Abstract
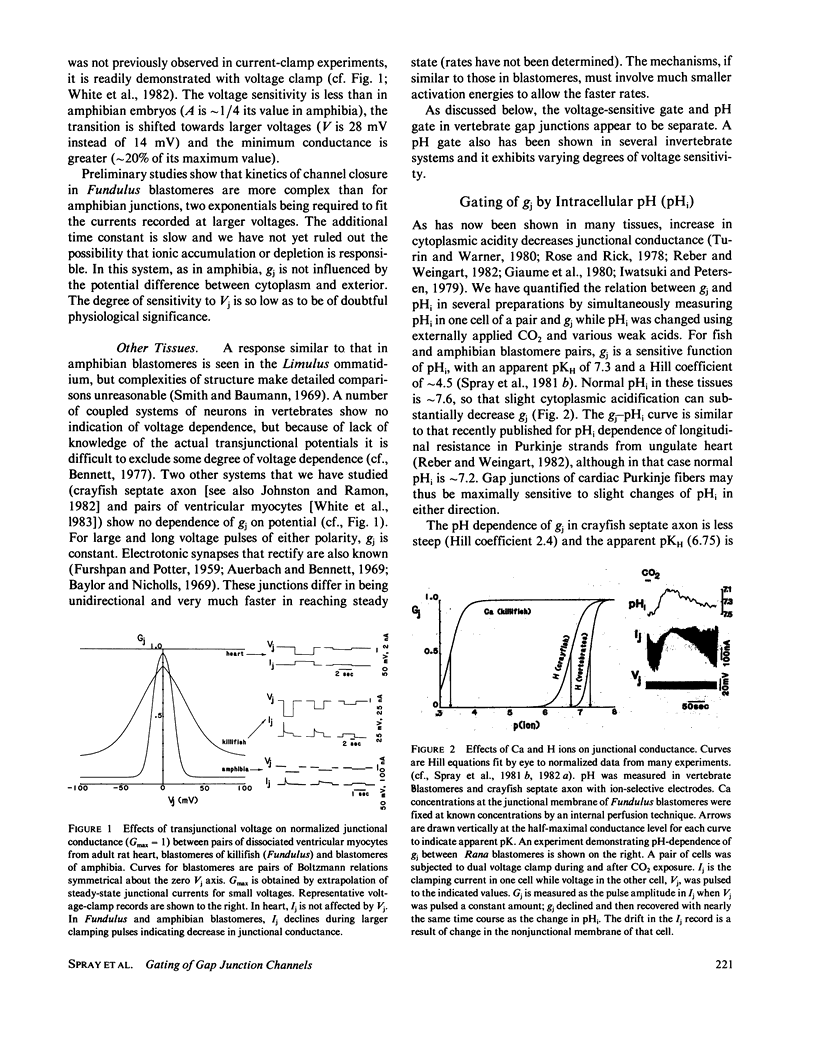
Gap junctional conductance ( gj ) in various species is gated by voltage and intracellular pH (pHi). In amphibian embryos, gj is reduced to half by a 14 mV transjunctional voltage ( Vj ), a change that in fish embryo requires approximately 28 mV. Crayfish septate axon and pairs of dissociated rat myocytes show no voltage dependence of gj over a range of Vj greater than +/- 50 mV. In fish and amphibian blastomeres , gj is steeply decreased by decrease in pHi (n, Hill coefficient: 4.5) and the apparent pKH (7.3) is in the physiological range. In crayfish septate axon the pKH is lower (6.7) and the curve is less steep (n = 2.7). Rises in cytoplasmic Ca can also decrease gj but much higher concentrations are required (greater than 0.1 mM in fish blastomeres). Voltage and pH gates on gap junctions in amphibian embryos appear independent. In squid blastomeres pH gates exhibit some sensitivity to potential, both transjunctional and between inside and outside. A pharmacology of gap junctions is being developed: certain agents block gj directly (aldehydes, alcohols, NEM in crayfish); others block by decreasing pHi (esters that are hydrolyzed by intrinsic esterases, NEM in vertebrates, and, as in the experiments demonstrating the effect of pHi, weak acids). Certain agents block pH sensitivity without affecting voltage dependence (retinoic acid, glutaraldehyde, EEDQ), further indicating separateness of pH and voltage gates. These studies demonstrate a dynamics of gap junctional conductance and variability in gating in a series of possibly homologous membrane channels.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A. A., Bennett M. V. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969 Feb;53(2):211–237. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Simonneau M., Tauc L., Segundo J. P. Uncoupling of electrotonic synapses by calcium. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4577–4581. doi: 10.1073/pnas.75.9.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Chemical and electrical synaptic connexions between cutaneous mechanoreceptor neurones in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):591–609. doi: 10.1113/jphysiol.1969.sp008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Goodenough D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978 Sep;16(3):1–486. [PubMed] [Google Scholar]
- Bennett M. V., Spira M. E., Pappas G. D. Properties of electrotonic junctions between embryonic cells of Fundulus. Dev Biol. 1972 Dec;29(4):419–435. doi: 10.1016/0012-1606(72)90082-6. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Dahl G., Isenberg G. Decoupling of heart muscle cells: correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J Membr Biol. 1980 Mar 31;53(1):63–75. doi: 10.1007/BF01871173. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C., Spira M. E., Korn H. Uncoupling of invertebrate electrotonic synapses by carbon dioxide. Neurosci Lett. 1980 Apr;17(1-2):197–202. doi: 10.1016/0304-3940(80)90084-1. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1104–1122. [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Control of intercellular communication by voltage dependence of gap junctional conductance. J Neurosci. 1983 Jan;3(1):79–100. doi: 10.1523/JNEUROSCI.03-01-00079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the effect of carbon dioxide, ammonium chloride and acetylcholine on intercellular communication. J Physiol. 1979 Jun;291:317–326. doi: 10.1113/jphysiol.1979.sp012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. F., Ramón F. Voltage independence of an electrotonic synapse. Biophys J. 1982 Jul;39(1):115–117. doi: 10.1016/S0006-3495(82)84497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Kanno Y., Socolar S. J. Quantum jumps of conductance during formation of membrane channels at cell-cell junction. Nature. 1978 Jul 13;274(5667):133–136. doi: 10.1038/274133a0. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Takemoto L. J., Hunkapiller M. W., Hood L. E., Revel J. P. Differences between liver gap junction protein and lens MIP 26 from rat: implications for tissue specificity of gap junctions. Cell. 1983 Mar;32(3):967–978. doi: 10.1016/0092-8674(83)90081-8. [DOI] [PubMed] [Google Scholar]
- Nishiye H., Mashima H., Ishida A. Ca binding of isolated cardiac nexus membranes related to intercellular uncoupling. Jpn J Physiol. 1980;30(1):131–136. doi: 10.2170/jjphysiol.30.131. [DOI] [PubMed] [Google Scholar]
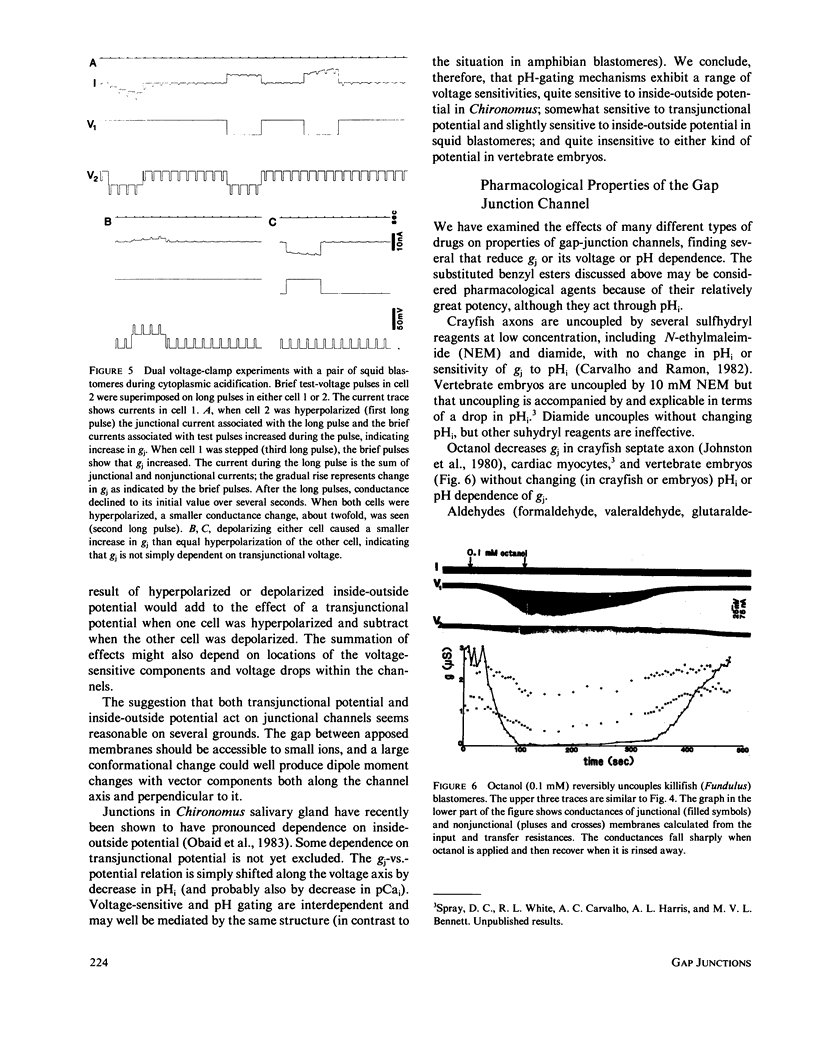
- Obaid A. L., Socolar S. J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J Membr Biol. 1983;73(1):69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Reber W. R., Weingart R. Ungulate cardiac purkinje fibres: the influence of intracellular pH on the electrical cell-to-cell coupling. J Physiol. 1982 Jul;328:87–104. doi: 10.1113/jphysiol.1982.sp014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Warner A. E. Free calcium in Xenopus embryos measured with ion-selective microelectrodes. Nature. 1980 Feb 14;283(5748):658–660. doi: 10.1038/283658a0. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Rose B., Rick R. Intracellular pH, intracellular free Ca, and junctional cell-cell coupling. J Membr Biol. 1978 Dec 29;44(3-4):377–415. doi: 10.1007/BF01944230. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Goodenough D. A. Dye transfer between cells of the embryonic chick lens becomes less sensitive to CO2 treatment with development. J Cell Biol. 1982 Mar;92(3):694–705. doi: 10.1083/jcb.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. G., Baumann F. The functional organization within the ommatidium of the lateral eye of limulus. Prog Brain Res. 1969;31:313–349. doi: 10.1016/S0079-6123(08)63248-3. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Voltage dependence of junctional conductance in early amphibian embryos. Science. 1979 Apr 27;204(4391):432–434. doi: 10.1126/science.312530. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Stern J. H., Harris A. L., Bennett M. V. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci U S A. 1982 Jan;79(2):441–445. doi: 10.1073/pnas.79.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. E. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980 Mar;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]



