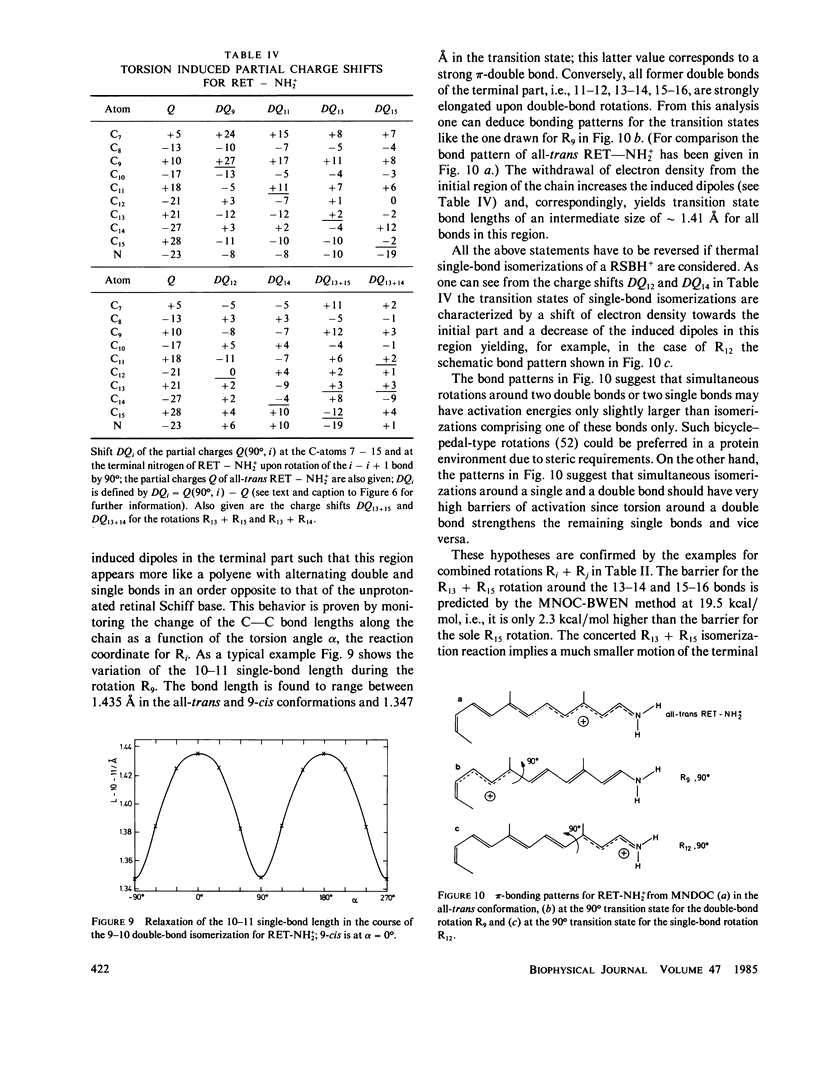
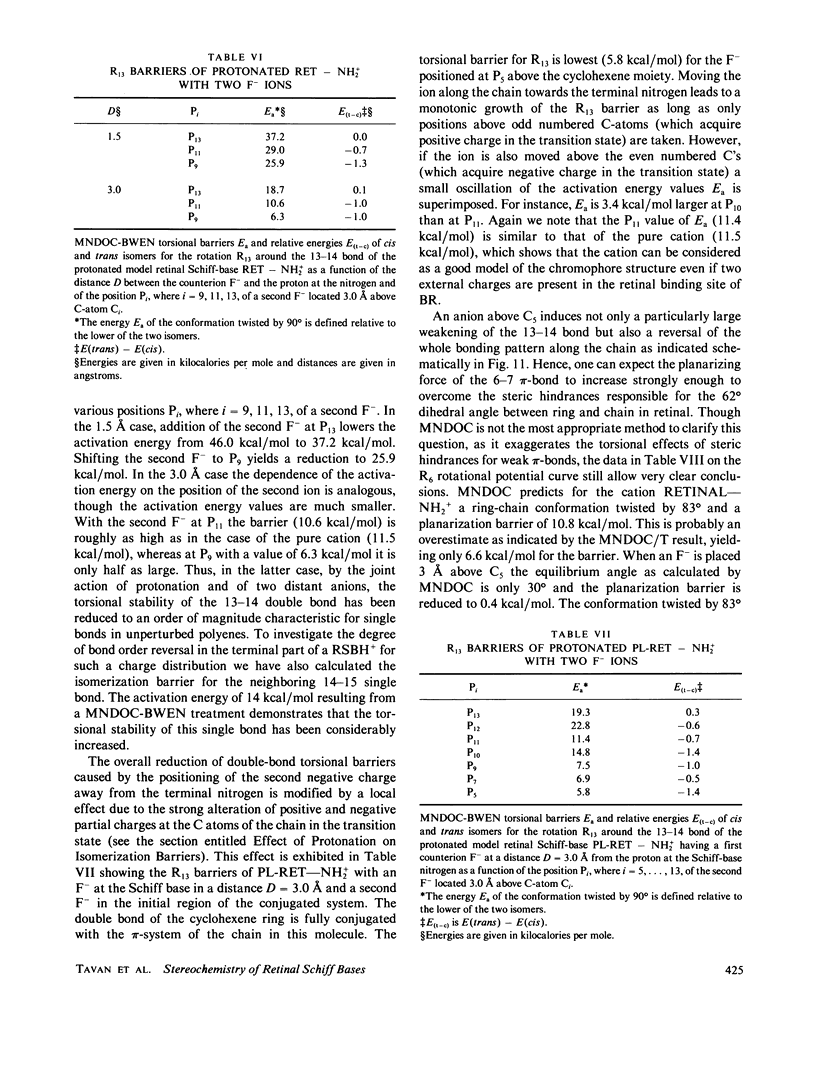
Abstract
Based on quantumchemical MNDOC calculations it is shown that the ground-state properties of a retinal Schiff base depend sensitively on its protonation state and charge environment. This is exemplified for the equilibrium geometry, for the distribution of partial charges and, in particular, for the thermal isomerization barriers around the π-bonds. It is demonstrated that a protein, by protonating the retinal Schiff base and by providing one or two negative ions in its environment, can reduce double-bond isomerization barriers from 50 kcal/mol for the unprotonated compound to ∼ 5 kcal/mol and can increase single bond barriers from 5 kcal/mol to ∼ 20 kcal/mol. Thereby, the specific location of the ions relative to the polyene chain of the protonated retinal Schiff base determines the barrier heights. The results explain the ground-state isomerization reactions of retinal observed in bacteriorhodopsin and in squid retinochrome.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard G. D. Red-absorbing visual pigment of butterflies. Science. 1979 Mar 16;203(4385):1125–1127. doi: 10.1126/science.203.4385.1125. [DOI] [PubMed] [Google Scholar]
- Birge R. R., Cooper T. M. Energy storage in the primary step of the photocycle of bacteriorhodopsin. Biophys J. 1983 Apr;42(1):61–69. doi: 10.1016/S0006-3495(83)84369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz P. E., Mohler J. H. Effect of selected anions and solvents on the electron absorption, nuclear magnetic resonance, and infrared spectra of the N-retinylidene-n-butylammonium cation. Biochemistry. 1975 Jun 3;14(11):2304–2309. doi: 10.1021/bi00682a005. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Ottolenghi M., Pande A., Pande J., Callender R. H. Acid-base equilibrium of the Schiff base in bacteriorhodopsin. Biochemistry. 1982 Sep 28;21(20):4953–4959. doi: 10.1021/bi00263a019. [DOI] [PubMed] [Google Scholar]
- Erickson J. O., Blatz P. E. N-retinylidene-1-amino-2-propanol: a Schiff base analog for rhodopsin. Vision Res. 1968 Oct;8(10):1367–1375. doi: 10.1016/0042-6989(68)90056-4. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Byers G. W., Leermakers P. A. Spectroscopic model for the visual pigments. Influence of microenvironmental polarizability. Biochemistry. 1970 Feb 17;9(4):858–864. doi: 10.1021/bi00806a020. [DOI] [PubMed] [Google Scholar]
- KROPF A., HUBBARD R. The mechanism of bleaching rhodopsin. Ann N Y Acad Sci. 1959 Nov 12;74(2):266–280. doi: 10.1111/j.1749-6632.1958.tb39550.x. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Hara R., Hara T. Dependency of absorption characteristics of retinochrome on pH and salts. Exp Eye Res. 1982 Apr;34(4):499–508. doi: 10.1016/0014-4835(82)90022-7. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Hara R., Hara T., Kakitani T. Squid retinochrome. Configurational changes of the retinal chromophore. Biophys J. 1983 Oct;44(1):127–137. doi: 10.1016/S0006-3495(83)84285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Ebrey T. The blue membrane: the 3-dehydroretinal-based artificial pigment of the purple membrane. Biochemistry. 1978 May 16;17(10):1915–1922. doi: 10.1021/bi00603a018. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Conversion of light energy to electrostatic energy in the proton pump of Halobacterium halobium. Photochem Photobiol. 1979 Aug;30(2):285–290. doi: 10.1111/j.1751-1097.1979.tb07148.x. [DOI] [PubMed] [Google Scholar]


