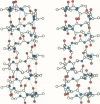
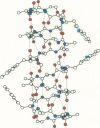
Abstract
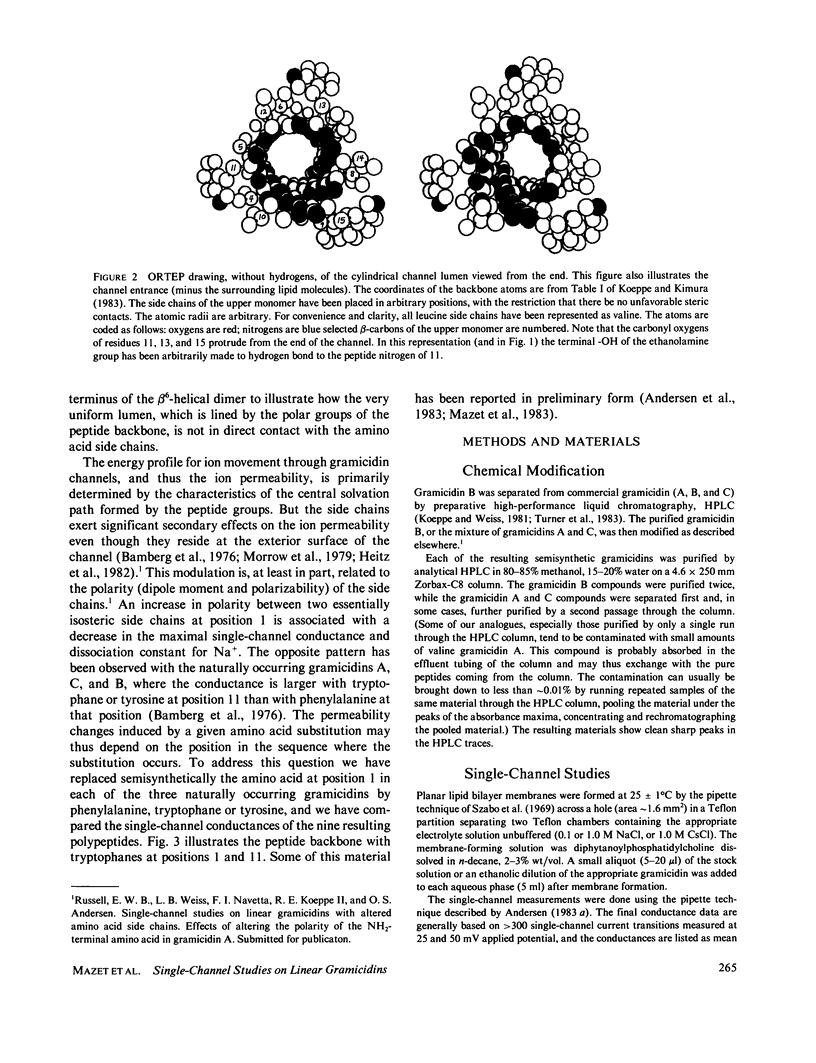
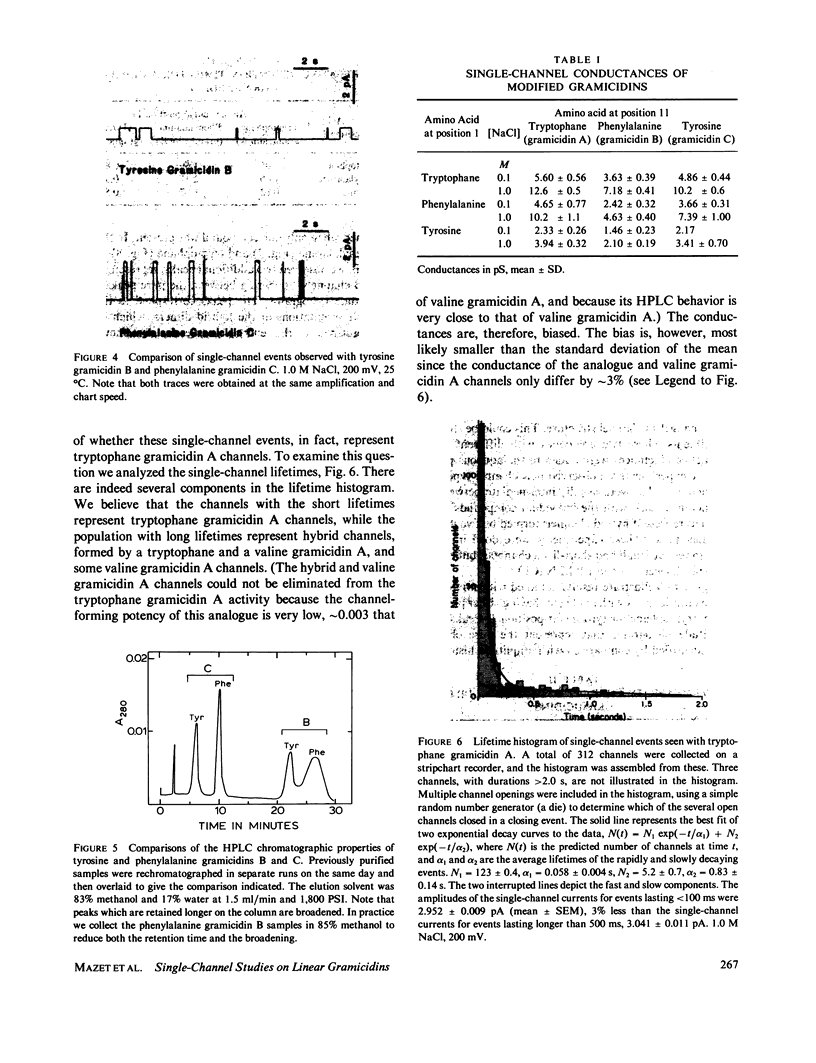
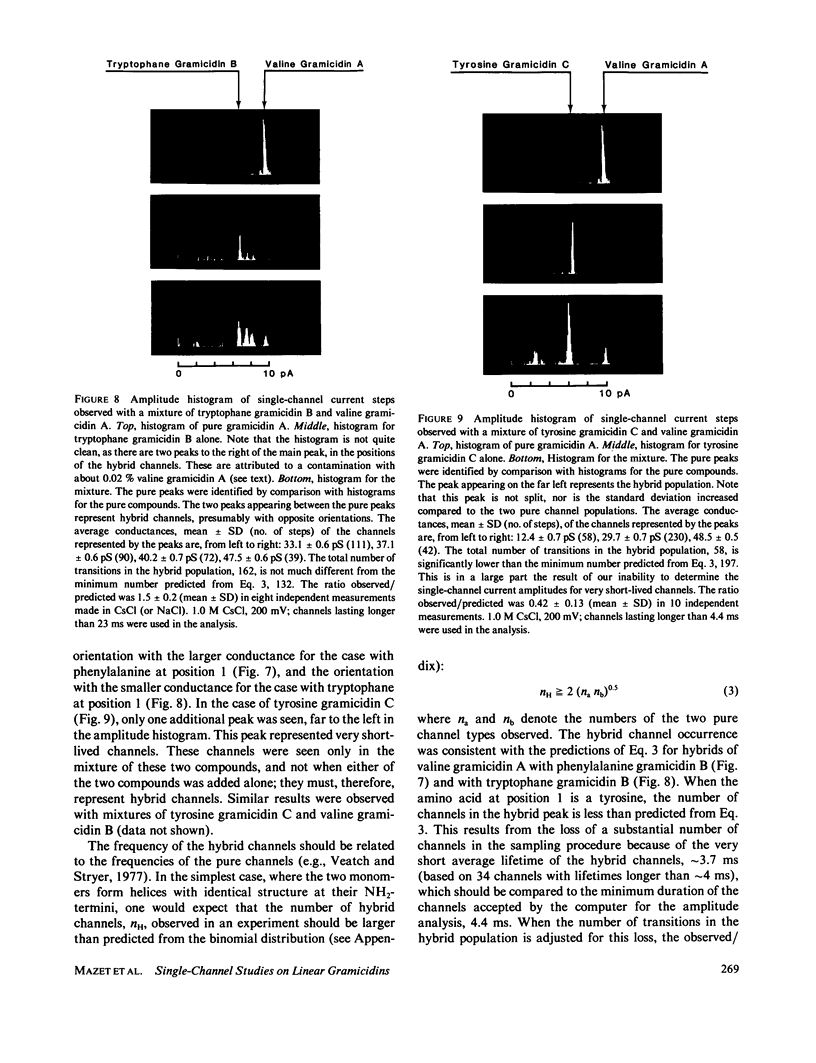
The relation between chemical structure and permeability characteristics of transmembrane channels has been investigated with the linear gramicidins (A, B, and C), where the amino acid at position 1 was chemically replaced by phenylalanine, tryptophane or tyrosine. The purity of most of the compounds was estimated to be greater than 99.99%. The modifications resulted in a wide range of conductance changes in NaCl solutions: sixfold from tryptophane gramicidin A to tyrosine gramicidin B. The conductance changes induced by a given amino acid substitution at position 1 are not the same as at position 11. The only important change in the Na+ affinity was observed when the first amino acid was tyrosine. No major conformational changes of the polypeptide backbone structure could be detected on the basis of experiments with mixtures of different analogues and valine gramicidin A (except possibly with tyrosine at position 1), as all the compounds investigated could form hybrid channels with valine gramicidin A. The side chains are not in direct contact with the permeating ions. The results were therefore interpreted in terms of modifications of the energy profile for ion movement through the channel, possibly due to an electrostatic interaction between the dipoles of the side chains and ions in the channel.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys J. 1983 Feb;41(2):119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S. Ion movement through gramicidin A channels. Studies on the diffusion-controlled association step. Biophys J. 1983 Feb;41(2):147–165. doi: 10.1016/S0006-3495(83)84416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Apell H. J., Alpes H., Gross E., Morell J. L., Harbaugh J. F., Janko K., Läuger P. Ion channels formed by chemical analogs of gramicidin A. Fed Proc. 1978 Oct;37(12):2633–2638. [PubMed] [Google Scholar]
- Bamberg E., Apell H. J., Alpes H. Structure of the gramicidin A channel: discrimination between the piL,D and the beta helix by electrical measurements with lipid bilayer membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2402–2406. doi: 10.1073/pnas.74.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Janko K. The action of a carbonsuboxide dimerized gramicidin A on lipid bilayer membranes. Biochim Biophys Acta. 1977 Mar 17;465(3):486–499. doi: 10.1016/0005-2736(77)90267-x. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Noda K., Gross E., Läuger P. Single-channel parameters of gramicidin A,B, and C. Biochim Biophys Acta. 1976 Jan 21;419(2):223–228. doi: 10.1016/0005-2736(76)90348-5. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Fischer W., Brickmann J., Läuger P. Molecular dynamics study of ion transport in transmembrane protein channels. Biophys Chem. 1981 Apr;13(2):105–116. doi: 10.1016/0301-4622(81)80009-9. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz F., Spach G., Trudelle Y. Single channels of 9, 11, 13, 15-destryptophyl-phenylalanyl-gramicidin A. Biophys J. 1982 Oct;40(1):87–89. doi: 10.1016/S0006-3495(82)84462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Jordan P. C. Electrostatic modeling of ion pores. Energy barriers and electric field profiles. Biophys J. 1982 Aug;39(2):157–164. doi: 10.1016/S0006-3495(82)84503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Berg J. M., Hodgson K. O., Stryer L. Gramicidin A crystals contain two cation binding sites per channel. Nature. 1979 Jun 21;279(5715):723–725. doi: 10.1038/279723a0. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Hodgson K. O., Stryer L. Helical channels in crystals of gramicidin A and of a cesium--gramicidin A complex: an x-ray diffraction study. J Mol Biol. 1978 May 5;121(1):41–54. doi: 10.1016/0022-2836(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. I. Energy and potential profiles and interactions between ions. Biophys J. 1978 May;22(2):209–219. doi: 10.1016/S0006-3495(78)85485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. II. Kinetic behavior of the gramicidin A channel. Biophys J. 1978 May;22(2):221–248. doi: 10.1016/S0006-3495(78)85486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G., Elias S. R., Hautman J. M. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim Biophys Acta. 1978 Sep 22;512(2):436–451. doi: 10.1016/0005-2736(78)90266-3. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Veatch W. R., Stryer L. Transmembrane channel activity of gramicidin A analogs: effects of modification and deletion of the amino-terminal residue. J Mol Biol. 1979 Aug 25;132(4):733–738. doi: 10.1016/0022-2836(79)90386-3. [DOI] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Sachs F. Single ionic channels observed in tissue-cultured muscle. Nature. 1979 Dec 20;282(5741):861–863. doi: 10.1038/282861a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- Renugopalakrishnan V., Urry D. W. A theoretical study of Na+ and Mg+2 binding to the carbonyl oxygen of N-methyl acetamide. Biophys J. 1978 Dec;24(3):729–738. doi: 10.1016/S0006-3495(78)85416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P. A., Finkelstein A. Interaction of ions and water in gramicidin A channels: streaming potentials across lipid bilayer membranes. J Gen Physiol. 1978 Sep;72(3):327–340. doi: 10.1085/jgp.72.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P. A., Finkelstein A. Water permeability of gramicidin A-treated lipid bilayer membranes. J Gen Physiol. 1978 Sep;72(3):341–350. doi: 10.1085/jgp.72.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN A. V. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN A. J Am Chem Soc. 1965 May 5;87:2011–2020. doi: 10.1021/ja01087a027. [DOI] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN. VII. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN B. J Am Chem Soc. 1965 May 5;87:2027–2030. doi: 10.1021/ja01087a029. [DOI] [PubMed] [Google Scholar]
- Schagina L. V., Grinfeldt A. E., Lev A. A. Interaction of cation fluxes in gramicidin A channels in lipid bilayer membranes. Nature. 1978 May 18;273(5659):243–245. doi: 10.1038/273243a0. [DOI] [PubMed] [Google Scholar]
- Szabo G., Urry D. W. N-acetyl gramicidin: single-channel properties and implications for channel structure. Science. 1979 Jan 5;203(4375):55–57. doi: 10.1126/science.83000. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B., Haydon D. A. Ion movements in gramicidin pores. An example of single-file transport. Biochim Biophys Acta. 1980 Nov 4;602(2):331–354. doi: 10.1016/0005-2736(80)90316-8. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B. Ion transport in the simplest single file pore. Biochim Biophys Acta. 1979 Jul 5;554(2):410–429. doi: 10.1016/0005-2736(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Goodall M. C., Glickson J. D., Mayers D. F. The gramicidin A transmembrane channel: characteristics of head-to-head dimerized (L,D) helices. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1907–1911. doi: 10.1073/pnas.68.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Prasad K. U., Trapane T. L. Location of monovalent cation binding sites in the gramicidin channel. Proc Natl Acad Sci U S A. 1982 Jan;79(2):390–394. doi: 10.1073/pnas.79.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Walker J. T., Trapane T. L. Ion interactions in (1-13C)D-Val8 and D-Leu14 analogs of gramicidin A, the helix sense of the channel and location of ion binding sites. J Membr Biol. 1982;69(3):225–231. doi: 10.1007/BF01870401. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Fossel E. T., Blout E. R. The conformation of gramicidin A. Biochemistry. 1974 Dec 17;13(26):5249–5256. doi: 10.1021/bi00723a001. [DOI] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Morrow J. S., Veatch W. R. Conformation of the gramicidin A transmembrane channel: A 13C nuclear magnetic resonance study of 13C-enriched gramicidin in phosphatidylcholine vesicles. J Mol Biol. 1980 Oct 15;143(1):1–19. doi: 10.1016/0022-2836(80)90121-7. [DOI] [PubMed] [Google Scholar]
- Winter W. T., Smith P. J., Arnott S. Hyaluronic acid: structure of a fully extended 3-fold helical sodium salt and comparison with the less extended 4-fold helical forms. J Mol Biol. 1975 Dec 5;99(2):219–235. doi: 10.1016/s0022-2836(75)80142-2. [DOI] [PubMed] [Google Scholar]