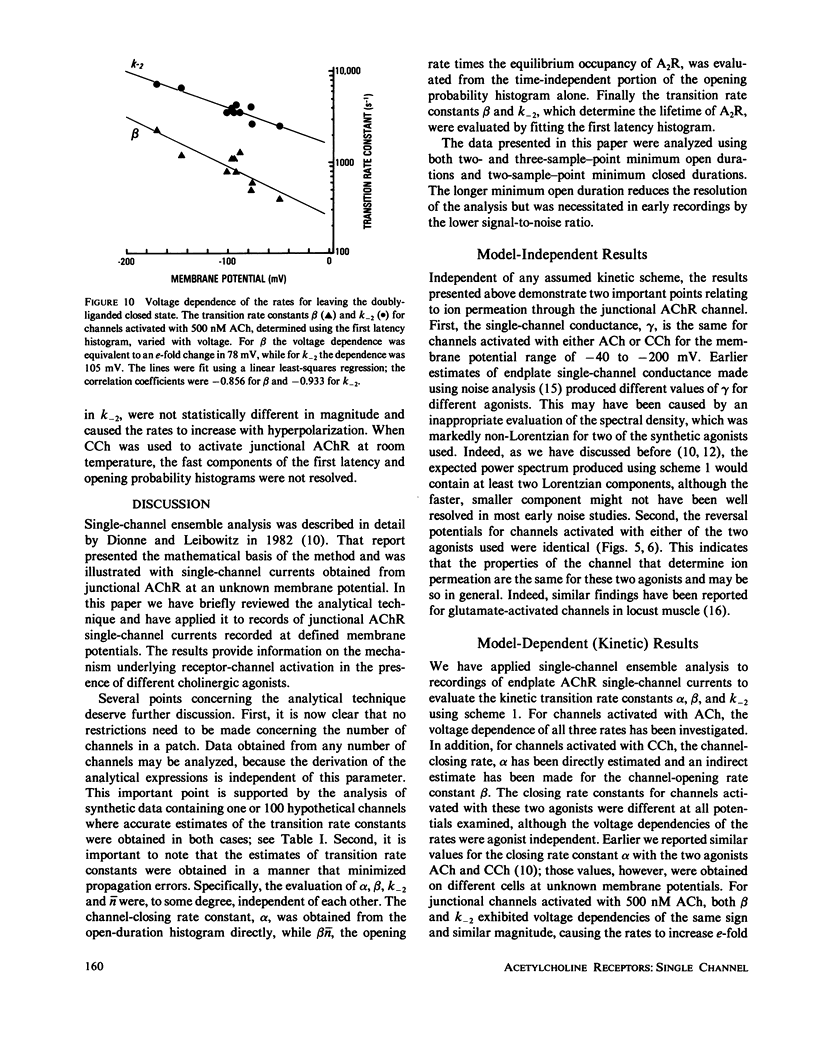
Abstract
The temporal relationships among junctional acetylcholine receptor single-channel currents have been examined to probe the mechanism of channel activation. We have presented an analytical approach, termed single-channel ensemble analysis, that allows one to estimate the kinetic transition rate constants for channel-opening and closing as well as the rate of leaving the specific doubly-liganded, closed state from which opening occurs. This approach may be applied to data produced by any number of independent channels as long as the probability of channel opening is low, a condition that is experimentally verifiable. The method has been independently validated using simulated single-channel data generated by computer from one or 100 hypothetical channels. Typical experimental values for the transition rate constants estimated from acetylcholine-activated single channels at the garter snake neuromuscular junction were: opening = 1,200 s-1, closing = 455 s-1, back rate for leaving the doubly-liganded, closed state = 3,200 s-1 at a transmembrane potential of -92 mV at room temperature. Each of these three rate constants was voltage dependent, with the closing rate decreasing e-fold for 173 mV of hyperpolarization, the opening rate increasing e-fold for 78 mV, and the unbinding rate increasing e-fold for 105 mV. The channel-closing rate was agonist dependent, being greater at all potentials for channels activated with carbamylcholine than for channels activated with acetylcholine. However, the single-channel conductance and reversal potential were the same for these two agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E. The kinetics of slow muscle acetylcholine-operated channels in the garter snake. J Physiol. 1981 Jan;310:159–190. doi: 10.1113/jphysiol.1981.sp013542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gration K. A., Lambert J. J., Ramsey R. L., Rand R. P., Usherwood P. N. Agonist potency determination by patch clamp analysis of single glutamate receptors. Brain Res. 1981 Dec 28;230(1-2):400–405. doi: 10.1016/0006-8993(81)90423-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. M., Dionne V. E. Temperature dependence of ion permeation at the endplate channel. J Gen Physiol. 1983 May;81(5):687–703. doi: 10.1085/jgp.81.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]


