Abstract
This study was conducted to test the validity of a measure that has potential to bridge research on the addictive liability of drugs and on individuals' liability to addiction, which to date have evolved in largely parallel arenas. The length of time between onset of abuse and dependence (LOTAD) has evolved from recent findings on transitions through levels of addiction; it was hypothesized that shorter LOTAD is indicative of greater addictive liability. Hypotheses were based on animal studies and human studies. Retrospective data from the DSM-IV Substance Use Disorders Work Group were reanalyzed using configural frequency analysis, survival curves, bivariate Kendall's tau associations, and linear regression. The sample consisted of participants recruited from community and clinical settings. The measure was the Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM). The shortest LOTADs were observed for disorders related to use of cocaine and opiates, followed by cannabis and then alcohol regardless of the subsample that was analyzed. As hypothesized, females and early initiators of drug use had shorter LOTADs compared to men and other initiators of drug use; no consistent differences in LOTAD were observed between African-Americans and Caucasians. None of the LOTAD variance associated with differences between drugs could be accounted for by gender, early use of the drug, or ethnicity. Specific areas of research where LOTAD might be useful as well as how LOTAD might be improved are discussed.
Keywords: Abuse, Dependence, Abuse liability, Etiology
1. Introduction
Estimation of drugs' addictive potential has helped clarify how drug use can lead to drug use-related disorders and in the U.S. has informed drug control procedures as well as Food and Drug Administration (FDA) policy. Impressive strides have been made in the last century toward understanding differences in the addictive potential between substances. However, important gaps in the literature remain (Balster and Bigelow, 2003). For example, addictive liability research is mostly based on studies conducted in laboratory settings. Substances that are not developed by pharmaceutical manufacturers, substances that are not regulated by the FDA(e.g., inhalants), “street” uses of prescription medications, settings where drug abuse takes place, and dosages of medications often do not match laboratory settings. In a conceptually related literature, individuals' liabilities to addiction have been researched in terms of the factors that contribute to persons' risk for addiction (Tarter et al., 2002) as well as factors that contribute to transitions through levels of addiction (Hasin and Grant, 2004; Ridenour et al., 2003; Schuckit et al., 2001). However, literatures focusing on the addictive liability of drugs and on persons' liability to addiction have evolved largely in parallel arenas. A measure of substances' addictive liabilities that could be used in research regarding individuals' liability for addiction might provide a more widely used bridge between the two literatures.
This study was an initial validity test of a measure that might be useful for post-marketing surveillance of pharmaceutical substances as well as addictive potential research of non-FDA regulated substances, using large samples, or with etiological or clinical foci. The term “abuse potential” is used to describe the concept of a drug's potential to generate addiction in users of the drug. Because the term “abuse” refers to a specific psychiatric diag osis and the abuse diagnosis is included in the analyses of this paper, the term “addictive potential” is used henceforth. Moreover, the term “abuse potential” refers to the potential for experiencing any of a number of problems due to drug use; the measure examined in the present study is theoretically different from “abuse potential” because it is composed of levels of addiction.
Balster and Bigelow, (2003) summarize recent calls for clinically relevant evaluations of addictive liability by arguing that “epidemiological experience—the extent of actual abuse—is the ultimate gold-standard criterion index that other approaches are trying to predict” (p. S26) (Ator and Griffiths, 2003; Fischman and Foltin, 1991; Griffiths et al.,2003). Abuse, defined as DSM-IV substance-related abuse diagnoses, appears to not provide an adequate gauge of addictive potential. Arfken and Cicero (2003) reported that of the 932 Medwatch surveillance reports that were suggestive of tramadol abuse, only 30% could be definitely diagnosed as at least meeting DSM-IV abuse criteria (some users met criteria for dependence). Follow-up data indicated that abuse diagnoses turned out to be “mostly due to transient experimentation” rather than clinically significant abuse or dependence. Ridenour et al. (2003) argued that the environmental element of each abuse criterion makes the abuse criteria impure measures of an individual's misuse of the drug. To illustrate, the abuse criterion of substance use leading to social problems might be avoided by socializing only with persons who misuse substances or do not view drug use as problematic. Using the prevalence of substance use-related dependence diagnoses as an alternative metric for the drug's addictive liability also has limitations. Users of different drugs differ for reasons that do not reflect drug characteristics such as availability, legality, or social acceptability. Hence, alcohol users differ considerably from opiate users and prevalence estimates of dependence for different drugs also are likely to be influenced by factors other than characteristics of the drug. A third alternative for estimating addictive potential of different substances in people is the length of time from first use to the onset of a disorder (e.g., Ridenour et al., 2003; Wagner and Anthony, 2002); however, time from first use to onset of a disorder has similar shortcomings as using prevalence to estimate addictive potential.
Numerous studies have examined sequences between levels of addiction such as (a) initiating use, (b) onset of criteria and diagnoses, and (c) length of time between levels of drug involvement such as abuse or dependence (Hasin and Grant, 2003; Langenbucher et al., 2004; Mackesy-Amiti et al., 1997; Ridenour et al., 2003; Wagner and Anthony, 2002). These studies largely have been limited to addressing nosological issues; investigation of characteristics that might be associated with the rate of transition through levels of addiction has been neglected. However, results from these studies suggest that a fourth alternative to estimating the addictive liability of substances in people may be the length of time between onset of abuse and dependence (LOTAD). Using abuse rather than use as the threshold to select a baseline sample identifies individuals who have already endorsed certain problems due to their use of substances. LOTAD measures the pace that a drug generates dependence, potentially a reflection of drugs' addictive liability. It is assumed that a greater addictive liability of a drug is indicated by a smaller time lapse between experiencing abuse and experiencing dependence. Hence, very high addictive liability would be indicated if, on average, people experience dependence at about the same time as experiencing abuse (LOTAD = 0), which might be interpreted as: a person experiences dependence on a drug at about the same time his or her functioning within the environment is impeded by use of the drug.
Results from a recent study of the order of onset of abuse and dependence criteria suggested that LOTAD rank orders substances consistently with the addictive potential of different drugs based on animal studies (Ridenour et al.,2003). The general impression of the addictive potential of different drugs from animal studies is that cocaine and opiates have very high addictive liability, followed by alcohol, and cannabis, respectively (Erdtmann-Vourliotis et al., 1999; Gardner, 1997; Stafford et al., 1998; Winger et al.,1983). Table 1 presents the rank orders of drugs based on the four options for estimating addictive liability of substances in people national estimates of prevalences of abuse and dependence, length of time from initiation to abuse and dependence, and LOTAD using survival curves and configural frequency analysis based on data from Ridenour et al. (2003) findings. The first column of Table 1 indicates the rank order of addictive liability of substances based on impressions from animal studies. The median number of years from use to abuse and the survival curve LOTAD most closely match the rank order of animal studies. Results for alcohol were only partly consistent with hypotheses of addictive liability of drugs for people based on animal studies; alcohol LOTAD was longer than the cannabis LOTAD (the opposite order is expected). Alcohol patterns might deviate somewhat from the hypothesized patterns because of its legality, availability, controlled and known dosage (i.e., proof), or social acceptability. For example, persons who do not even sample other drugs might use or abuse alcohol.
Table 1.
Rank orders of possible addictive liability indicators for different drugs compared to animal studies results
| Animal studies | Prevalence of abuse | Prevalence of dependence | Years from initiation to abuse | Years from initiation to dependence | Survival curve LOTAD | Configural frequency analysis | |
|---|---|---|---|---|---|---|---|
| Alcohol | 3 | 2 | 2 | 4 | 4 | 4 | 4 |
| Cannabis | 4 | 4 | 4 | 3 | 3 | 3 | 3 |
| Opiates | 1 | 3 | 3 | 1 | 2 | 1 | 2 |
| Cocaine | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Notes: Rank orders indicate the addictive liability that would be assigned to the substances in rows, based on the metric of the column. Rank orders for “prevalence of abuse or dependence” indicate the greatest to least prevalence of abuse among users of the substance. Rank orders of “years from initiation to abuse or dependence” indicate the median number of years from initial use to onset of the disorder, from shortest to longest length of time. Rank order of “survival curve LOTAD” indicates the size of the gap between the abuse survival curve and the dependence survival curve from smallest to largest. Rank order of “configural frequency analysis” indicates the proportion of persons with an abuse or dependence diagnosis who experienced abuse before dependence, from least to greatest.
There are a number of advantages to using LOTAD, if it proves to be a valid measure of addictive potential in people. Standard criteria are used for comparisons between drugs, LOTAD is clinical in nature because it is based on DSM-IV criteria, large sample studies and clinical studies of addictive potential could be conducted, LOTAD studies could be conducted with numerous existing datasets, and psychiatric interviews on which LOTAD is based have been translated into numerous languages and psychometric estimates of the translations are available (Cottler et al., 1997; Hasin et al., 1997; Pull et al., 1997; Ustun et al., 1997), and LOTAD may prove useful in etiological research on addictive liability or as a clinical trials outcome measure including research regarding interactions between characteristics of drugs, individuals, and drug ingesting environments (as demonstrated in the present study).
However, there are potential shortcomings to using LOTAD to measure addictive liability. Results from Ridenour et al. (2003) study suggest that DSM-IV substance use-related diagnoses could be improved by developing criteria specifically for each substance rather than basing all substance diagnoses on the Alcohol Dependence Syndrome (Edwards and Gross, 1976). Their argument was partly based the very short time lapses between the abuse and dependence diagnoses for cocaine use and opiate use (which is inconsistent with the DSM-IV intent that abuse diagnosis reflects an earlier stage of addiction than dependence). They also argued that abuse criteria inadequately assess problems related to substance use because they dually require environmental conditions. Sizable proportions of persons with DSM-IV dependence on different substances either do not meet criteria for DSM-IV abuse or experience abuse after dependence (Hasin and Grant, 2004; Ridenour et al., 2003). Moreover, longitudinal studies suggest that many persons who meet alcohol abuse criteria do not later qualify for alcohol dependence (Hasin et al., 1997; Schuckit et al., 2001). For these reasons, some of the analyses used by Ridenour et al. (2003) and the present analyses were designed to include all persons with diagnoses, including those with only abuse or only dependence.
Although the DSM-IV criteria appear less than optimal for diagnoses, using the same criteria across different substances may be useful for comparing substances with regard to their relative addictive liability. For example, the rate of transition from onset of use to abuse and to dependence varies greatly between different drugs (Ridenour et al., 2003; Wagner and Anthony, 2002), and therefore, may provide a technique to measure drugs' relative addictive liability in people. Further evidence that LOTAD might provide a measure of addictive liability of substances in people was Ridenour et al. (2003) finding that LOTAD was not associated with experiencing a different substance use disorder earlier in life, which suggests that individuals' vulnerability to addiction has relatively little impact on LOTAD.
In the present study, the validity of LOTAD was tested using hypotheses based on animal and human studies. One hypothesis was that LOTAD will be shorter for persons who initiate use of a drug at a young age than other users (Brook et al., 2002; DeWitt et al., 2000). A second hypothesis was based on the finding that, among animals primed for selfadministration of an addictive substance, females consume greater volumes of the substance than males (Cicero et al., 2000; Grathwohl et al., 2001). Hence, it was hypothesized that LOTAD will be shorter in women than men. If LOTAD is shorter in women than men, it would demonstrate that LOTAD might improve measurement of addictive liability above substance because greater prevalences of the disorders occur in men than women (Anthony et al., 1994).
Additional analyses were designed to answer the following research questions. Are LOTAD scores for different substances correlated (based on the hypothesis that LOTAD also measures individuals' vulnerabilities to addiction across many drugs) or uncorrelated (based on the alternative hypothesis that LOTADs generally measure characteristics specific to each drug)? Are there LOTAD differences between races/ethnicities (no differences were expected)? How much do the characteristics that are associated with LOTAD (e.g., gender, early use of a drug) account for differences in LOTAD between different drugs?
2. Methods
Data collection, reduction, and storage are detailed elsewhere (Cottler et al., 1995). Hence, only a brief description of the sample and methodology is presented below.
2.1. Sample
Data from 1226 participants were taken from the DSM-IV field trials, which were conducted in five U.S. sites (Denver, Philadelphia, St. Louis, San Diego, and Vermont) by the American Psychiatric Association Substance Use Disorders Work Group between 1990 and 1994 (Cottler et al., 1995). Participants were recruited from clinical settings (63.4%) and the community (33.6%, from the entire Vermont sample or part of the St. Louis sample) and sample characteristics varied by location of data collection. Because of differences in samples between sites, the results could not be tested for differences between geographical locations. However, the sizable number of African-Americans (36% of total sample) and women (43% of total sample) facilitated tests for differences between races/ethnicities and genders. All data collection, editing, and entry efforts underwent extensive quality control procedures. For sites where clinical participants were recruited, participants were approached and interviewed within two weeks after admission to the program to allow stabilization to begin prior to interviews.
The number of participants who had used alcohol regularly in their lifetime was 1200 and the numbers of users of illicit drugs were 823 for cannabis, 329 for opiates, and 613 for cocaine. The proportion of participants with abuse and dependence diagnoses, respectively, were 65% and 46% for alcohol, 47% and 37% for cannabis, 60% and 54% for opiates, and 81% and 79% for cocaine. Because of missing age of onset data, slightly fewer participants were eligible for LOTAD analyses (n = 1193 for alcohol, 806 for cannabis, 328 for opiates, and 611 for cocaine). The number of African-Americans eligible for analyses was 270 for alcohol, 232 for cannabis, 112 for opiates, and 225 for cocaine. The number of women eligible for analyses was 509 for alcohol, 313 for cannabis, 97 for opiates, and 227 for cocaine.
2.2. Assessment
Interviews were conducted using the paper and pen version of the Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM) (Robins et al., 1990). The CIDI-SAM is a structured, non-clinician administered interview of substance use characteristics including DSM and ICD symptomotology for many classes of substances. Good reliability and validity has been reported for CIDI-SAM diagnoses, specific criteria, and retrospective recall of age of onsets (Cottler et al., 1989; Cottler and Compton, 1993; Horton et al., 2000), even for test-retest periods of six months (Langenbucher et al., 1994). Age of onset for criteria of substance use-related diagnoses was assessed for year of onset. The CIDI-SAM requires a threshold of six or more lifetime uses before illicit substance abuse and dependence are queried. A threshold of regular alcohol use (using alcohol at least once a month for six or more consecutive months) was required before alcohol diagnosis questions were asked. Diagnoses were determined using computer algorithms.
DSM-IV abuse disorders are not diagnosed if an individual meets criteria for dependence on the same substance (i.e., the “exclusion criterion”). The exclusion criterion was omitted from algorithms for abuse diagnoses because it would defeat the purpose of these analyses. The “clustering” criterion, which requires that at least three dependence criteria occur in a 12-month period was assessed only in a small proportion of participants from St. Louis. Hence, onset of the dependence diagnoses was set to equal the onset of participants' third dependence criterion.
Early use was operationalized as the age at which the earliest quartile of use (or regular use for alcohol) occurred because there is no widely agreed upon age threshold to identify early users. Hence, slightly more than 25% of users of each substance were designated as early users because a greater number of users of each substance used at the same age or earlier than the age at which the 25th percentile occurred. The proportion of users of each substance that was categorized as an early user was 27.8% for regular use of alcohol, 35.5% for use of cannabis, 28.9% for use of opiates, and 25.4% for use of cocaine.
2.3. Analyses
Data analyses were conducted to test for differences between subgroups and to attempt to account for differences in LOTAD between drugs. Because of the potential for discrepant results using different analytical approaches, two techniques were used for each of the data analyses.
2.3.1. Subgroup LOTAD differences
In the first data analytic approach, configural frequency analyses and survival curve analyses were used to test hypotheses regarding hypothesized subgroup differences. Each of these techniques permits the inclusion of persons meeting criteria for only abuse or dependence. Configural frequency analyses were used to test differences between subgroups regarding the relative probability of occurrence of each sequence of diagnoses (abuse before dependence, both onset at about the same time, or dependence before abuse; Ridenour et al., 2003; von Eye, 1990). Omnibus χ2s were used to test whether the proportion of sequences of abuse and dependence deviated significantly from random order within a subgroup. Z-tests based on the standard normal approximation of the binomial test (von Eye, 1990, pp. 16-23) were conducted to test the relative commonality of each sequence. χ2 tests were conducted to test for differences between subgroups in the proportions of sequences of abuse and dependence.
Ridenour et al. (2003) reported that the abuse-beforedependence sequence was most common for alcohol and cannabis use-related disorders but that the most common sequence for cocaine and opiates was abuse and dependence onsets during the same 12-month period. For the configural frequency analyses conducted in the present study, it was hypothesized that these patterns would be replicated in each of the subgroups studied, with two additional expectations. A smaller proportion of women (compared to men) and of early users (compared to other users) were hypothesized to be in the abuse-before-dependence onset sequence for each drug.
Survival curve analysis was the second type of analysis used to test differences between genders, early users versus other users, and ethnic groups. Survival curve analyses provided a visual summary of the prevalence of abuse and dependence disorders as well as the change in this prevalence over time following five uses of the illicit drugs or regular use of alcohol. Longer LOTADs were indicated by dependence curves that were higher above abuse curves. Women and early substance users were expected to have shorter LOTADs (smaller gaps between the abuse and dependence survival curves) than men and other users, respectively.
The same individuals contributed to generating the survival curves for abuse and dependence. Traditionally, interpretation of survival curve results would be limited to the method of inspection because statistical tests assume curves are computed from independent samples. Moreover, population distributions of LOTADs are unknown. A bootstrap technique was used to approximate statistical tests of differences in LOTAD between groups. Because the size of LOTAD appears to change over time (i.e., the difference between survival probabilities of abuse and dependence decrease or increase over time), differences between survival curves and subgroup LOTADs were tested at five-year intervals (5, 10, 15, 20, and 25 years after baseline). Details and an example of this procedure are presented in the Appendix A. Detailed results of the additional bootstrap analyses are available from the first author upon request.
2.3.2. Between-drug LOTAD differences associated with personal characteristics
Two analytical techniques were used to investigate how between-drug differences in LOTAD might be accounted for by personal characteristics. LOTAD for an individual was calculated by subtracting the age of onset of abuse from the age of onset of dependence for the same substance. First, bivariate associations between LOTAD for different drugs were hypothesized to be either small or nonsignificant because LOTAD was hypothesized to measure addictive potential associated with drug characteristics and not individuals' liability to addiction. The distribution of LOTAD is unknown and may not be normal; hence, bivariate associations were estimated using Kendall's tau with pairwise deletion of missing cases. The sample sizes for Kendall's tau estimates were smaller than other analyses in this study because fewer participants had two or more LOTADs. Second, we attempted account for the between-drug LOTAD differences using multiple regression analyses with the predictors consisting of drug type, gender, early onset, and ethnicity and LOTAD as the criterion. The drug type predictors consisted of dummy coded variables using alcohol as the referent drug type. Data from persons with more than one LOTAD were in separate rows of the dataset, a separate row for each drug that (s)he had a LOTAD. The “cluster” option in STATA (Huber/White sandwich estimator) was used to account for repeated measures (two or more LOTADs from the same individuals).
3. Results
3.1. Is LOTAD shorter in women than men?
Table 2 presents the configural frequency analyses results regarding gender differences. Smaller proportions of women (compared to men) experienced abuse before dependence for alcohol, cannabis, and opiates. Moreover, a greater proportion of women (than men) experienced dependence before abuse. Nearly two-fold the proportion of women (compared to men) experienced dependence before abuse related to alcohol and cannabis use.
Table 2.
Configural frequency analysis of gender differences in the sequence of onset of abuse and dependence diagnoses
| Substance | Abuse onset |
Total N | Omnibus row χ2 | χ2 Difference in sequences | ||
|---|---|---|---|---|---|---|
| Before dependence | Same year as dependence | After dependence | ||||
| Gender differences | ||||||
| Alcohol | ||||||
| Malesa | 410(151),78%b | 72,14%c | 43(10),8%c | 525 | 475.76d | 7.65d |
| Femalesa | 168(93),76%b | 21,9%c | 31(7),14%c | 220 | 183.99d | |
| Cannabis | ||||||
| Malesa | 198(119),61%b | 72,22%c | 52(20),16%c | 322 | 116.75d | 10.85d |
| Femalesa | 89(55),59%b | 21,14%c | 42(22),28% | 152 | 47.86d | |
| Cocaine | ||||||
| Malesa | 75(24),24%c | 186,60%b | 50(17),16%c | 311 | 101.10d | 2.29 |
| Femalesa | 53(16),28% | 100,53%b | 36(8),19%c | 189 | 34.89d | |
| Opiates | ||||||
| Malesa | 53(26),35% | 71,46%b | 29(9),20%c | 153 | 17.41d | 1.61 |
| Females | 15(6),29% | 23,44% | 14(5),27% | 52 | 2.81 | |
Notes: Separate configural frequency analyses were conducted for each substance among persons with either or both abuse and dependence. Percentiles indicate the proportion of the total number of participants that experienced a disorder associated with the row substance in the column sequence. Some row percentiles may not sum to 100% because of rounding error. Parenthetical values indicate the ‘n’ experiencing either abuse only (in the “before dependence” column) or dependence only (in the “after dependence” column). “Total N” is slightly lower than number of persons meeting lifetime criteria for both abuse and dependence in Table 1 because of missing age of onset data.
Frequencies of the sequences differed significantly from random order.
Sequence was common, compared to chance (Type).
Sequence was rare, compared to chance (Antitype).
Difference in sequences of onset of disorders was significant(p< .025).
Fig. 1 presents the abuse and dependence survival curves for women and men. The cannabis LOTAD appears shorter for women than men; however, bootstrap tests were nonsignificant. Regarding alcohol, the survival curve LOTAD appears longer for men. The bootstrap test at five years after regular use approached statistical significance (p = .075), but tests were non-significant thereafter. The opiates and cocaine survival curve LOTADs appeared to not differ between men and women; abuse and dependence curves were indistinguishable for opiate and cocaine diagnoses in both genders. Bootstrap tests suggested that opiate LOTADs were shorter for women than for men at 15 years (p < .05) and 25 years (p < .005) after initiating opiate use. Bootstrap tests suggested that cocaine LOTADs were shorter for women than for men at five years (p < .05) and 25 years (p < .005) after initiating cocaine use.
Fig. 1.
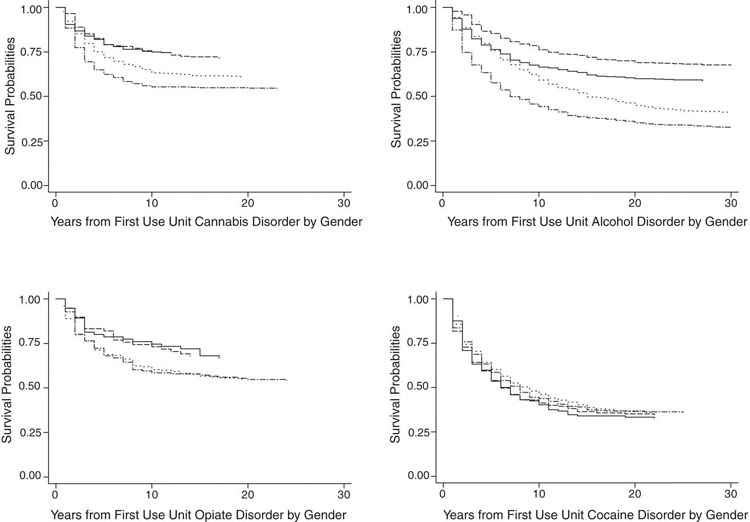
Gender differences in length of time between abuse and dependence (LOTAD) for DSM-IV disorders related to use of cannabis, alcohol, opiates, and cocaine. Notes: In each of the four subfigures are presented two pairs of survival curves, one pair for women (abuse and dependence) and one pair for men (abuse and dependence). The size of the gap between the abuse and dependence curve for each gender is the LOTAD for that gender related to the substance depicted in the subfigure. Legend: (—) women abuse; (- - -) women dependence; (-·-·-) men abuse; (···) men dependence.
In sum, results of configural frequency analyses and survival curve LOTADs generally were consistent with the hypothesis that women's addictive liability is greater than men's. However, the difference in addictive liability associated with drug characteristics appears much greater than the gender difference. These results are consistent with our hypothesis that LOTAD might provide a measure of the addictive liability of substances.
3.2. Is LOTAD shorter for early users of a drug?
Table 3 presents configural frequency analyses results comparing early users to other users. Statistically significant differences were found related to use of alcohol, cannabis, and cocaine. Results regarding alcohol were small and not consistently in the hypothesized direction. Results for cannabis users and cocaine users were consistent with the hypothesis that fewer early users would experience abuse before dependence compared to other users.
Table 3.
Configural frequency analysis of differences between early users vs. other users in the sequence of onset of abuse and dependence diagnoses
| Substance | Abuse onset |
Total N | Omnibus row χ2 | χ2 Difference in sequences | ||
|---|---|---|---|---|---|---|
| Before dependence | Same year as dependence | After dependence | ||||
| Onset differences | ||||||
| Alcohol | ||||||
| Early a | 191(43),76%b | 44,18% | 15(1),6%c | 250 | 213.70d | 6.37d |
| Othersa | 405(201),75%b | 73,14%c | 59(16),11%c | 537 | 428.56d | |
| Cannabis | ||||||
| Earlya | 123(62),55% | 57,26% | 43(13),19% | 223 | 49.11d | 9.66d |
| Othersa | 164(112),65%b | 36,14% | 51(29),20% | 251 | 83.67d | |
| Cocaine | ||||||
| Earlya | 27(9),11% | 193,80%b | 21(8),9%c | 241 | 237.24d | 38.61d |
| Others a | 101(31),27% | 209,56%b | 65(17),17% | 375 | 89.86d | |
| Opiates | ||||||
| Earlya | 21(9),38% | 24,43% | 11(5),20% | 56 | 4.96d | 0.65 |
| Others | 47(23),32% | 70,47% | 32(9),21% | 149 | 14.75d | |
Notes: Separate configural frequency analyses were conducted for each substance among persons with either or both abuse and dependence. Percentiles indicate the proportion of the total number of participants that experienced a disorder associated with the row substance in the column sequence. Some row percentiles may not sum to 100% because of rounding error. Parenthetical values indicate the ‘n’ experiencing either abuse only (in the “before dependence” column) or dependence only (in the “after dependence” column). “Total N” is slightly lower than number of persons meeting lifetime criteria for both abuse and dependence in Table 1 because of missing age of onset data.
Frequencies of the sequences differed significantly from random order.
Sequence was common, compared to chance (Type).
Sequence was rare, compared to chance (Antitype).
Difference in sequences of onset of disorders was significant (p< .025).
Fig. 2 presents LOTAD survival curves for early initiators versus other initiators. Regarding cannabis, LOTAD appeared longer for early users than other users; however, none of the bootstrap tests were statistically significant. Regarding alcohol, LOTAD appeared slightly longer in early users than other users, but again, bootstrap tests were all non-significant. For users of opiates and cocaine, no visual differences were found between early users versus other users. Bootstrap tests suggested a shorter LOTAD for early cocaine initiators only at five years following cocaine initiation. Bootstrap tests for opiates suggested that early initiators had shorter LOTAD than other users from 10 years through 25 years following initiation of opiate use.
Fig. 2.
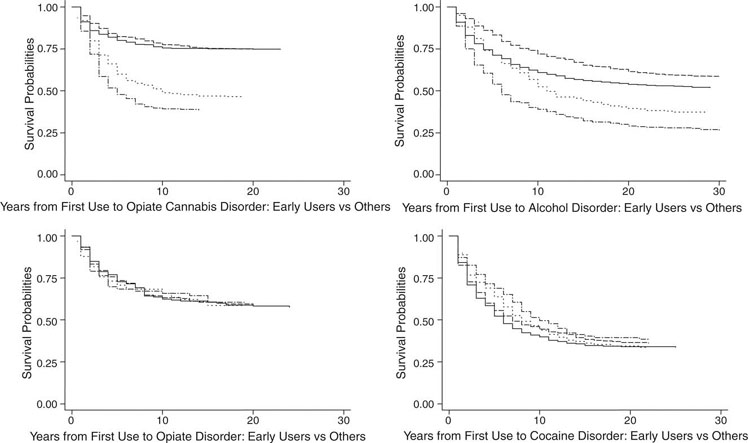
Length of time between abuse and dependence (LOTAD) differences between early initiators and other initiators for DSM-IV disorders related to use of cannabis, alcohol, opiates, and cocaine. Notes: In each of the four subfigures are presented two pairs of survival curves, one pair for early initiators (abuse and dependence) and one pair for other initiators (abuse and dependence). The size of the gap between the abuse and dependence curve for each type of initiator is the LOTAD related to the substance depicted in the subfigure. Legend: (—) other initiators abuse; (- - -) other initiators dependence; (-·-·-) early initiators abuse; (···) early initiators dependence.
These results generally were consistent with the hypothesis that early initiators have greater liability to addiction than other users. Results of configural frequency and survival curve analyses were consistent with the notion that LOTAD could provide a measure of addictive liability; differences between drugs were greater than differences between early and other users.
3.3. Are there LOTAD differences between ethnicities?
Configural frequency analyses results for comparisons between Caucasians and African-Americans are presented in Table 4. The only statistically significant difference between the ethnic groups in the configural frequency analyses was regarding cannabis. About two-fold the proportion of Caucasians (compared to African-Americans) experienced abuse onset and dependence onset during the same year. Half of the proportion of Caucasians (compared to African-Americans) experienced abuse onset after dependence onset.
Table 4.
Configural frequency analysis of ethnic differences in the sequence of onset of abuse and dependence diagnoses
| Substance | Abuse onset |
Total N | Omnibus row χ2 | χ2 Difference in sequences | ||
|---|---|---|---|---|---|---|
| Before dependence | Same year as dependence | After dependence | ||||
| Ethnic differences | ||||||
| Alcohol | ||||||
| Afr.—Am.a | 133(58),79%b | 20,12% | 16(4),9%c | 169 | 156.65d | 0.53 |
| Cauc.a | 312 (125),77%b | 57,14% | 36(9),9%c | 405 | 349.73 | |
| Cannabis | ||||||
| Afr.—Am.a | 76(43),61%b | 15,12% | 33(15),27% | 124 | 47.53d | 14.64d |
| Cauc. a | 105(63),58%b | 51,28% | 26(9),14% | 182 | 53.75d | |
| Cocaine | ||||||
| Afr.—Am.a | 56(11),28%c | 107,54%b | 36(6),18%c | 199 | 40.41d | 2.79 |
| Cauc.a | 37(17),26% | 89,62%b | 18(9),13% | 144 | 56.29d | |
| Opiates | ||||||
| Afr.—Am.a | 25(8),32% | 36,46% | 18(6),23% | 79 | 6.25d | 0.66 |
| Cauc. | 28(18),36% | 35,45% | 14(4),18% | 77 | 8.91d | |
Notes: Separate configural frequency analyses were conducted for each substance among persons with either or both abuse and dependence. Percentiles indicate the proportion of the total number of participants that experienced a disorder associated with the row substance in the column sequence. Some row percentiles may not sum to 100% because of rounding error. Parenthetical values indicate the ‘n’ experiencing either abuse only (in the “before dependence” column) or dependence only (in the “after dependence” column). “Total N” is slightly lower than number of persons meeting lifetime criteria for both abuse and dependence in Table 1 because of missing age of onset data.
Frequencies of the sequences differed significantly from random order.
Sequence was common, compared to chance (Type).
Sequence was rare, compared to chance (Antitype).
Difference in sequences of onset of disorders was significant (p< .025).
Fig. 3 presents survival curve LOTADs for African—Americans and Caucasians. The cannabis LOTAD appeared shorter for African—Americans than for Caucasians in the survival curve analysis; however, bootstrap estimates suggested no statistically significant LOTAD differences occurred between African-American and Caucasian cannabis users. The alcohol survival curve LOTADs were overlapping for African-Americans and Caucasians and none of the bootstrap tests were statistically significant. The opiates survival curve LOTAD appears longer for Caucasians than for African-Americans, however, no statistically significant differences were found. Regarding cocaine users, the survival curve LOTAD appears to be greater for African-Americans than Caucasians; the only significant difference in cocaine LOTAD occurred at five years after cocaine initiation (p < .005), when Caucasians' cocaine LOTAD was shorter than African-Americans' cocaine LOTAD.
Fig. 3.
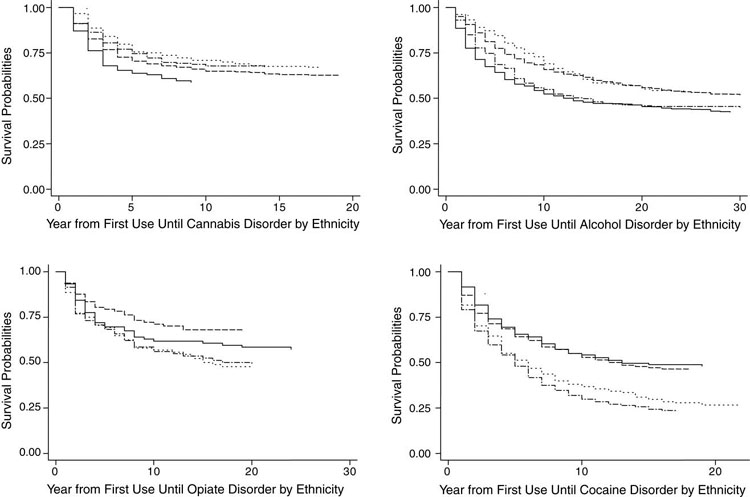
Race/ethnicity differences in length of time between abuse and dependence (LOTAD) for DSM-IV disorders related to use of cannabis, alcohol, opiates, and cocaine. Notes: In each of the four subfigures are presented two pairs of survival curves, one pair for African-Americans (abuse and dependence) and one pair for Caucasians (abuse and dependence). The size of the gap between the abuse and dependence curve for each ethnic group is the LOTAD for that ethnicity related to the substance depicted in the subfigure. Legend: (—) Caucasians abuse; (- - -) Caucasians dependence; (-·-·-) African-Americans abuse; (···) African-Americans dependence.
In sum, a few inconsistent differences between races/ethnicities were found suggesting that addictive liability is not different between African-Americans and Caucasians. These results are consistent with the notion that LOTAD might measure the addictive liability of substances.
3.4. Are LOTAD scores for different substances correlated?
Table 5 presents the results of Kendall's tau correlations between LOTADs for different drugs. None of the betweendrug LOTAD correlations were significant, suggesting that LOTAD associated with a particular drug is independent of LOTADs associated with other drugs.
Table 5.
Kendall’s tau estimates of between-drug LOTAD associations
| Cannabis | Alcohol | Opiates | |
|---|---|---|---|
| Alcohol | .095 (114) | ||
| Opiates | -.072 (55) | -.137 (66) | |
| Cocaine | .065 (126) | .048 (213) | -.022 (113) |
Notes: Parenthetical values contain the number of participants available for reanalysis. Sample sizes were considerably smaller than for other analyses because participants had to have met criteria for both abuse and dependence pertaining to the use of two or more drugs. None of the Kendall's taus were significant (p> .05).
3.5. Can LOTAD differences between drugs be accounted for by other characteristics?
Table 6 presents the concluding results of a series of regression equations predicting LOTAD. The “drug type and covariates” regression (R = .311) was only slightly more accurate than the “drug type” regression (R = .286) in terms of accounting for variance in LOTAD. Beta weights of drug types did not change from the “drug type” regression to the “drug type and covariates” regression. These results suggest that although the personal characteristics that were studied accounted for variance in LOTAD, they did not account for variance in LOTAD that was associated with differences between drug types. In summary, the results of all analyses were consistent with LOTAD providing a measure of the addictive liability of drugs in people.
Table 6.
Prediction of LOTAD by drug type is not accounted for by gender, ethnicity, and early use
| Regression | Predictor | Beta wt. | ‘p’ for unique var. |
|---|---|---|---|
| Cannabis diagnoses | -.091 | .010 | |
| Drug type (R2=0.0817, R=0.29) | Cocaine diagnoses | -.178 | .001 |
| Opiate diagnoses | -.254 | .000 | |
| Male | .122 | .001 | |
| Female × African—American | .072 | .035 | |
| Early use of predicted drug | -.113 | .003 | |
| Drug type and covariates(R2=0.097, R= 0.31) | Cannabis diagnoses | -.091 | .010 |
| Cocaine diagnoses | -.178 | .001 | |
| Opiate diagnoses | -.254 | .000 | |
| Female × cannabis diagnoses | .084 | .022 | |
| Early Use × cocaine diagnoses | .106 | .062 |
Notes: Conducted a series of linear regressions to predict LOTAD. Alcohol diagnosis was the referent group for dummy coded drug factors. All predictors were dummy coded. The ‘cluster’ option in STATA (Huber/White/sandwich estimator) was used to account for repeated measures from the same individuals (those with LOTADs for more than one drug). Negative beta weights = shorter LOTAD.
4. Discussion
This is the first study to our knowledge to investigate characteristics associated with rates of progression through levels of addiction. The results reported for drugs in general (Ridenour et al., 2003) were replicated for men and women, early users and other users of the drugs, and African-Americans and Caucasians. Consistent with recent animal studies, being female was associated with shorter LOTAD. The finding that early drug use initiation is associated with shorter LOTAD was expected based on previous studies of people. Although some LOTAD differences were found between African-Americans and Caucasians, they were inconsistent between drugs and were not replicated in the linear regression analysis. Each of these findings suggest that LOTAD might measure the addictive liability of substances in people.
Clearly, the proposal that LOTAD be considered a measure of addictive liability is preliminary. Other factors that could impact LOTAD that deserve further consideration include variance in level of psychoactive drug contained in street purchased substances, environmental reinforcers and triggers, contaminants, and other individual risk factors. Other factors that might impact LOTAD also might clarify individuals' vulnerability to addiction. Results from behavioral genetic studies generally suggest large overlap in familial risk that contributes to experiencing different substance use disorders, including overlap in the environmental and genetic factors(Bierut et al.,1998; Handelsman et al.,1993; Merikangas et al., 1998; Tsuang et al., 1998). Whether these factors also contribute to LOTAD is a research question that has not been addressed. Clarification of the factors that contribute to individuals' addictive liability might clarify epigenetic risks that contribute to addictive liability, and eventually, learning how to reduce individuals' vulnerability to addiction.
4.1. Limitations
Discussion of the results of this study ought to be considered in the context of the study limitations. One limitation was that data were based on retrospective reports of symptoms and their ages at onset. In the context of this study, it was fortunate that the CIDI-SAM was used to collect data. Good reliability and validity has been reported for CIDI-SAM diagnoses, specific criteria, and age of onsets (Cottler et al., 1989; Cottler and Compton, 1993; Horton et al., 2000). Langenbucher et al.(1994) reported good to excellent reliabilities of CIDI-SAM dependence criteria and diagnoses for a test-retest period of six months. Participants in Langenbucher et al. (1994) study dated each diagnosis and nearly all of the criteria at an earlier age at the second interview of the study, suggesting that errors in recall of age of onset are made consistently across substance use criteria. Hopefully, this consistent bias in recall of age of onset minimizes the potential errors in LOTAD estimates that might occur due to retrospective recall because LOTAD is a measure of the differences in onset rather than the age of onset, per se. Nevertheless, the purpose for collecting the DSM-IV Work Group data was not to study the age of onset of disorders. Techniques designed to more accurately ascertain recall of age of onset were not used. A prospective study would generate the most accurate estimates of LOTAD and analyses that were conducted in the present study ought to be replicated when such data become available.
Further limitations occurred as a result of the data not being collected for the purpose of studying LOTAD. One limitation was that onset data were only specified to the year of onset (rather than using finer decrements of age of onset). Although it is doubtful that retrospective data such as those collected for the DSM-IV Task Force could be made more accurate than the year of onset by asking for specific months or dates of onset, certain results of the present study might have been impacted by only specifying year of onset. To illustrate, it is possible that the LOTADs for cocaine and opiates might be differentiated by having a finer metric of time between onset of abuse and dependence in a prospective study. A study designed specifically to test LOTAD might include a broader range of measures of individual characteristics, estimates of supply of drug during the time of the study, or alternative measures of LOTAD. An alternative study design also might provide comparisons of LOTAD to traditional measures of addictive liability such as the ARCI. The sample was largely limited to clinical populations; data collected from general population samples might generate different results particularly for drugs that are relatively widely used such as cannabis and alcohol. To illustrate, perhaps a greater proportion of general population cannabis abusers do not experience dependence or receive treatment than is estimated in these results. Moreover, many individuals from the general population who experience dependence do not meet criteria for abuse (Hasin and Grant, 2004) indicating that abuse does not provide an adequate marker for dependence. Using configural frequency analysis and survival analysis permitted us to include persons meeting criteria for only abuse or only dependence in our tests of LOTAD. However, the relationship of abuse to dependence may vary between treatment and general population samples. An additional limitation of this study was that the clustering criterion was not included in the dependence diagnoses. Inclusion of the clustering criterion might lengthen LOTAD for some individuals because the onset of the third dependence criterion does not necessarily equal the age at which three dependence criteria occur within a 12-month period.
4.2. LOTAD refinements needed
Refinements to LOTAD might produce a more accurate measure of addictive liability than was used in this study. Specifically, LOTAD might be more accurately measured if abuse is replaced with an alternative indicator of problematic use that is more directly linked to substance use. Two possible indicators are a threshold level of the number of days in a month during which a drug is consumed or binge use of a drug. A more homogenous marker of dependence, such as physiological dependence, also might improve LOTAD measurement.
Even if LOTAD proves to be a valid measure of addictive potential, it will have limitations. For example, LOTAD cannot be estimated for substances that are either new to the drug using “scene” or which are used by few individuals(e.g., LOTAD for ecstasy cannot be estimated at this time because it is currently used by too few persons to obtain estimates of differences between abuse onset and dependence onset). Prospective studies of LOTAD that are of sufficient time length and frequency of assessment are nonexistent and would require extensive resources to conduct.
One of the results of the present study was unexpected: early cannabis users' LOTAD was longer than other cannabis users' LOTAD. Developmental differences between early users and other users might be one factor that led to a longer LOTAD occurring for early users. Early users of cannabis generally first used cannabis before entering high school (the mean age of first cannabis use was 12.6 years for early cannabis users and 18.4 years for other cannabis users, respectively). Perhaps early users experience a greater prevalence of social or legal problems (abuse) associated with the same amount of cannabis use than do other cannabis users because of the greater amount of monitoring by adults (e.g., greater teacher and parental supervision), which also could result in a greater proportion of the early cannabis users receiving intervention prior to experiencing dependence compared to other users, which in turn might delay the onset of dependence relative to the onset of abuse.
4.3. Further testing of LOTAD is warranted
A practical use of addictive liability research has been to provide scientific data on which to base drug control policies prior to the marketing of new medications (Balster and Bigelow, 2003; Schuster and Henningfield, 2003). LOTAD will not be a useful measure to meet this purpose of addictive liability research because it would have to be conducted after novel substances are approved and marketed. However, there are a number of niches in the study of addictive liability where LOTAD could potentially provide important contributions.
4.3.1. LOTAD for etiological research
Having a measure of addictive potential that could be used in clinical and large sample research could expand our understanding of addictive potential. The liability-threshold model of drug addiction suggests that persons who cross a critical threshold of overall liability to addiction are the persons who experience addiction (Tarter and Vanyukov, 1994). The ability to combine the addictive potential associated with specific drugs with liability to addiction associated with personal characteristics (e.g., Table 6) could be informative for developing strategies regarding preventive, clinical, and public health efforts. It is feasible that certain characteristics (such as genotypes or neurophysiological characteristics) interact with the addictive liability of certain drugs to place some individuals at heightened or reduced risk for addiction to a particular drug. A similar issue that might be studied is estimation of individuals' overall liability to addiction for a drug based on personal characteristics, accounting for the addictive potential of the drug itself. For illustration, results of the regression analyses (Table 6) suggested that although women's LOTADs were shorter than men's LOTADs on average, an interaction occurred such that women's LOTAD tended to be longer related to cannabis than to use of other drugs. Assuming that the drug a person uses contributes to their overall liability to addiction, users of cocaine and opiates might be considered at greater risk for addiction than users of other drugs (all other factors being equal).
Results presented in Table 6 indicated that African-American women's LOTADs tend to be longer than Caucasian women's LOTADs. Perhaps LOTAD can be helpful for clarifying population differences such as differences between the United States population (in which substance use is highly prevalent) versus the Malaysian population (in which substance use is relatively rare), or between populations with highly prevalent use of different substances. Using linear regression, LOTAD might provide a useful tool to estimate unique addictive liability for combinations of drugs, such as the combination of cocaine and heroin, over and above the addictive liability of the use of the two drugs alone.
4.3.2. LOTAD for clinical trials and theoretical research
In clinical studies, LOTAD might provide a useful outcome measure that also could advance theory. One overarching goal of treatment for drug dependence is to prevent relapse. In an experimental study, it is generally hypothesized that compared to control participants, fewer participants who receive a novel treatment will relapse. Using LOTAD, further data about the efficacy of the novel treatment might be garnered from the outcomes of participants who relapse. Suppose the participants who receive the novel treatment but relapse have longer LOTADs than control participants who relapsed. This result would be consistent with the hypothesis that the novel treatment reduces the addictive liability of the drug. LOTAD could be useful as an outcome measure of pharmaceutical as well as behavioral clinicaltrials. Moreover, lengthening LOTAD might serve as a useful clinical goal because persons treated for drug dependence who relapse might be afforded more time after abuse occurs to have insight of their relapse and seek help before dependence occurs.
Clinical trials outcome data using LOTAD also could have theoretical implications. Illustrations of this use for LOTAD focus on pharmaceutical clinical trials only because of the current depth of research on neural mechanisms of drugs' effects. Similar uses for LOTAD might be applied to behavioral interventions. Data from recent cocaine reinstatement studies using laboratory animals have demonstrated that (a) D1-like dopamine receptor agonists attenuated cocaine-primed reinstatement (Self et al., 1996, 2000),(b) a nonselective dopamine antagonist had no effect on cocaine primed reinstatement (Cornish and Kalivas, 2000), and(c) cocaine primed reinstatement was potentiated by both an amphetamine (a dopamine reuptake blocker and dopamine releaser, Lynch et al., 1998) and a D2-like dopamine receptor agonist (Self et al., 1996). Suppose that D1-receptor agonists lengthened LOTAD and nonselective dopamine agonists had no effect on LOTAD during clinical trial studies of people dependent on cocaine. (Presumably, amphetamine and D2like receptor agonists would not be studied in people because of the evidence from animals suggesting that they increase risk for relapse.) The results would be consistent with being able to generalize the knowledge regarding the dopaminergic neural mechanisms of cocaine dependence from animal studies to people. On the other hand, suppose that findings from animal studies on cocaine reinstatement were not replicated in people. This result would suggest that knowledge from animal studies regarding the dopaminergic neural mechanisms of cocaine dependence could not be transferred to people.
4.3.3. LOTAD for transfer of animal research to human treatment
A medium to investigate how well the findings from addictive potential research conducted with animals generalize to people might yield great benefits to people; this possibility is illustrated with a progressive ratio break point study. Consistent with other animal studies of the effect that buprenorphine has on cocaine use (Kosten et al., 1991; Mello et al., 1989), Carroll et al. (1992) break point study suggested that ingesting buprenorphine reduces the addictive potential of cocaine, alcohol, and other substances. In a clinical trial study investigating the efficacy of using buprenorphine to treat cocaine addiction, the outcome measures might consist of comparing the control and treatment groups in terms of (a) the traditional rate of relapse and (b) LOTAD among persons who relapse at follow-up. One hypothesis would be that compared to control participants who relapse, LOTAD would be longer among persons who received buprenorphine but nevertheless relapse. If cocaine LOTAD was lengthened by buprenorphine treatment, as could be expected from studies on people (Foltin and Fischman, 1994; Rothman et al., 2000; Schottenfeld et al., 1993), evidence of buprenorphine's lowering of cocaine's addictive potential in people would be provided. Moreover, the result would strengthen the evidence that our knowledge of the neural mechanisms altered by buprenorphine that underlie cocaine dependence in animals is generalizable to people.
In summary, LOTAD or some alternative measure of the rate at which progression through levels of addiction occurs, may prove a useful construct if it is found to validly measure addictive potential. The results of the present study are consistent with the validity of LOTAD as a measure of drug's addictive potential by replicating patterns of addictive liability generated by animal studies using data collected from people. These results suggest that further testing of LOTAD as a measure of addictive potential is warranted.
Acknowledgements
Thanks to James C. Anthony, Ph.D. and three anonymous reviewers for comments on a previous draft of this manuscript. This investigation was supported by grants from NIDA (DA00430, DA00434, DA15984), NIAAA (AA12111), and NIMH (MH17104). Dr. Compton's work on this study was conducted prior to his appointment at the National Institute on Drug Abuse (NIDA). The views presented in this paper are of the authors' and do not necessarily represent the views of NIDA, the National Institutes of Health or the federal government.
Appendix A
Bootstrap analysis can be used to approximate a test for statistical differences and is useful when a population distribution is unknown (Efron and Tibshirani, 1986). One thousand bootstrap resamplings of the original sample were used to recalculate the survival curve probabilities (i.e., the point on the survival curve) at each of the five-year intervals. Then, the distribution of these 1000 recalculated survival probabilities were used to estimate standard deviations of survival probabilities at each of the five-year intervals. The survival probabilities and their standard deviations could then be used for t-tests of differences between abuse and dependence curves within each subgroup as well as the differences in LOTAD between subgroups. In Table 7, the bootstrap procedure is demonstrated using results that were generated to test for gender differences in alcohol LOTAD (Fig. 1). Table 7 presents the results for tests of (a) the difference in survival probabilities between abuse and dependence (i.e., is LOTAD different from zero?) for men (column 1) and women (column2); (b) the difference in LOTADs between men and women (column 3); (c) the t-value of the LOTAD difference between men and women (column 4); and (d) the probability of obtaining the gender difference in LOTAD by chance (p-value) in column 5. Rows of Table 1 are the different time points at which the tests were conducted (five-year intervals from 5 to 25 years after baseline). Men's and women's LOTADs were statistically greater than zero at all time points (columns 1 and 2 of Table 1). Five years after the initial regular use of alcohol (row 1), the difference between men's abuse and dependence survival probabilities was large enough to meet the p < .005 criterion (survival probability difference = 0.129); for women, the difference was smaller (survival probability difference = 0.066) but met the p < .05 criterion. Men's survival probability LOTAD was greater than women's LOTAD by 0.063 (standard deviation = 0.035) which resulted in a t-value of 1.785; a difference that approached statistical significance (p = .075). None of the other alcohol LOTAD gender differences even approached statistical significance. Hence, although the gap between women's abuse and dependence curves visually appears to be smaller than the gap between men's abuse and dependences curves (Fig. 1), the LOTADs based on survival probability appear to not be statistically significant.
Table 7.
Example results of bootstrap estimates of survival probabilities and t-tests of gender difference in alcohol LOTAD
| Number of years following regular use | Size of LOTAD |
Gender differences in LOTAD (StdDev) | t-Value of LOTAD gender differences | p-Value of LOTAD gender differences | |
|---|---|---|---|---|---|
| Men | Woment | ||||
| 5 | 0.129** | 0.066* | 0.063 (0.0351) | 1.785 | 0.075 |
| 10 | 0.140** | 0.099** | 0.041 (0.0425) | 0.955 | 0.340 |
| 15 | 0.113** | 0.086** | 0.026 (0.0430) | 0.610 | 0.542 |
| 20 | 0.082** | 0.087* | -0.004 (0.0430) | -0.103 | 0.918 |
| 25 | 0.064* | 0.082* | -0.018 (0.0428) | -0.411 | 0.681 |
Notes: LOTAD is the length of time between onset of abuse and dependence. ATest of the difference between abuse survival probability and dependence survival probability (i.e., is LOTAD significantly different from zero?). StdDev: standard deviation. C.I.: confidence interval.
p<.005.
p<.05.
References
- DSM-IV. fourth ed. American Psychiatric Association; Washington, D.C.: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Arfken CL, Cicero TJ. Postmarketing surveillance for drug abuse. Drug Alcohol Depend. 2003;70:S97–S105. doi: 10.1016/s0376-8716(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 2003;70:S13–S40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Brook DW, Brook JS, Zhang C, Cohen P, Whiteman M. Drug use and the risk of major depressive disorder, alcohol dependence and substance use disorders. Arch. Gen. Psychiatry. 2002;59:1039–1044. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine and habitual smoking. Arch. Gen. Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Carmona GN, May SA, Buzalsky S, Larson C. Buprenorphine's effects on self-administration of smoked cocaine base and orally delivered phencyclidine, ethanol, and saccharin in rhesus monkeys. J. Pharmacol. Exp. Ther. 1992;26:26–37. [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharmacol. Biochem. Behav. 2000;65:91–96. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J. Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Compton WM. Advantages of the CIDI family of instruments in epidemiological research of substance use disorders. Int. J. Methods Psychiatr. Res. 1993;3:109–119. [Google Scholar]
- Cottler LB, Robins L, Helzer J. The reliability of the SAM. Br. J. Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Schuckit MA, Helzer JE, Crowley T, Woody G, Nathan P, Hughes J. The DSM-IV field trial for substance use disorders: Major results. Drug Alcohol Depend. 1995;38:59–69. doi: 10.1016/0376-8716(94)01091-x. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Grant BF, Blaine J, Mavreas B, Pull C, Hasin D, Compton WM, Rubio-Stipec M, Mager D. Concordance of DSM-IV alcohol and drug use disorder criteria and diagnoses as measured by AUDADIS-ADR, CIDI and SCAN. Drug Alcohol Depend. 1997;47:195–205. doi: 10.1016/s0376-8716(97)00090-2. [DOI] [PubMed] [Google Scholar]
- DeWitt DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am. J. Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Edwards G, Gross GG. Alcohol dependence: provisional description of a clinical syndrome. Br. Med. J. 1976;1:1058–1061. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–77. [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Riechert U, Voker Hollt V. Acute injection of drugs with low addictive potential ( Δ9-tetrahydrocannabinol, 3,4-methylenedioxymethamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than highly addicting drugs (cocaine and morphine) Mol. Brain Res. 1999;71:313–324. doi: 10.1016/s0169-328x(99)00207-7. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br. J. Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the selfadministration of cocaine by humans. Behav. Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Gardner EJ. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langord JG, editors. Substance abuse: A comprehensive textbook. Williams & Wilkins; Baltimore, MD.: 1997. [Google Scholar]
- Grathwohl C, Dadmarz M, Vogel WH. Oral self-administration of ethanol and cocaine in rats. Pharmacology. 2001;63:160–165. doi: 10.1159/000056128. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(Suppl):S41–S54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Branchey MH, Buydens-Branchey L, Gribomont B, Holloway K, Silverman J. Morbidity risk for alcoholism and drug abuse in relatives of cocaine addicts. Am. J. Drug Alcohol Abuse. 1993;19:347–357. doi: 10.3109/00952999309001624. [DOI] [PubMed] [Google Scholar]
- Hasin D, Grant BF. The co-occurrence of DSM-IV alcohol abuse in DSM-IV alcohol dependence. Arch. Gen. Psychiatry. 2004;61:891–896. doi: 10.1001/archpsyc.61.9.891. [DOI] [PubMed] [Google Scholar]
- Hasin D, Grant BF, Cottler L, Blaine J, Towle L, Ustun B, Sartorius N. Nosological comparisons of alcohol and drug diagnoses: a multisite, multi-instrument international study. Drug Alcohol Depend. 1997;47:217–226. doi: 10.1016/s0376-8716(97)00092-6. [DOI] [PubMed] [Google Scholar]
- Horton J, Compton WM, Cottler LB. Reliability of substance use disorder diagnoses among African-Americans and Caucasians. Drug Alcohol Depend. 2000;57:203–209. doi: 10.1016/s0376-8716(99)00050-2. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Marby DW, Nestler EJ. Cocaine conditioned place preference is attenuated by chronic buprenorphine treatment. Life Sci. 1991;49:PL201–PL206. doi: 10.1016/0024-3205(91)90490-3. [DOI] [PubMed] [Google Scholar]
- Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. J. Abnorm. Psychol. 2004;113:72–80. doi: 10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- Langenbucher J, Morgenstern J, Labouvie E, Nathan PE. Lifetime DSM-IV diagnosis of alcohol, cannabis, cocaine and opiate dependence: six-month reliability in a multi-site clinical sample. Addiction. 1994;89:1115–1127. doi: 10.1111/j.1360-0443.1994.tb02788.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Heaser WA, Carroll ME. Effects of amphetamine, butorphanol, and morphine pretreatment on the maintenance and reinstatement of cocaine-reinforced responding. Exp. Clin. Psychopharmacol. 1998;6:255–263. doi: 10.1037//1064-1297.6.3.255. [DOI] [PubMed] [Google Scholar]
- Mackesy-Amiti ME, Fendrich M, Goldstein PJ. Sequence of drug use among serious drug users: typical vs. atypical progression. Drug Alcohol Depend. 1997;45:185–196. doi: 10.1016/s0376-8716(97)00032-x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine suppresses cocaine self-administration by rhesus monkeys. Science. 1989;245:859–862. doi: 10.1126/science.2772637. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch. Gen. Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Pull CB, Saunders JB, Mavreas V, Cottler LB, Grant BF, Hasin DS, Blaine J, Mager D, Ustun BT. Concordance between ICD-10 alcohol and drug use disorder criteria and diagnoses as measured by the AUDADIS-ADR, CIDI, and SCAN: results of a crossnational study. Drug Alcohol Depend. 1997;47:207–216. doi: 10.1016/s0376-8716(97)00091-4. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Cottler LB, Compton WM, Spitznagel EL, Cunningham-Williams RM. Is there a progression from abuse disorders to dependence disorders? Addiction. 2003;98:635–644. doi: 10.1046/j.1360-0443.2003.00350.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Babor T. WHO/ADAMHA; St. Louis: 1990. WHO/ADAMHA Composite International Diagnostic Interview-Substance Abuse Module (SAM) [Google Scholar]
- Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, Liberto JG. An open-label study of a functional opioid antagonist in the treatment of opioid dependence. J. Subst. Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaineabusing opioid-dependent humans. Biol. Psychiatry. 1993;34:66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Bucholz KK, Reich T, Bierut L. Five-year clinical course associated with DSM-IV alcohol abuse or dependence in a large group of men and women. Am. J. Psychiatry. 2001;158:1084–1090. doi: 10.1176/appi.ajp.158.7.1084. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Henningfield J. Conference on abuse liability assessment of CNS drugs. Drug Alcohol Depend. 2003;70:S1–S4. doi: 10.1016/s0376-8716(03)00095-4. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJU, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1 and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Self DW, Karanian DA, Spencer JJ. Effects of the novel D1 dopamine receptor agonist ABT-431 on cocaine self-administration and reinstatement. Ann. N. Y. Acad. Sci. 2000;909:133–144. doi: 10.1111/j.1749-6632.2000.tb06679.x. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- von Eye A. Cambridge University Press; New York: 1990. Introduction to Configural Frequency Analysis. [Google Scholar]
- Tarter RE, Soledad S, Dunn MG. Predictor variables by developmental stages: A Center for Substance Abuse Prevention multisite study. Psychol. Addict. Behav. 2002;16:S3–S10. doi: 10.1037/0893-164x.16.4s.s3. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M. Alcoholism: a developmental disorder. J. Cons. Clin. Psychol. 1994;62:1096–1107. doi: 10.1037//0022-006x.62.6.1096. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer J, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch. Gen. Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Ustun B, Compton W, Mager D, Babor T, Baiyewu O, Chatterji S, Cottler L, Gogus A, Mavreas V, Peters L, Pull C, Saunders J, Smeets R, Stipec MR, Vrasti R, Hasin D, Room R, Van den Brink W, Regier D, Blaine J, Grant BF, Sartorius N. WHO study on the reliability and validity of the alcohol and drug use disorder instruments: overview of methods and results. Drug Alcohol Depend. 1997;47:161–169. doi: 10.1016/s0376-8716(97)00087-2. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Winger G, Young AM, Woods JH. Ethanol as a reinforcer: comparison with other drugs. In: Kissin B, Begleiter H, editors. The Pathogenesis of Alcoholism: Biological Factors. Plenum Press; New York: 1983. pp. 107–131. [Google Scholar]


