Abstract
The expected changes on CT or MRI after treatment of a head and neck cancer are described; it is important not to confuse such expected changes with persisting or recurrent tumour, or a treatment complication. Post-treatment CT or MRI is of value when a recurrent tumour is suspected, to confirm the presence of such a lesion and to determine its extent; this is important information for determining the possibility of salvage therapy. More rarely, imaging may be of use in the differentiation between tumour recurrence and a treatment complication. In patients with a high-risk profile for tumour recurrence after treatment, imaging is of value for surveillance of the patient, as an adjunct to clinical follow-up. The baseline study should be obtained about 3 to 4 months after the end of therapy. There is evidence that tumour recurrences can be detect earlier by systematic follow-up imaging.
Keywords: Tumour recurrence, treatment complication, laryngeal necrosis, osteoradionecrosis, chondroradionecrosis, fistula
Introduction
After treatment of a head and neck cancer, a number of tissue changes become visible on CT and MR images of the neck. These expected alterations should be known, so that they are not misinterpreted as evidence of persistent or recurrent tumour.
Imaging may be used to monitor tumour response and to try to detect recurrent or persistent disease before it becomes clinically evident, possibly with a better chance for successful salvage.
Treatment complications, such as soft tissue or bone necrosis, are less frequent than tumour recurrences, but these conditions may be clinically sometimes difficult to distinguish. Although definitive distinction between necrosis and recurrent tumour may also radiologically be difficult, imaging findings may be helpful in guiding the choice of treatment and assessing the response to specific treatment.
Expected tissue changes after radiotherapy
After irradiation of a neck cancer, a number of tissue changes become visible on CT and MR images of the neck. Within the first 2 weeks after radiotherapy, there is an acute inflammatory reaction within the deep tissues. Increased permeability, due to detachment of the lining endothelial cells within small blood and lymphatic vessels, results in interstitial oedema. After this initial period of a few weeks, there is progressive thickening of the connective tissue. Endothelial proliferation is also seen, eventually resulting in complete obstruction of the vessels. The reduction in venous and lymphatic drainage results in further accumulation of interstitial fluid. Then the fibrosis becomes progressively more advanced, but the interstitial oedema may be reduced by formation of collateral capillary and lymphatic channels.
The changes visible on post-treatment CT and MR images depend on the radiation dose and rate, the irradiated tissue volume, and the time elapsed since the end of radiation therapy [1, 2]. Changes which may be seen include (Fig. 1(a)–(j)):
Thickening of the skin and platysma muscle.
Reticulation of the subcutaneous fat and the deep tissue fat layers.
Oedema in the retropharyngeal space.
Increased enhancement of the major salivary glands, followed by size reduction of these glands: post-irradiation sialadenitis.
Atrophy of lymphatic tissue, in both the lymph nodes and Waldeyer’s ring.
Thickening and increased enhancement of the pharyngeal walls.
Thickening of the laryngeal structures, with increased density of the fat in the pre-epiglottic and paralaryngeal spaces.
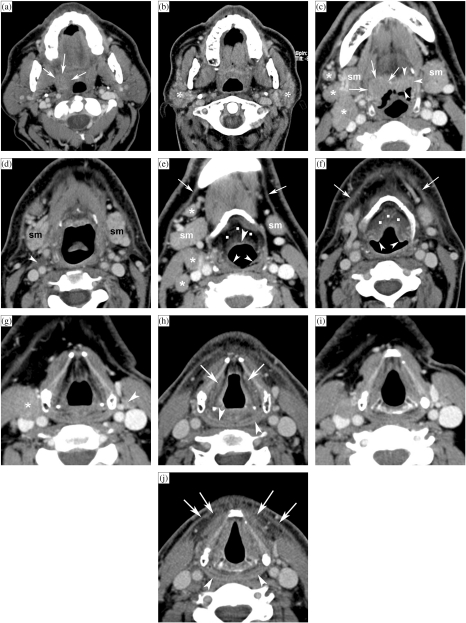
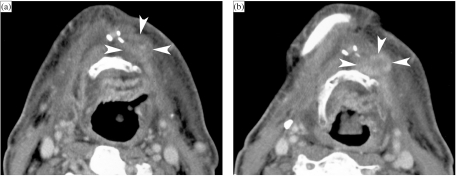
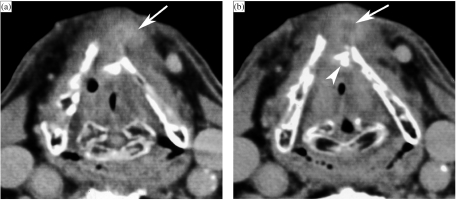
Figure 1.
Patient with a right-sided T3N2b oropharyngeal tonsillar cancer, treated by definitive radiotherapy (RT). Anatomically corresponding axial contrast-enhanced CT images are shown, obtained just before and 6 months after completion of radiation treatment. (a, b) Level of oropharyngeal tonsil. Before RT (a), part of the oropharyngeal cancer is seen (arrows). After RT (b), the oropharyngeal tissues appear symmetrical. Volume reduction and increased enhancement of the parotid salivary glands (asterisks) correspond to radiation sialadenitis. (c, d) Level of tongue base. Before RT (c), asymmetry is seen in the tongue base tissues, appearing thicker on the right side; this corresponds to tumour extension along the tongue base, into the vallecula on the right side (arrows), and normal lingual tonsil on the left side (arrowheads). Several adenopathies are seen (asterisks). After RT (d), the tumoural tissue as well as the lingual tonsil disappeared. Also the adenopathies disappeared, leaving some strands of residual tissue (arrowhead). Note signs of radiation sialadenitis in both submandibular salivary glands (sm). (e, f) Level of hyoid bone. Before RT (e), several adenopathies are seen (asterisks). Note the normal appearance of submandibular salivary glands (sm), epiglottis (arrowhead) and ary-epiglottic folds (small arrowheads), pre-epiglottic fat plane (dots), and platysma muscles (arrows). After RT (f), the adenopathies disappeared; the submandibular salivary glands became smaller due to radiation sialadenitis. Thickening of the epiglottis and ary-epiglottic folds (arrowheads) is seen, as well as increased density in the pre-epiglottic space (dots). Note the thickening of the platysma muscles (arrows). (g, h) Level of false vocal cords. Before RT (g), adenopathy is seen on the right side (asterisk), as well as normal sized lymph node on the left side (arrowhead). After RT (h), both the adenopathies as the normal-appearing lymph node disappeared. Within the larynx, increased density is seen with the paraglottic spaces; the walls of the laryngeal ventricles appear thickened (arrows). There is also thickening and increased enhancement of the posterior hypopharyngeal walls (arrowheads). (i, j) Level of true vocal cords before (i) and after RT (j). Within the larynx, at this level very few changes are visible. The hypopharyngeal walls show slightly increased enhancement and thickening (arrowheads). More pronounced changes are visible within the anterior neck soft tissue, showing streaky infiltration of the fatty tissues, and platysma muscle thickening (arrows).
These tissue changes are most pronounced during the first few months after the end of radiation therapy, and diminish or even resolve with time. It is important to note that the expected tissue changes after radiation therapy appear symmetrical, unless the neck is irradiated using asymmetric radiation portals.
The laryngeal cartilages do not show changes after irradiation. Reduction in the degree of cartilage sclerosis in the neighbourhood of the tumour has been described, and this seems to correlate with local control [3].
Expected tissue changes after surgery
The limits of surgical therapy are determined by the balance between obtaining cure by radical resection of the tumour, and leaving the patient in a functionally and aesthetically acceptable situation. More extensive resections are possible by the introduction of various reconstructive materials, such as pedicled or free soft tissue flaps, grafts and prostheses.
Tissue transferred to close a defect, while maintaining its original vascular pedicle, is called a pedicled flap. Pedicled flaps may be harvested locally (in the immediate area of the defect), regionally (from the same area, but not contiguous to the defect), or at distance from the defect. The most commonly used distant flaps are musculocutaneous flaps, such as the pectoralis major flap. The pectoralis major flap has an excellent blood supply and gives acceptable functional and cosmetic results. It is commonly used to reconstruct the pharyngeal defect after laryngectomy with partial pharyngectomy; it is also very useful for closing defects in the irradiated neck, as it introduces tissue with a fresh blood supply.
On imaging studies, the pectoralis major flap appears initially as a bulky soft tissue structure, showing the characteristics of muscle; gradually, denervation atrophy appears, causing volume loss and fatty replacement of the muscle (Fig. 2). At the time of imaging, the muscle denervation may be incomplete; fibre-like structures with muscle density within the flap should not be confused with tumour recurrence.
Figure 2.
Axial contrast-enhanced CT image. Situation after total laryngectomy. At a mid-cervical position, the neopharynx is seen to be constructed by residual pharyngeal tissue (black arrows) and a musculocutaneous soft tissue flap (pectoralis major flap, containing skin (arrowheads), subcutaneous fat (black asterisk), and muscle (white asterisk)).
When a flap is vascularised by local vessels, anastomosed to the flap by microvascular techniques, it is called a free flap. Different kinds of free flaps are in use, for example cutaneous flaps to reconstruct defects in the oral cavity, osseous flaps (e.g. fibula) to reconstruct mandibular defects, and free jejunal interposition to reconstruct the defect created by a total laryngopharyngectomy.
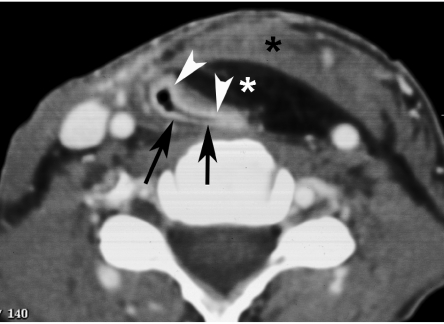
Neck dissection is a surgical procedure to remove neck nodal metastasis. Several procedures are distinguished. In radical neck dissection, unilateral en bloc removal of the neck lymph nodes levels I–V [4], including the sternocleidomastoid muscle, internal jugular vein, submandibular gland and spinal accessory nerve, is performed (Fig. 3). If the spinal accessory nerve is preserved, the procedure is called a modified radical neck dissection. Prelevation of the right sternocleidomastoid muscle may cause hypertrophy of the ipsilateral scapula levator muscle.
Figure 3.
Axial contrast-enhanced CT image. Soft tissue loss in the right side of the neck after radical neck dissection, including resection of the sternocleidomastoid muscle and internal jugular vein (labelled by arrows and arrowhead on the opposite side).
If the spinal accessory nerve and one more of the above mentioned structures, removed in a radical neck dissection, are preserved, the procedure is called a functional or conservative neck dissection. This type of dissection is performed when there are no or only small clinically or radiographically positive lymph nodes present in the neck.
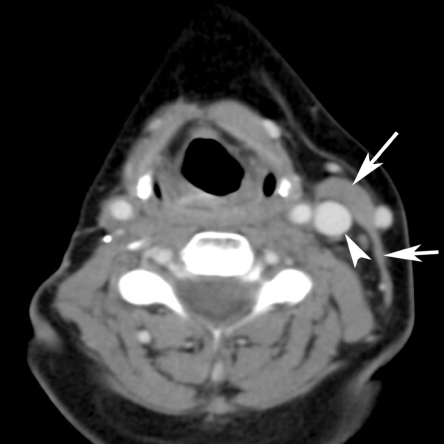
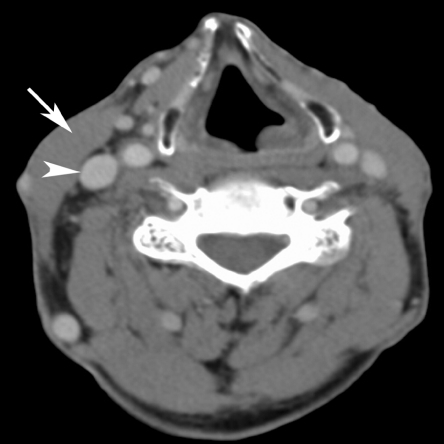
In a selective neck dissection, a more limited number of lymph nodes levels are removed. A commonly performed selective neck dissection is a supraomohyoid neck dissection: this includes removal of levels I, II and III and is performed when a small cancer of the oral cavity is removed and no positive lymph nodes are present (Fig. 4).
Figure 4.
Axial contrast-enhanced CT image. Limited soft tissue loss in the left side of the neck after selective neck dissection; essentially, removal of the lymph nodes underneath the sternocleidomastoid muscle and surrounding fatty tissue has been performed, leaving the muscle and internal jugular vein (labelled by an arrow and arrowhead on the opposite side).
Tumour recurrence
On CT or MRI, tumour recurrence appears after radiation therapy as a soft tissue mass at the primary site and/or as an enlarged (and/or centrally liquefied) neck adenopathy. After surgical treatment, tumour recurrence typically appears as a soft tissue mass along the surgical resection margins. Bone or cartilage erosion may be seen in large recurrent tumours. Also perivascular or perineural spread may be seen on imaging studies.
Early tumour recurrence may be difficult to distinguish from tissue changes induced by therapy. Therefore, it is recommended to obtain a follow-up CT or MR study after surgical, radiation or combined treatment for a head and neck neoplasm with high-risk profile [5, 6]. Probably the best time to obtain such a baseline study is about 3 to 6 months after the end of treatment. Such a baseline study allows treatment-caused changes in the head and neck tissues to be documented. By comparing subsequent studies with the baseline study, it becomes possible to detect with more confidence tumour recurrences or treatment complications, and this at an earlier stage than is possible with clinical follow-up alone (Fig. 5). In most patients, CT is an adequate imaging modality for pre- and post-treatment imaging; MR is preferred in patients with nasopharyngeal, sinonasal and skull base tumours.
Figure 5.
Patient surgically treated for a left-sided floor of the mouth cancer, abutting the mandible; rim resection of the mandible was included, and the patient received postoperative irradiation. Baseline CT study, 6 months after completion of therapy, shows expected post-therapeutic changes, but also a more or less nodular area in front of the hyoid bone, without clear enhancement (A, arrowheads); based on these findings, a follow-up CT study was recommended. Three months later, a larger and enhancing nodular lesion is seen in the same region: suspect for recurrent tumour (B, arrowheads). Clinically no evidence of recurrent tumour was present. Based on the radiological findings, resection was performed about a month later and confirmed tumour recurrence.
There is evidence that the baseline study itself carries important predictive information regarding the eventual local outcome: several studies show that CT may be useful in the early differentiation of treatment responders from non-responders in irradiated laryngeal and hypopharyngeal cancer [5, 7]. In this regard, the value of MRI is less well established.
Based on the appearance of the larynx/hypopharynx on an early post-radiotherapy CT study, a prediction of long-term local outcome can be made according to the following scores: 1=expected post-radiotherapy changes, i.e. complete resolution of the tumour at the primary site and symmetrically appearing laryngeal and hypopharyngeal tissues, as described above; 2=focal mass with a maximal diameter of <1 cm and/or asymmetric obliteration of laryngeal tissue planes; 3=focal mass with a maximal diameter of >1 cm, or <50% estimated tumour volume reduction [3, 5].
The post-radiotherapy CT-score 1 was shown to be a very strong predictor of long-term local control; patients with such findings on post-radiotherapy CT will probably not benefit from further follow-up imaging studies. Conversely, patients with a first follow-up examination classified as CT-score 3 do very poorly; almost all these patients will develop a local failure [3]. Further exploration in such post-radiotherapy CT-score 3 patients is warranted. FDG or thallium PET or SPECT imaging may prove to be a useful intermediate step in cases where biopsy is considered too risky, or if a biopsy result is returned as negative. Indeed, the predictive value of a negative biopsy for local control is reported to be only 70% [8]; this is likely due to sampling error, as tumour recurrences initially develop submucosally and can therefore not be accurately targeted. In cases of contradiction between the clinical findings, CT findings, results of radionuclide studies and/or biopsy, close clinical follow-up and repeat imaging studies are needed.
The local outcome of patients initially classified as post-radiotherapy CT-score 2 is indeterminate. Unless clinical examination is already suspect for local failure, further follow-up CT studies are needed in these patients; a time interval of 3–4 months is recommended, to be continued up to 2 years after completion of radiation treatment (Fig. 6).
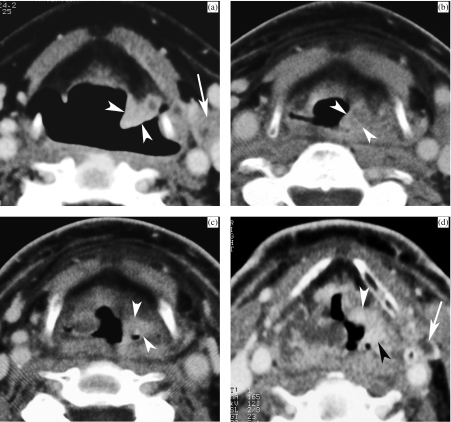
Figure 6.
(a) Pre-treatment CT image of a patient with T2 supraglottic squamous cell carcinoma, showing infiltrating lesion within the left ary-epiglottic fold (arrowheads). A pathologic lymph node is seen along the left internal jugular vein (arrow). (b) Three months after radiation treatment. Clinical examination showed pronounced laryngeal oedema, but no evidence of tumour. On CT, thickening of the supraglottic soft tissues is seen, more pronounced in the left ary-epiglottic fold (arrowheads); the density within the left ary-epiglottic fold is also somewhat higher than in the surrounding tissues. This is a non-specific finding (score 2), warranting further imaging follow-up. (c) Nine months after radiation treatment. Clinically favourable evolution. However, CT shows more pronounced enhancement in the left ary-epiglottic fold compared to the previous study (arrowheads). This was reported as suspicious for tumour recurrence. Direct laryngoscopy was performed but showed no mucosal abnormalities; biopsies were negative. (d) One year after radiation treatment. Apart from increasing generalised laryngeal oedema, the enhancing mass in the ary-epiglottic fold is now extending more anteriorly into the left paraglottic space (arrowheads). Also note the appearance of small necrotic lymph node in the left neck (arrow). Direct laryngoscopy, performed after the CT study, revealed caking of necrotic tissue over the left ary-epiglottic fold, suspect for tumour recurrence. Biopsy revealed squamous cell carcinoma. The patient died with progressive locoregional disease 7 months later.
Some authors recommend FDG–PET as the initial baseline study in patients treated with advanced disease with low clinical suspicion of recurrence and in patients with non-specific symptoms that could indicate recurrence but without a clinically obvious mass; cross-sectional imaging should then be performed for an equivocal or positive PET study, or as the initial study in patients with a suspicious palpable mass or biopsy proved recurrent tumour [9]. Further study is needed to elucidate the most efficient use of PET and CT/MR in post-treatment situations; also the potential role of combined PET-CT systems has to be evaluated.
Potential value of imaging surveillance
Use of this imaging-based information could lead to more prompt salvage surgery and potentially improve the survival of these patients [5]. However, few data regarding the value of post-treatment surveillance in patients with head and neck cancer are available. Some authors argue that routine follow-up is indispensable, as patients with asymptomatic locoregional recurrences, discovered during surveillance, have a significantly better post-recurrence survival than those patients where recurrent disease was found by symptoms [10]. Other authors point out that the apparently longer survival of patients with recurrent tumour diagnosed by testing may be due to lead time bias (i.e. early diagnosis falsely appears to prolong survival) [6]. This statement probably is true for patients treated by combined-modality therapy for advanced head and neck cancer, who are known to do extremely poorly after relapse and rarely have an effective treatment option available [11]. However, in single modality treated patients, where a reasonable chance of salvage exists after locoregional recurrence (e.g. 35–60% surgical salvage rate for irradiated laryngeal cancer), imaging surveillance may be worthwhile to add to the clinical follow-up in order to further improve the salvage rate. More studies are required to elucidate this question.
Treatment complications
Complications after surgery
Most surgical complications occur early after treatment, and are dealt with on a clinical basis. Imaging may be required in the detection and visualisation of fistula originating from the oral cavity or pharynx. Many of these fistulas will close spontaneously, but some may need reintervention. Imaging may also be of use in the confirmation of flap failure (necrosis) (Fig. 7).
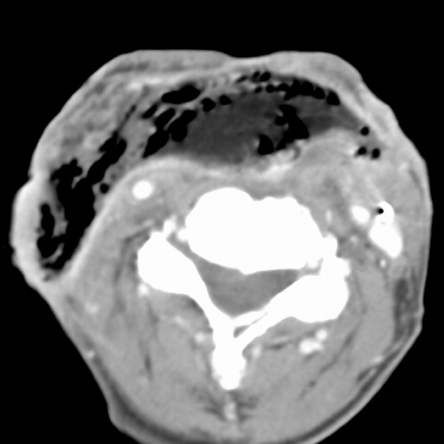
Figure 7.
Axial contrast-enhanced CT image. A few weeks before this CT study, total laryngectomy was performed, with neopharyngeal reconstruction by a pectoralis major flap. The patient suffers now from persistent fistulisation. Throughout the pectoralis major flap, large, confluent gas bubbles are visible, indicating flap necrosis. Flap necrosis was surgically confirmed.
Complications after radiotherapy
Acute effects of radiotherapy (skin and mucosal reactions) occur during or immediately after treatment, and usually settle spontaneously. Tissue necrosis is a rare complication of radiotherapy in the head and neck region, usually appearing months to years after the end of radiation treatment. Several risk factors have been identified, including high radiation doses and large radiation fields. Soft tissue, cartilage and bone necrosis may be encountered; several tissue types may be involved at the same time.
Mandibular osteoradionecrosis
Osteoradionecrosis may involve the mandible, the ossified cartilage of the larynx, the temporal bone and the hyoid bone [12, 13]. The pathogenesis is not fully understood. A popular theory is that irradiation produces hypoxic, hypocellular and hypovascular tissue, unable to remodel following tissue loss, thus leading to breakdown [14]. Trauma is often associated with the appearance of osteoradionecrosis as it creates a demand for tissue repair beyond the capabilities of the irradiated tissue. The CT findings include bony abnormalities (cortical interruptions and loss of spongiosa trabeculation), sequestration, pathological fractures, soft tissue thickening (sometimes gas-containing), and fistula formation (Fig. 8). Radiologically, differentiation from tumour recurrence may be difficult. In the mandible, some findings, such as the association with cortical defects away from the position of the original tumour (at the buccal surface or in the heterolateral side of mandible), make the diagnosis of mandibular osteoradionecrosis more likely [15]. Apart from cortical interruptions, MRI also shows altered bone marrow signal intensities in the mandibular parts involved by osteoradionecrosis. These areas show abnormal homogenous low marrow signal intensity on T1-weighted images; increased signal intensity on T2-weighted images may also be observed. The pathological bone marrow shows diffuse and intense enhancement after intravenous administration of contrast agent [16, 17].
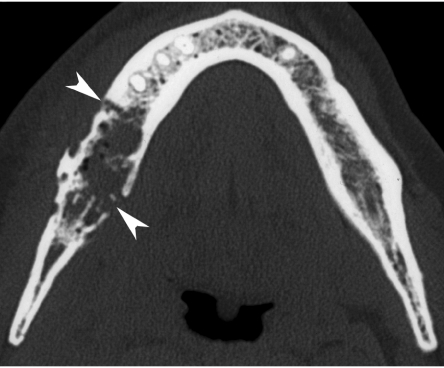
Figure 8.
Axial CT image, bone window. A patient treated 10 years earlier by external irradiation for a right-sided parotid malignancy, now presenting with oral pain and mucosal dehiscence. Extensive resorption of spongiosa in right mandibular body (compare to opposite side), and destruction of both lingual and buccal cortex, complicated by pathologic fracture (arrowheads). Intra-osseous air bubbles are seen. Histopathological study showed necrotic bone with signs of osteomyelitis.
Laryngeal necrosis
Persisting severe oedema and radionecrosis of the larynx are uncommon treatment complications, with an incidence of about 1%. The occurrence of laryngeal necrosis peaks during the 12 months following treatment, which is more or less contemporaneous with the peak incidence of tumour recurrence. However, cases of laryngeal necrosis occurring more than 10 years after radiation treatment do occur [18]. These late effects after radiation treatment are largely due to impaired vascular and lymphatic flow, caused by endothelial damage and fibrosis [19]. Cartilage itself is resistant to the effect of irradiation (see above). Cartilage changes usually occur when the perichondrium is broached by trauma or tumour, exposing the underlying irradiated cartilage to micro-organisms in the airway [20]; this may lead to infectious perichondritis, which may result in necrosis and laryngeal collapse.
Patients with laryngeal necrosis often have neck and/or ear pain, some degree of dysphagia, and anterior neck swelling. Hoarseness and dyspnea are caused by increasing oedema with impairment of vocal cord mobility, resulting in cord fixation. Inflammatory changes in the overlying skin or cutaneous fistulae may be present. Palpation of the laryngeal region usually is painful. On imaging studies, a variable degree of laryngeal soft tissue swelling is seen [13]. These soft tissue changes surrounding the necrotic cartilage can be very pronounced and may be the only visible abnormality, making the differentiation with recurrent tumour very difficult. Furthermore, laryngeal necrosis and tumour recurrence may occur simultaneously. In laryngeal necrosis, some fluid may be seen surrounding the cartilages. Cartilaginous abnormalities are often visible, but in some patients they may only become apparent on follow-up CT studies.
Necrosis of the thyroid cartilage may cause fragmentation and collapse of this cartilage with or without gas bubbles visible adjacent to it (Fig. 9). Patients with arytenoid cartilage necrosis may show anterior dislocation of this cartilage; this could be due to crico-arytenoidal joint effusion, secondary to inflammation or infection. Progressive lysis of the arytenoid is possible, showing a crumbly aspect evolving to complete disappearance [21]. Also, sloughing of the arytenoid cartilage into the airway has been described [13]. The adjacent part of the cricoid cartilage may appear sclerotic. Cricoidal sclerosis may be also seen in association with lysis of the thyroid cartilage.
Figure 9.
Patient presenting with hoarseness, dyspnea and pain in the laryngeal region, 14 years after radiotherapy for laryngeal cancer. The CT images (section B was obtained 5 mm below section A) show fragmentation of the anterior part of the thyroid cartilage (arrowhead), intra- and extralaryngeal soft tissue thickening, including a small prelaryngeal enhancing area with central fluid density, possibly corresponding to a small abscess (arrow). This study was reported as suggestive for laryngeal necrosis. A FDG–PET study at that time was negative. Conservative treatment was initiated, with gradual improvement of the symptoms; this evolution corroborates the hypothesis of (limited) laryngeal necrosis.
On MR studies, laryngeal necrosis may appear as focal swelling of the laryngeal soft tissues, loss of the normal high signal in the medullary space of ossified laryngeal cartilage on T1-weighted images, and enhancement of the affected cartilage after injection of gadolinium [22].
In some cases, the imaging findings allow better differentiation between tumour recurrence and chondronecrosis than clinical examination alone. Studies on post-radiotherapy surveillance of laryngeal and hypopharyngeal cancer [3, 7] showed that progressive cartilage alterations on post-radiotherapy CT studies predicted poor local outcome, either due to tumour recurrence or chondroradionecrosis. In these studies gas bubbles in the vicinity of cartilage and cartilage collapse were not observed in cases of tumour recurrence. Such findings can be regarded as suggestive of radionecrosis; nevertheless, a co-existent tumour recurrence may be difficult to exclude, depending on the associated tissue alterations (Fig. 10).
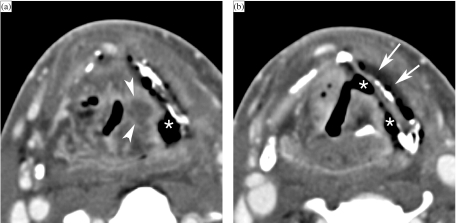
Figure 10.
Axial contrast-enhanced CT images. A patient treated 5 months earlier by irradiation for T3 supraglottic cancer, now presenting with progressive dysphagia. Laryngoscopy shows a fixed left vocal cord, suspect for tumour recurrence. (a) On a background of expected changes after radiation therapy, a centrally hypodense nodular area of soft tissue thickening is seen in the left ary-epiglottic fold (arrowheads). Furthermore, a large soft tissue defect (asterisk), connecting the left piriform sinus with the denuded thyroid cartilage lamina, is seen. The thyroid lamina appears slightly irregular, and is abutted by air. (b) At a lower level, soft tissue defects are seen to connect to the left piriform sinus, as well as to the laryngeal ventricle (asterisks). Note the fluid layer at the outer side of the thyroid cartilage (arrows). A FDG–PET study was strongly positive at the level of the supraglottis. Because of a rapidly deteriorating clinical situation, total laryngectomy was performed. Histologic examination revealed extensive tissue necrosis, but no laryngeal tumour recurrence.
It has been suggested that FDG–PET may allow differentiation between tumour recurrence and tissue necrosis as a complication of therapy [23, 24]. However, false positive results may occur as tissue necrosis may be associated with an important inflammatory reaction, increased metabolism and thus increased uptake of the tracer.
Other complications after radiotherapy
Fibrosis after radiotherapy may lead to contraction and hardening of the cervical tissues. Fibrosis-induced laryngeal dysfunction may lead to aspiration due to immobilisation of the epiglottis and/or delayed closure of the laryngeal vestibulum and glottis; secondary aspiration may be caused by ineffective clearance of the pharynx. Dysphagia may be caused by pharyngeal stenosis, which is most often seen in the neopharynx after total laryngectomy.
Fibrosis of the masticatory muscles may occur, particularly if they were involved by the cancer. The MRI signal characteristics of fibrosis are variable; often, follow-up studies are needed to rule out tumour recurrence with a sufficient degree of confidence.
Other long-term complications of radiotherapy include arteriopathy, delayed central nervous system reaction, radiation myelopathy, cranial nerve palsy and secondary tumours [25].
Conclusion
Post-treatment CT or MR imaging is of value when a recurrent tumour is suspected, to confirm the presence of such a lesion and to determine its extent. More rarely, imaging may be of use in the differentiation between tumour recurrence and treatment complications.
In patients with a high-risk profile for tumour recurrence after treatment, imaging is of value for surveillance of the patient, as an adjunct to clinical follow-up. The baseline study should be obtained about 3 to 6 months after the end of therapy. There is evidence that tumour recurrences can be detected earlier by systematic follow-up imaging.
Key points
Irradiation produces tissue changes on post-treatment imaging studies, not to be misinterpreted as evidence of persistent or recurrent disease.
Early imaging identification of recurrent tumour is facilitated by obtaining a baseline study about 3 to 6 months after therapy.
Tissue necrosis after radiotherapy may be difficult to differentiate from recurrent tumour; in such circumstances, patient management mainly depends on the combination of clinical and imaging findings, including evolution of time.
Biopsy results may be false-negative in cases of recurrent tumour; in case of contradiction between clinical, imaging and/or biopsy findings, close follow-up and repeat imaging studies are needed.
References
- 1.Mukherji SK, Mancuso AA, Kotzur IM, et al. Radiologic appearance of the irradiated larynx. Part I. Expected changes. Radiology. 1994;193:141–8. doi: 10.1148/radiology.193.1.8090882. [DOI] [PubMed] [Google Scholar]
- 2.Nömayr A, Lell M, Sweeney R, et al. MRI appearance of radiation-induced changes of normal cervical tissues. Eur Radiol. 2001;11:1807–17. doi: 10.1007/s003300000728. [DOI] [PubMed] [Google Scholar]
- 3.Pameijer FA, Hermans R, Mancuso AA, et al. Pre- and post-radiotherapy computed tomography in laryngeal cancer: imaging-based prediction of local failure. Int J Radiat Oncol Biol Phys. 1999;45:359–66. doi: 10.1016/s0360-3016(99)00149-2. [DOI] [PubMed] [Google Scholar]
- 4.Som PM, Curtin HD, Mancuso AA. An imaging-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classifications. Arch Otolaryngol Head Neck Surg. 1999;125:388–96. doi: 10.1001/archotol.125.4.388. [DOI] [PubMed] [Google Scholar]
- 5.Hermans R, Pameijer FA, Mancuso AA, et al. Laryngeal or hypopharyngeal squamous cell carcinoma: can follow-up CT after definitive radiotherapy be used to detect local failure earlier than clinical examination alone? Radiology. 2000;214:683–7. doi: 10.1148/radiology.214.3.r00fe13683. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz DL, Barker J Jr, Chansky K, et al. Postradiotherapy surveillance practice for head and neck squamous cell carcinoma—too much for too little? Head Neck. 2003;25:990–9. doi: 10.1002/hed.10314. [DOI] [PubMed] [Google Scholar]
- 7.Mukherji SK, Mancuso AA, Kotzur IM, et al. Radiologic appearance of the irradiated larynx. Part II. Primary site response. Radiology. 1994;193:149–54. doi: 10.1148/radiology.193.1.8090883. [DOI] [PubMed] [Google Scholar]
- 8.Keane TJ, Cummings BJ, O’Sullivan B, et al. A randomized trial of radiation therapy compared to split course radiation therapy combined with Mitomycin C and 5 Fluorouracil as initial treatment for advanced laryngeal and hypopharyngeal squamous carcinoma. Int J Radiat Oncol Biol Phys. 1993;25:613–8. doi: 10.1016/0360-3016(93)90006-h. [DOI] [PubMed] [Google Scholar]
- 9.Mukherji SK, Wolf GT. Evaluation of head and neck squamous cell carcinoma after treatment (editorial) AJNR Am J Neuroradiol. 2003;24:1743–6. [PMC free article] [PubMed] [Google Scholar]
- 10.de Visscher AV, Manni JJ. Routine long-term follow-up in patients treated with curative intent for squamous cell carcinoma of the larynx, pharynx and oral cavity. Arch Otolaryngol Head Neck Surg. 1994;120:934–9. doi: 10.1001/archotol.1994.01880330022005. [DOI] [PubMed] [Google Scholar]
- 11.Cooney TR, Poulsen MG. Is routine follow-up useful after combined-modality therapy for advanced head and neck cancer? Arch Otolaryngol Head Neck Surg. 1999;125:379–82. doi: 10.1001/archotol.125.4.379. [DOI] [PubMed] [Google Scholar]
- 12.Hermans R, Fossion E, Ioannides C, et al. CT-findings in osteoradionecrosis of the mandible. Skelet Radiol. 1996;25:31–6. doi: 10.1007/s002560050028. [DOI] [PubMed] [Google Scholar]
- 13.Hermans R, Pameijer FA, Mancuso AA, et al. Computed tomography findings in chondroradionecrosis of the larynx. AJNR Am J Neuroradiol. 1998;19:711–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–8. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 15.Hermans R. Imaging of mandibular osteoradionecrosis. Neuroimag Clin North Am. 2003;13:597–604. doi: 10.1016/s1052-5149(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 16.Bachmann G, Rössler R, Klett R, et al. The role of magnetic imaging and scintigraphy in the diagnosis of pathologic changes of the mandible after radiation therapy. Int J Oral Maxillofac Surg. 1996;25:189–95. doi: 10.1016/s0901-5027(96)80027-0. [DOI] [PubMed] [Google Scholar]
- 17.Chong J, Hinckley LK, Ginsberg LE. Masticator space abnormalities associated with mandibular osteoradionecrosis: MR and CT findings in five patients. AJNR Am J Neuroradiol. 2000;21:175–8. [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien PC. Tumour recurrence or treatment sequelae following radiotherapy for larynx cancer. J Surg Oncol. 1996;63:130–5. doi: 10.1002/(SICI)1096-9098(199610)63:2<130::AID-JSO11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Alexander FW. Micropathology of radiation reaction in the larynx. Ann Otol Rhinol Laryngol. 1963;72:831–41. doi: 10.1177/000348946307200316. [DOI] [PubMed] [Google Scholar]
- 20.Keene M, Harwood AR, Bryce DP, et al. Histopathological study of radionecrosis in laryngeal carcinoma. Laryngoscope. 1982;92:173–80. doi: 10.1002/lary.1982.92.2.173. [DOI] [PubMed] [Google Scholar]
- 21.De Vuysere S, Hermans R, Delaere P, et al. CT findings in laryngeal chondroradionecrosis. J Belge Radiol. 1999;82:16–8. [PubMed] [Google Scholar]
- 22.Bousson V, Marsot-Dupuch K, Lashiver X, et al. Nécrose post-radique du cartilage cricoï de: un cas inhabituel. J Radiol. 1995;76:517–20. [PubMed] [Google Scholar]
- 23.Anzai Y, Carroll WR, Quint DJ, et al. Recurrence of head and neck cancer after surgery or irradiation: prospective comparison of 2-deoxy-2-[F-18]fluoro-D-glucose PET and MR imaging diagnoses. Radiology. 1996;200:135–41. doi: 10.1148/radiology.200.1.8657901. [DOI] [PubMed] [Google Scholar]
- 24.McGuirt WF, Greven KM, Keyes JW Jr, et al. Laryngeal radionecrosis versus recurrent cancer: a clinical approach. Ann Otol Rhinol Laryngol. 1998;107:293–6. doi: 10.1177/000348949810700406. [DOI] [PubMed] [Google Scholar]
- 25.Becker M, Schroth G, Zbären P, et al. Long-term changes induced by high-dose irradiation of the head and neck region: imaging findings. RadioGraphics. 1997;17:5–26. doi: 10.1148/radiographics.17.1.9017796. [DOI] [PubMed] [Google Scholar]