Abstract
The cranial nerves are often involved in head and neck malignancies. Some malignancies have a strong propensity to show perineural spread. Cranial nerve palsy may be the presenting sign of metastatic disease to the skull base. Like metastatic disease to the lungs or liver, the cranial nerves themselves may be the site of metastatic disease. In addition, cranial nerves can be injured by radiation therapy or sacrificed during surgical treatment. This paper focuses on the imaging features of perineural infiltration, skull base neural foramen involvement and metastatic disease in the cranial nerves. It will also highlight the complications of radiation therapy, in particular radiation-induced optic neuritis.
Keywords: Perineural infiltration, cranial palsy, radiation-induced neuritis
Introduction
The cranial nerves are often involved in head and neck malignancies. Some malignancies such as adenoid cystic, squamous cell or mucoepidermoid carcinomas have a propensity to show perineural spread. Cranial nerve palsy may be the presenting sign of metastatic disease to the skull base. Metastatic breast, lung, renal or prostatic carcinomas form the majority of cancers that involve the skull base resulting in cranial nerve palsies. Like metastatic disease to the lungs or liver, the cranial nerves themselves may be the site of metastatic disease.
Cranial nerves can be injured or sacrificed during treatment. For instance, the optic nerve is sacrificed if the orbital apex periorbita is involved. The facial or accessory nerves are also frequently excised in parotid malignancies and neck dissection involving the posterior triangle. Cranial nerves may also be injured following radiation therapy.
This paper focuses on the imaging features of perineural infiltration, skull base neural foramen involvement in metastatic disease and the cranial nerve metastasis. It will also highlight the complications of radiation therapy, in particular radiation-induced optic neuritis.
Perineural spread
Perineural spread (PNS) refers to the tumour extension along nerve sheaths. This phenomenon is well described in both the surgical and imaging literature [1–4] and carries a grave prognosis. Imaging plays a critical role because this condition may be asymptomatic. PNS rarely presents before the detection of the primary head and neck tumour. More commonly, PNS occurs after the patient has been treated.
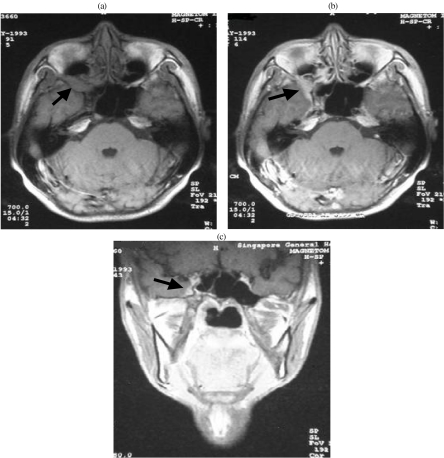
The clinical signs and symptoms vary with the type of nerves involved. Pain and paraesthesia are associated with sensory nerves. For instance, involvement of the maxillary nerve in the pterygopalatine fossa (PPF) results in pain or hypoesthesia over the face. Tumour involvement of the PPF is readily identified on MRI. The PPF is fat filled and tumour infiltration is seen as intermediate signal intensity replacing the high signal intensity fat on T1-weighted images (Fig. 1). Following the administration of contrast, the malignant infiltration of the maxillary nerve extending intracranially through the foramen rotundum can be demonstrated [5]. The earliest CT sign is the replacement of low density fat by intermediate density tumour. Later, the PPF may show widening and this erosion may also involve the foramen rotundum.
Figure 1.
Nasopharyngeal carcinoma with perineural infiltration of V2. (a) Axial T1-weighted MRI shows intermediate signal intensity tumour widening the right pterygopalatine fossa (arrow). (b) Axial contrast enhanced MRI shows tumour enhancement and extension into the cavernous sinus (arrow). (c) Coronal T1-weighted MRI shows thickened V2 (arrow).
PNS involving the mandibular nerve is most commonly seen in skull base cancers, especially nasopharyngeal carcinomas [6]. Foraminal enlargement can be seen on CT but this is a late sign. MRI can often demonstrate perineural thickening of the mandibular nerve before osseous changes in the foramen ovale are detectable [7]. At this stage, the patient may remain asymptomatic. Malignancies involving the submandibular gland, tongue or floor of the mouth may invade the lingual nerve and extend intracranially through the foramen ovale along the mandibular nerve. The malignant infiltration may continue proximally to the trigeminal nerve ganglion, the trigeminal nerve and the route entry zone.
Malignant tumours of the parotid gland often involve the small branches of the facial nerve (microscopic infiltration) at the time of diagnosis. However, frank facial nerve palsy is usually the result of recurrent disease. MRI is the modality of choice for the early detection of perineural infiltration of the facial nerve. Apart from parotid gland malignancies, the facial nerve may also be infiltrated as a result of the involvement of the Vidian nerve and the greater superficial petrosal nerve (GSPN). Tumour infiltrating the Vidian canal (which connects the PPF and the foramen lacerum) can spread along the GSPN to the geniculate ganglion [8]. At this point perineural infiltration can further proceed to the labyrinthine or tympanic portions of the facial nerve.
Denervation atrophy
Changes in the skeletal muscles following denervation can be divided into three phases: acute, subacute and chronic. Animal models show that in the first 4 weeks, there is a decrease in the calibre of muscle fibres but no change in the total amount of tissue water. There is, however, a relative decrease in intracellular water associated with a relative increase in extracellular water. In the chronic phase, muscular atrophy associated with fatty infiltration takes place. A mixture of features seen in the acute and chronic stages characterises the subacute phase. In addition, there is a relative increase in the perfusion of muscles following denervation [9–11].
In the acute to subacute phases, T2-weighted images show high signals thus producing an oedema-like appearance. This is because the T2 of extracellular water is longer than the T2 of intracellular water. In addition, there is a relative increase in perfusion and increased accumulation of contrast in the extracellular spaces in denervated muscles resulting in increased contrast enhancement (Fig. 2). In the chronic phase, there is muscle atrophy and increased signals on T1 and fast spin echo T2 weighted images due to fat accumulation. These MRI features may be confused with tumour infiltration of the muscles concerned. It should be noted that malignant infiltration produces an increase in size of the affected muscles, whereas denervation atrophy shows a decrease in muscle bulk. Additionally, the signal changes in denervation atrophy are generalised and higher in signal intensity. Malignant infiltration of the muscles on the other hand is more localised and lower in signal intensity.
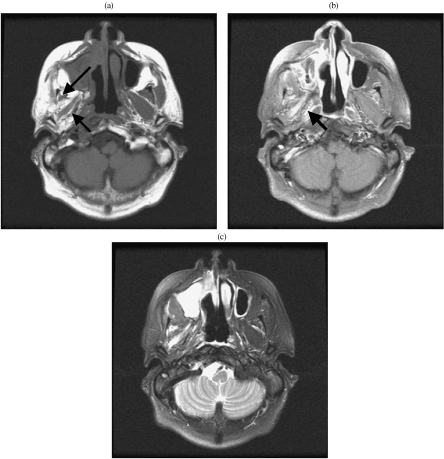
Figure 2.
Denervation atrophy of muscles of mastication following V3 perineural infiltration. (a) Axial T1-weighted MRI shows gross atrophy of the muscles of mastication (arrows). Compare with the normal muscles on the ipsilateral side. (b) Axial contrast enhanced MRI shows enhancement in the right lateral pterygoid muscle (arrow). (c) Axial T2-weighted MRI shows high signal intensity in the right lateral pterygoid muscle.
Denervation atrophy is most commonly encountered in the muscles innervated by the mandibular and the hypoglossal nerves. Paralysis of the tongue resulting in atrophy and fatty infiltration is usually easy to recognise clinically. The earliest clinical sign is fasciculation and atrophy may not be obvious. At this stage, radiological signs of atrophy and fatty infiltration may be subtle. However, the affected side of the tongue can be seen to drop backwards in axial images. This is a helpful sign. It is easily seen in lower sections showing the posterior aspect of the tongue. This sign does not appear to be widely appreciated in the radiological community [11]. It is important to note that posterior displacement of the tongue may give the impression of an apparent increase in its length. This could be mistaken as an increase in the size of the tongue but should be recognised for what it is. Harnsberger reported a patient who had hypoglossal nerve palsy who underwent repeated biopsies of the contralateral normal tongue for suspected tumour involvement [12].
Metastatic disease
Rarely, the cranial nerves themselves become sites of metastatic deposits. Malignant cells are carried to the perineurium and endoneurium via the vasa nervorum. These vas nervorum pierce the epineurium and perineurium to anastomose with the vascular system that runs parallel to the nerve fibres. These endoneural vessels typically have relatively large diameters [13]. Malignant cells deposited in these vascular beds often extend along the involved nerves through PNS. Malignant cells may also be deposited on cranial nerves as a result of meningeal carcinomatosis. In patients with meningeal carcinomatosis there is often multiple cranial nerve involvement (Fig. 3).
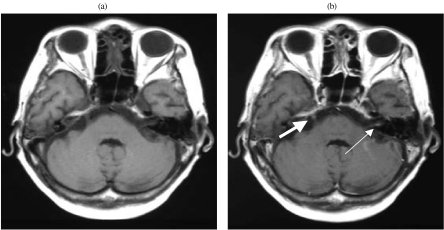
Figure 3.
Meningeal carcinomatosis with involvement of cranial nerves. (a) Axial T1-weighted MRI shows no enhancement in cranial nerves V and VII. (b) Axial contrast enhanced MRI shows contrast enhancement in the right cranial nerve V (thick arrow) and the left cranial nerve VII (thin arrow). (The patient had clinical left CN VII palsy.)
More commonly, metastatic disease involves the osseous structures around the neural foramen or the cavernous sinus (Fig. 4). These lesions compress the cranial nerves resulting in various combinations and degrees of cranial nerve palsies. Metastases in the sphenoid bone around the cavernous sinus or secondaries involving the jugular foramen are notorious for multiple cranial nerve palsies.
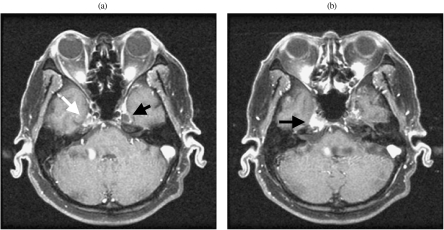
Figure 4.
Metastatic disease to the petrous apex and involvement of Meckel’s cave in a patient with cranial nerves V3 and VI palsy. (a) Axial contrast MRI shows enhancement in Meckel’s indicating trigeminal ganglion involvement (white arrow). Compare with the normal contralateral side (black arrow). (b) Axial contrast enhanced MRI shows enhancement of the petrous apex in the expected position of cranial nerve VI.
Radiation-induced neuritis
Compared with the grey and white matter of the brain, cranial nerves are relatively radioresistant. One of the reasons why cranial nerves are less prone to ischaemia is because of the relatively large endoneural vessels and the dual arterial supply with good anastomosis [13]. In clinical practice, the most commonly encountered cranial nerve palsy following irradiation for skull base malignancy is the sixth cranial nerve. This is presumably due to the small size of the nerve rendering it more vulnerable to injury. The twelve cranial nerve is also commonly involved. Radiation-induced optic neuritis has devastating consequences. Deterioration of vision is often gradual and imaging findings are often negative. However, sometimes optic nerve swelling with contrast enhancement may be seen on MRI [14, 15].
In summary, the cranial nerves are often involved in head and neck malignancies. Imaging plays a crucial role in identifying PNS, osseous skull base metastasis and cranial nerve metastasis. In addition, cranial nerves can be injured by radiation therapy or sacrificed during surgical treatment.
References
- 1.Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through peripheral nerves. Am J Surg. 1963;106:651–67. doi: 10.1016/0002-9610(63)90074-6. [DOI] [PubMed] [Google Scholar]
- 2.Soo KC, Carter RL, O’Brien CJ, et al. Prognostic implications of squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145–8. doi: 10.1288/00005537-198610000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Chong VF, Fan YF. Pterygopalatine fossa and maxillary nerve infiltration in nasopharyngeal carcinoma. Head Neck. 1997;19:121–5. doi: 10.1002/(sici)1097-0347(199703)19:2<121::aid-hed6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg LE. MR imaging of perineural tumor spread. Magn Reson Imaging Clin N Am. 2002;10:511–25. doi: 10.1016/s1064-9689(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 5.Chong VFH, Fan YF. Pictorial essay: maxillary nerve involvement in nasopharyngeal carcinoma. Am J Roentgenol. 1996;167:1309–12. doi: 10.2214/ajr.167.5.8911202. [DOI] [PubMed] [Google Scholar]
- 6.Chong VF, Fan YF, Khoo JB. Nasopharyngeal carcinoma with intracranial spread: CT and MR characteristics. J Comput Assist Tomogr. 1996;20:563–9. doi: 10.1097/00004728-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Laine FJ, Braun IF, Jensen ME, Nadel L, Som PM. Perineural tumor extension through the foramen ovale: evaluation with MR imaging. Radiology. 1990;174:65–71. doi: 10.1148/radiology.174.1.2152985. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg LE, Demonte F, Gillenwater AM. Greater superficial petrosal nerve: anatomy and MR findings in perineural tumor spread. AJNR Am J Neuroradiol. 1996;17:389–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Russo CP, Smoker WR, Weissman JL. MR appearance of trigeminal and hypoglossal motor denervation. AJNR Am J Neuroradiol. 1997;18:1375–83. [PMC free article] [PubMed] [Google Scholar]
- 10.Davis SB, Mathews VP, Williams DW III. Masticator muscle enhancement in subacute denervation atrophy. AJNR Am J Neuroradiol. 1995;16:1292–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Chong VF, Fan YF. Hypoglossal nerve palsy in nasopharyngeal carcinoma. Eur Radiol. 1998;8:939–45. doi: 10.1007/s003300050492. [DOI] [PubMed] [Google Scholar]
- 12.Harnsberger HR, Dillon WP. Major motor atrophic patterns in the face and neck: CT evaluation. Radiology. 1985;155:665–70. doi: 10.1148/radiology.155.3.4001368. [DOI] [PubMed] [Google Scholar]
- 13.Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MW, editors. Gray’s Anatomy. Structure of Peripheral Nerves. 38th edn. London: Churchill Livingstone; 1995. pp. 945–7. [Google Scholar]
- 14.Chong VF, Khoo JB, Chan LL, Rumpel H. Neurological changes following radiation therapy for head and neck tumours. Eur J Radiol. 2002;44:120–9. doi: 10.1016/s0720-048x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 15.Chong VF, Khoo JB, Fan YF. Imaging of the nasopharynx and skull base. Magn Reson Imaging Clin N Am. 2002;10:547–71. doi: 10.1016/s1064-9689(02)00010-7. [DOI] [PubMed] [Google Scholar]