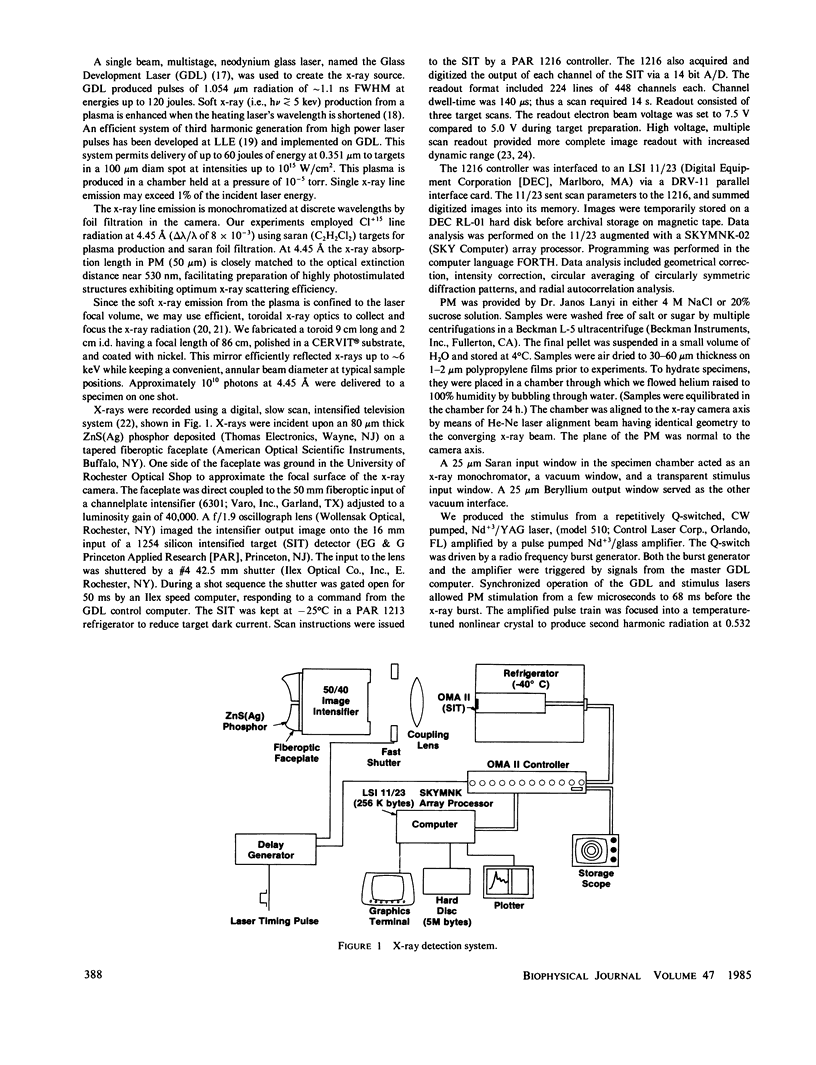
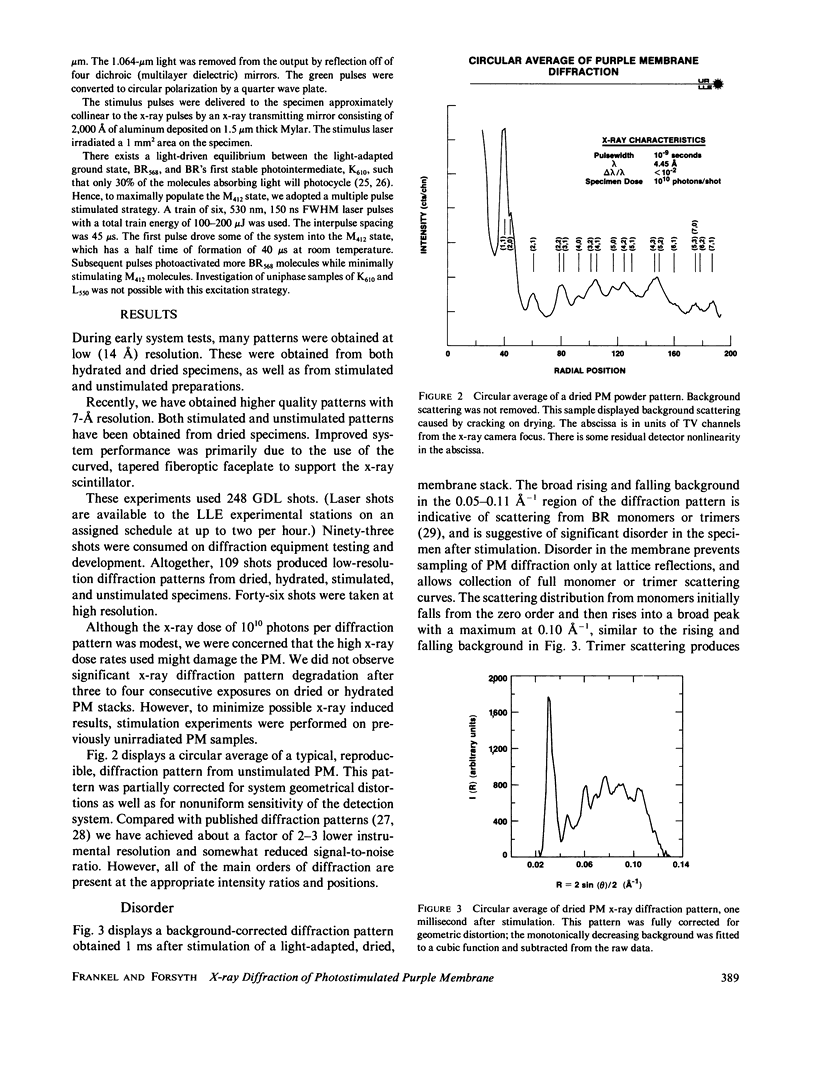
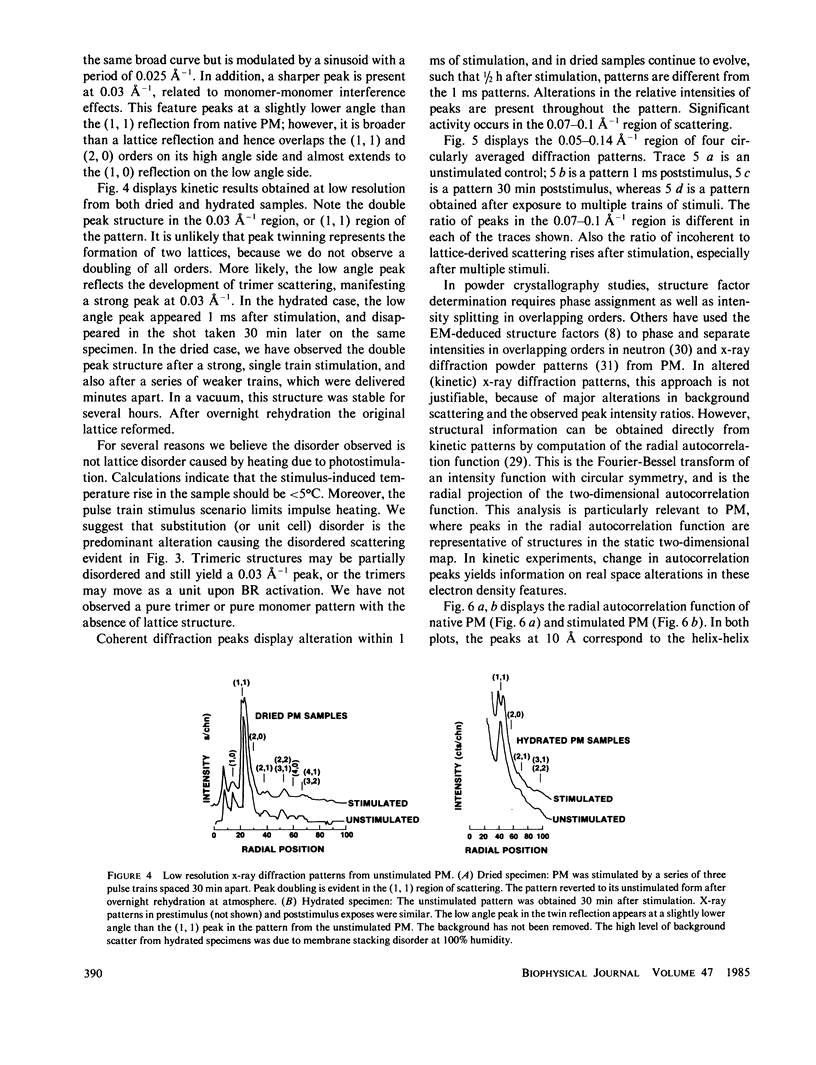
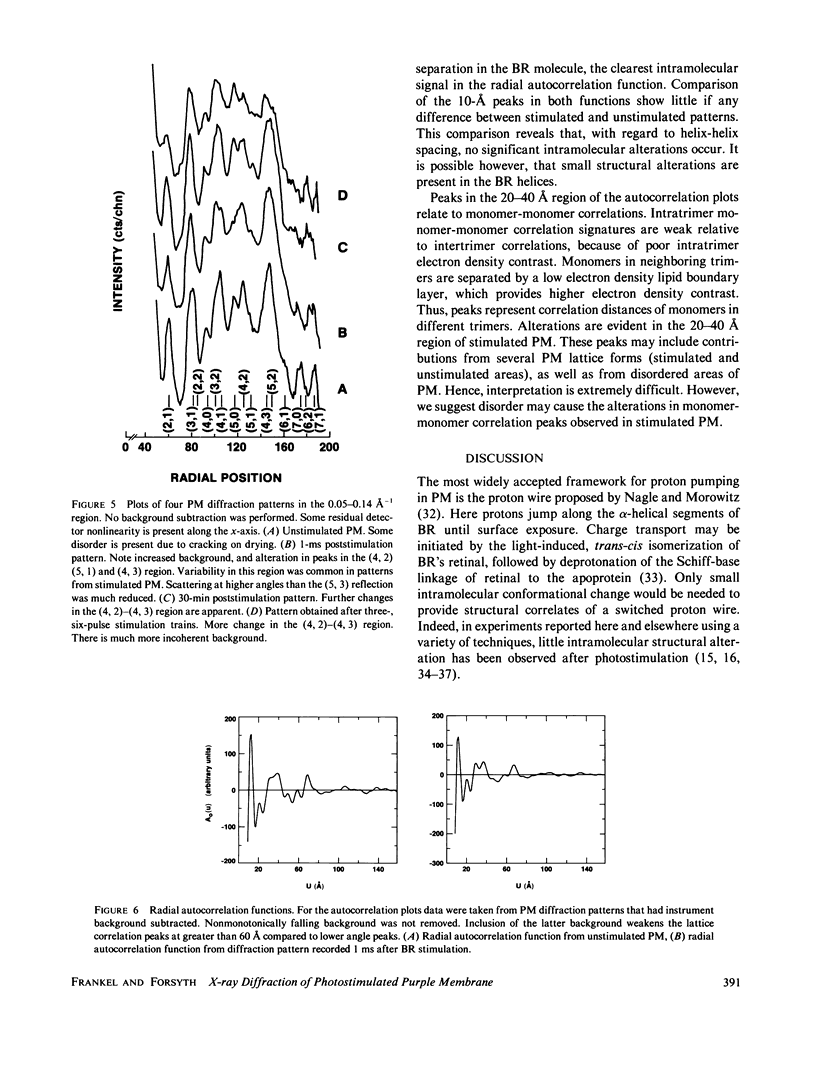
Abstract
A nanosecond resolution laser-driven x-ray source has been used to perform a time-resolved, x-ray diffraction study of the purple membrane of the Halobacterium halobium. Alterations in diffraction patterns have been observed 1 ms after photostimulation, and are interpreted to show disorder of bacteriorhodopsin packing in the plane of the membrane with little bacteriorhodopsin structural change.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K., Dollinger G., Eisenstein L., Singh A. K., Zimányi L. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4972–4976. doi: 10.1073/pnas.79.16.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of bleaching and regeneration on the purple membrane structure of Halobaterium halobium. Biophys J. 1977 Sep;19(3):285–297. doi: 10.1016/s0006-3495(77)85588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Tokunaga F., Ebrey T. G. Ultraviolet and visible absorption spectra of the purple membrane protein and the photocycle intermediates. Biochemistry. 1978 Jun 13;17(12):2293–2300. doi: 10.1021/bi00605a006. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Frankel R. D., Forsyth J. M. Nanosecond X-ray diffraction from biological samples with a laser-produced plasma source. Science. 1979 May 11;204(4393):622–624. doi: 10.1126/science.432665. [DOI] [PubMed] [Google Scholar]
- Goldschmidt C. R., Kalisky O., Rosenfeld T., Ottolenghi M. The quantum efficiency of the bacteriorhodopsin photocycle. Biophys J. 1977 Feb;17(2):179–183. doi: 10.1016/S0006-3495(77)85635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Bordas J., Koch M. H., Milch J. R. The use of synchrotron radiation in time-resolved X-ray diffraction studies of myosin layer-line reflections during muscle contraction. Nature. 1980 Mar 13;284(5752):140–143. doi: 10.1038/284140a0. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Korenbrot J. I., Stoeckenius W. Transient photovoltages in purple membrane multilayers. Charge displacement in bacteriorhodopsin and its photointermediates. Biochim Biophys Acta. 1978 May 18;509(2):300–317. doi: 10.1016/0005-2736(78)90049-4. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Goldschmidt C. R., Ottolenghi M. On the photocycle and light adaptation of dark-adapted bacteriorhodopsin. Biophys J. 1977 Aug;19(2):185–189. doi: 10.1016/S0006-3495(77)85579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on cis--trans isomerization of bacteriorhodopsin. FEBS Lett. 1977 Oct 1;82(1):7–11. doi: 10.1016/0014-5793(77)80874-0. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai G., Gilmore D. J. Areas of hydration in the purple membrane of Halobacterium halobium: a neutron diffraction study. J Mol Biol. 1979 Aug 5;132(2):181–191. doi: 10.1016/0022-2836(79)90390-5. [DOI] [PubMed] [Google Scholar]


