Abstract
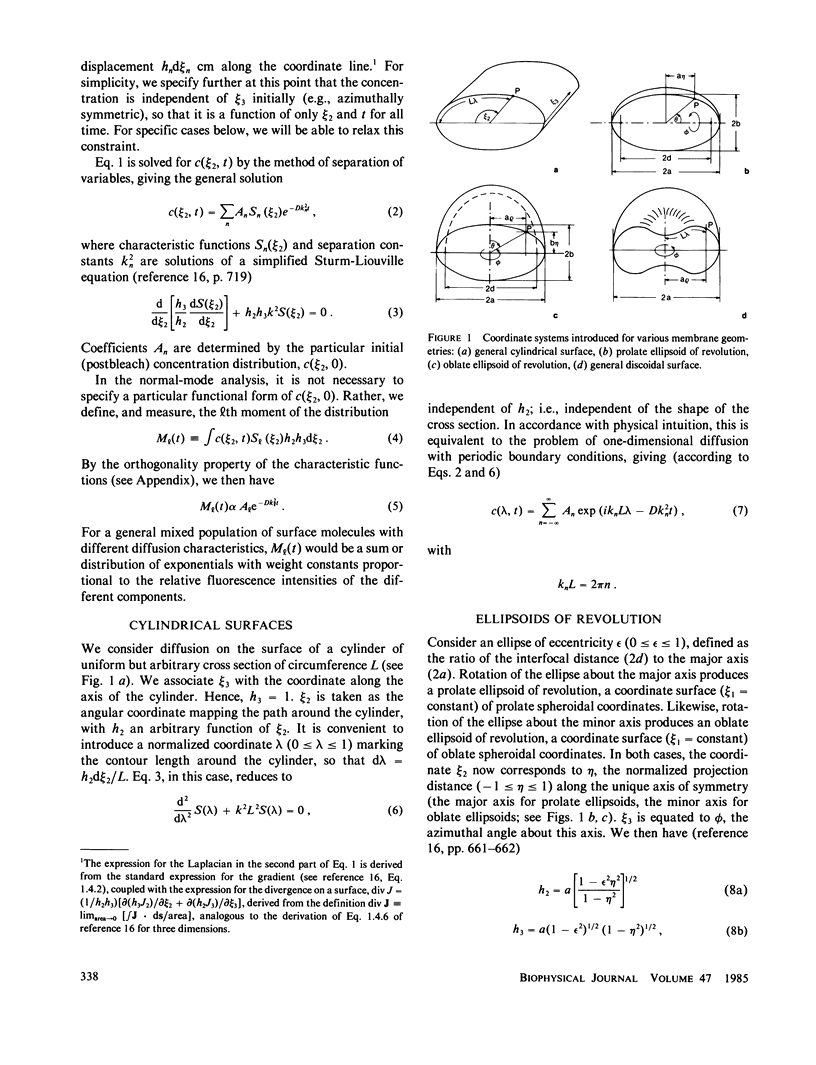
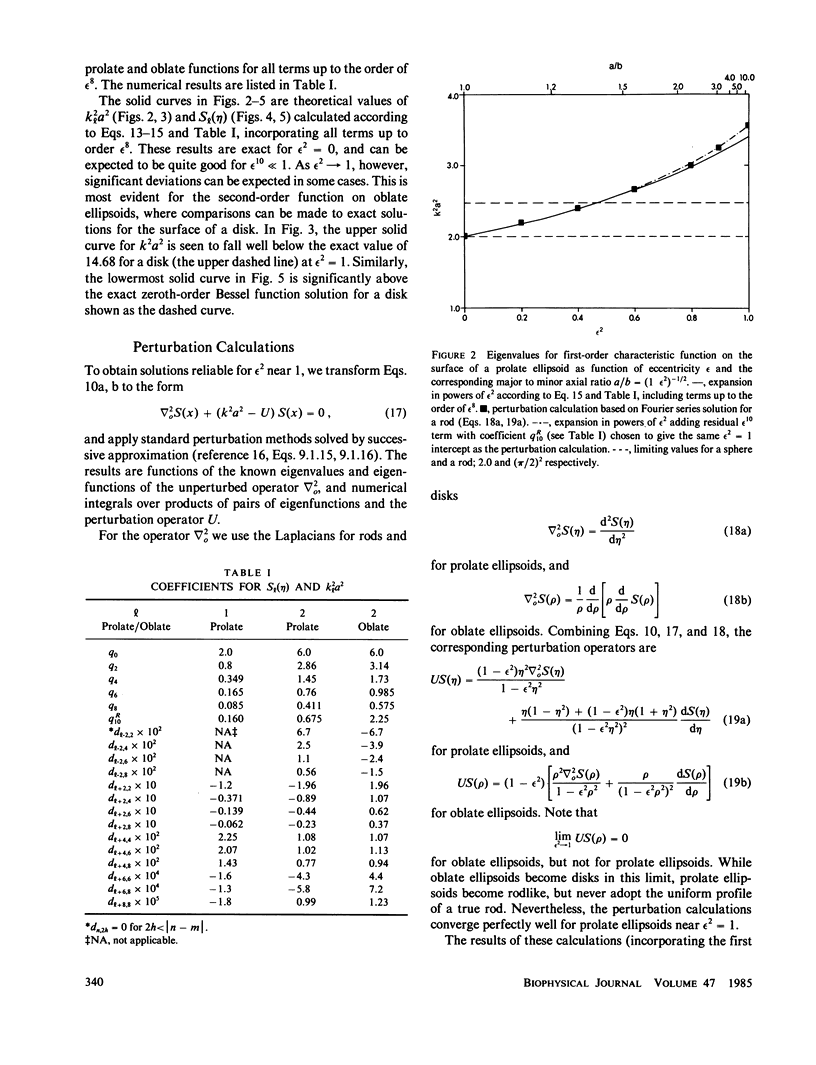
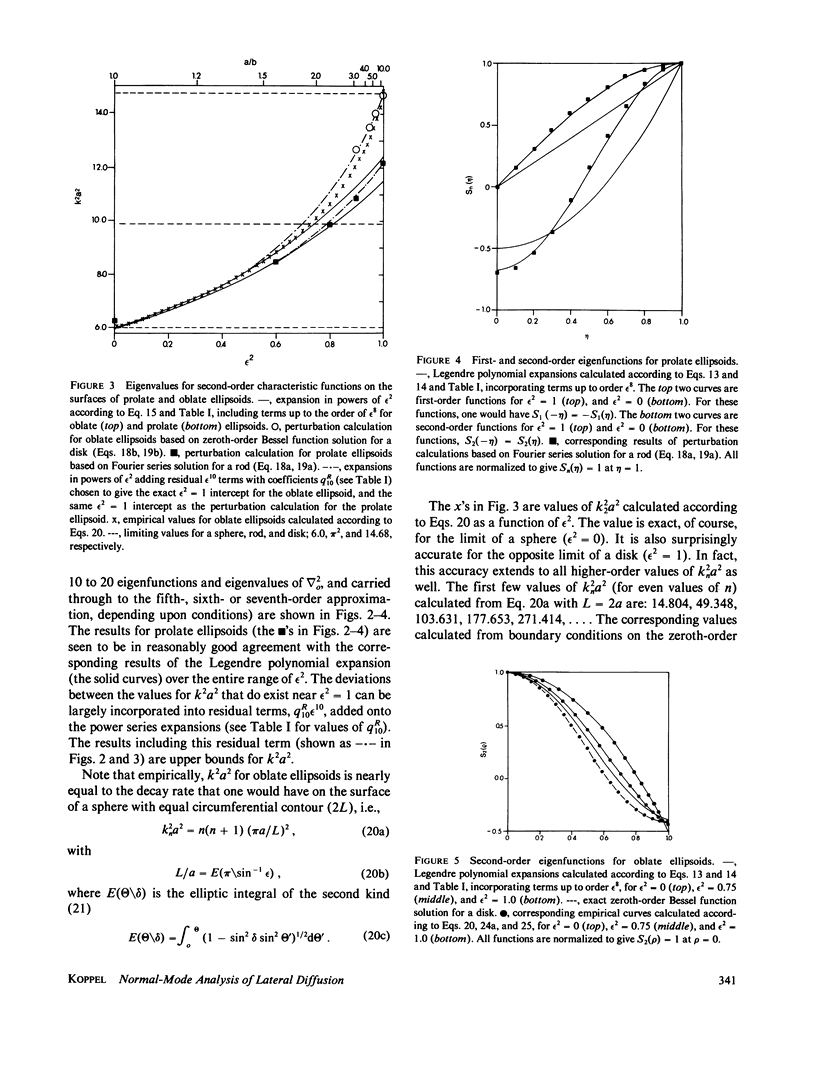
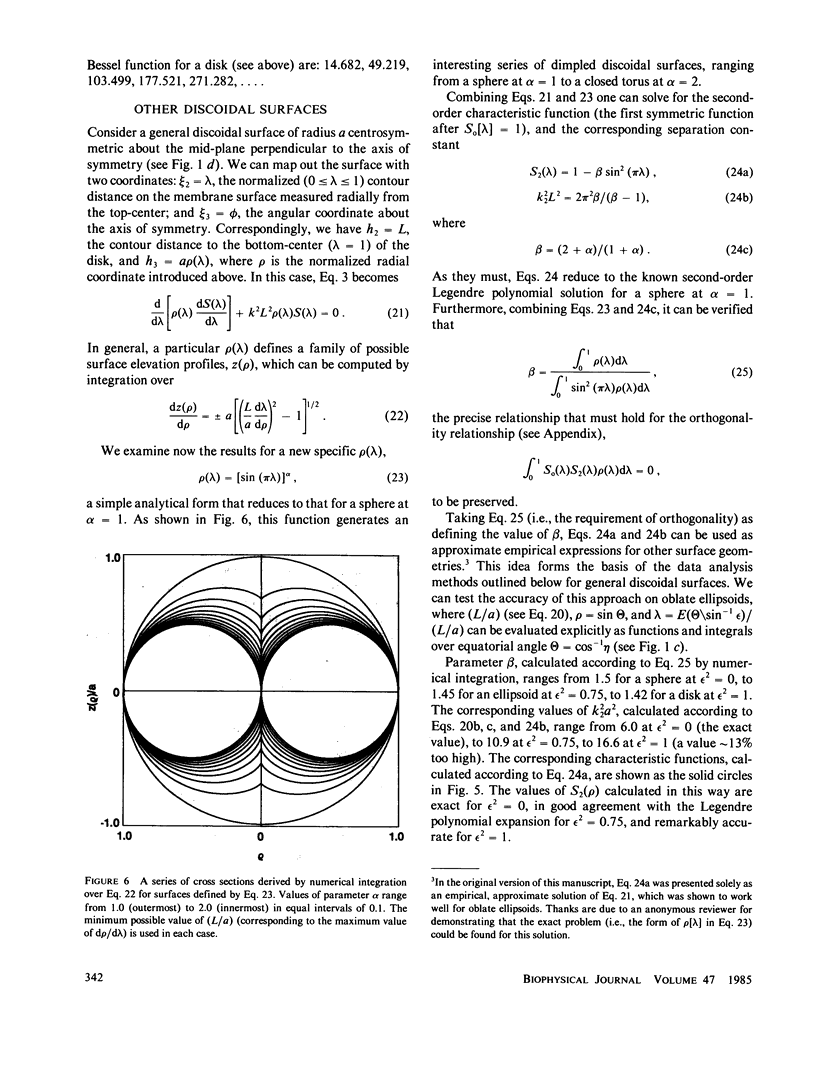
The normal-mode analysis of fluorescence redistribution after photobleaching, introduced for the characterization of lateral diffusion on spherical membrane surfaces, has been generalized and extended to other surface geometries. Theoretical expressions are derived for the characteristic values and orthogonal characteristic functions of the diffusion equations for cylindrical surfaces, ellipsoids of revolution and dimpled discoidal surfaces. On the basis of these results, a simple analytical function is proposed as an empirical solution for the analysis of photobleaching data on a variety of discoidal surfaces. Special experimental and computational methods for determining the surface-diffusion coefficient are described, and demonstrated with data for lipid diffusion in erythrocyte membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Hochman J. H., Schindler M., Lee J. G., Ferguson-Miller S. Lateral mobility of cytochrome c on intact mitochondrial membranes as determined by fluorescence redistribution after photobleaching. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6866–6870. doi: 10.1073/pnas.79.22.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. W. Mobility and diffusion in the plane of cell membrane. J Theor Biol. 1973 Jul;40(1):11–17. doi: 10.1016/0022-5193(73)90161-6. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P. A localized pattern photobleaching method for the concurrent analysis of rapid and slow diffusion processes. Biophys J. 1983 Aug;43(2):175–181. doi: 10.1016/S0006-3495(83)84338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P. Fluorescence photobleaching does not alter the lateral mobility of erythrocyte membrane glycoproteins. Nature. 1981 Sep 10;293(5828):159–161. doi: 10.1038/293159a0. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Lateral diffusion in biological membranes. A normal-mode analysis of diffusion on a spherical surface. Biophys J. 1980 Apr;30(1):187–192. doi: 10.1016/S0006-3495(80)85087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- Poo M. Rapid lateral diffusion of functional A Ch receptors in embryonic muscle cell membrane. Nature. 1982 Jan 28;295(5847):332–334. doi: 10.1038/295332a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., McConnell H. M., Smith Baron A., Parce J. W. Pattern photobleaching of fluorescent lipid vesicles using polarized laser light. Biophys J. 1981 Jan;33(1):139–146. doi: 10.1016/S0006-3495(81)84877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Parce J. W., Smith B. A., McConnell H. M. Antibodies bound to lipid haptens in model membranes diffuse as rapidly as the lipids themselves. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4177–4179. doi: 10.1073/pnas.76.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]


