Abstract
Colon cancer is the second leading cause of cancer-related death in the Western world. Approximately 80–90% of colon cancers develop in adenomas after mutations. The risk of encountering malignancy increases with the size of the adenomatous polyp. It is approximately 1% in adenomas <1 cm, and increases to 10% for adenomas 1–2 cm, and 20–53% for adenomas >2 cm. CT colonography (CTC) is a new technique, which allows, after bowel preparation and distension of the cleansed colon, to generate a volumetric display of the colon. Multi-detector CTC has a sensitivity of 93–100% and 70–83% for detection of polyps sized  10 mm and 6–9 mm, respectively. For detection of colo-rectal cancer, CTC has a sensitivity of 83–100%. CTC is especially of value in patients with incomplete colonoscopy due to stenosis or colon elongation. It reliably detects synchronous cancers proximal to occlusive colon cancers, when colonoscopy fails to evaluate the entire colon. First results of a colon cancer screening study have shown that CTC is equal or even slightly superior to conventional colonoscopy in detection of adenomatous polyps
10 mm and 6–9 mm, respectively. For detection of colo-rectal cancer, CTC has a sensitivity of 83–100%. CTC is especially of value in patients with incomplete colonoscopy due to stenosis or colon elongation. It reliably detects synchronous cancers proximal to occlusive colon cancers, when colonoscopy fails to evaluate the entire colon. First results of a colon cancer screening study have shown that CTC is equal or even slightly superior to conventional colonoscopy in detection of adenomatous polyps  8 mm. Moreover, CTC detects clinically significant extracolonic abnormalities not shown by colonoscopy. To increase the patient acceptance for wide-spread application of CTC cancer screening the issue of patient discomfort by bowel preparation and radiation exposure needs to be addressed further.
8 mm. Moreover, CTC detects clinically significant extracolonic abnormalities not shown by colonoscopy. To increase the patient acceptance for wide-spread application of CTC cancer screening the issue of patient discomfort by bowel preparation and radiation exposure needs to be addressed further.
Keywords: CT colonoscopy, virtual colonoscopy, colon cancer, polyp, screening
Introduction
Colorectal cancer is the second leading cause of cancer-related death in the Western world, where approximately 2.7–2.8% of the population die of colorectal cancer [1]. Most colorectal cancers develop within benign adenomatous polyps [2] that take on average 10 years to transform into invasive cancer. This is supported by the fact that colonoscopic polypectomy can reduce the incidence of colorectal cancer by 76–90% [3]. The risk of encountering malignancy in an adenoma depends on the size: it is 1% in adenomas <1 cm, 10% in adenomas 1–2 cm, and 20–53% for adenomas measuring >2 cm [2]. The slow growth of adenomatous polyps, which are the precursor lesions of colon cancer, gives a long time window to screen for this largely preventable disease. Several international organizations have endorsed guidelines for colorectal cancer screening programs, which include fecal occult blood testing, flexible sigmoidoscopy, colonoscopy, and double contrast barium enema [4]. However, public awareness of the disease is still low and patient compliance with screening programs has been disappointing, possibly because of poor acceptance of the diagnostic tests used. Colonoscopy and barium enema require cathartic bowel preparation before the examination and carry a small risk of perforation. In addition, colonoscopy requires sedation and recovery time after the procedure.
CT colonography (CTC) is a rapidly evolving technique in which the data of a helical or multi-slice CT examination are used to generate a volumetric display of the entire colon. CTC could provide an attractive alternative to colonoscopy, if it is able to detect precancerous adenomatous polyps with an accuracy similar to conventional colonoscopy. CTC has several advantages over conventional colonoscopy: it is noninvasive, requires no sedation, and no CTC-related perforation has been reported. To date, CTC examinations have required bowel cleansing prior to scanning, although preliminary studies have shown that bowel preparation may be eliminated in the future.
CTC examination technique
Bowel preparation
Bowel cleansing before CTC is mandatory. Several protocols for bowel reparation have been advocated for CTC, including 4 l of polyethelene glycol electrolyte solution and bisacodyl tablets (GoLytely ®, Braintree Laboratories, Braintree, USA), or a solution of sodium phosphate (Phophosoda ®, Fleet Pharmaceuticals, Lynchburg, USA) [5–7]. At our institution, 30 ml of sodium phosphate solution and bisacodyl tablets (Prepacol ®, Nicholas, Sulzbach, Germany) are used in most instances. Administration of sodium phosphate preparations may result in elevation of sodium and phosphate serum levels. It is, therefore, contraindicated in patients with congestive heart failure or renal failure. Administration of 4 l of wash-out solution, as used in conventional colonoscopy, may result in considerable amounts of residual fluid in the colon.
Fecal tagging
Fecal tagging in CTC has been used in two different ways. First, the addition of fecal tagging to cathartic bowel preparations by ingestion of barium two days before the examination improves the differentiation of polyps from barium-tagged fecal material. Residual stool is mixed with barium and can be easily differentiated from true polyps, which reduces false-positive results (Fig. 1) [8]. However, this regimen does not improve the patient’s willingness to repeat the examination. Second, fecal tagging without cathartic colon preparation uses ‘electronic’ colon cleansing, i.e. subtraction of the radiopaque colon contents from the CT image, to provide an ‘empty’ colon for endoluminal viewing [9].
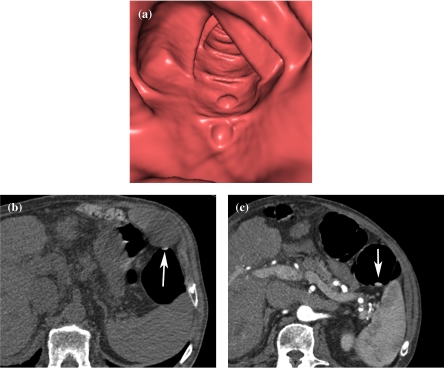
Figure 1.
Fecal tagging of stool with barium. (a) Endoluminal viewing shows two polypoid lesions. The true nature of these lesions can not be assessed with certainty. (b) Axial 2D source image of prone scan (the image is flipped to facilitate comparison with the scan in the supine position) shows fecal material to be very hyperdense (arrow), which allows easy differentiation from true polyps. (c) Axial image of supine scan shows mobility of the ‘polyp’ (arrow).
However, there are two limitations to this technique. Even with a low-fiber diet prior to the examination, it is difficult to achieve a homogenous mixture of orally ingested contrast material with fecal matter for complete subtraction of fecal material. Residual fecal material not mixed with orally ingested contrast material may be misinterpreted as a polypoid lesion. The second shortcoming of this technique is the fact that electronic subtraction of colon contents results in undesirable edge artifacts. The stair-step appearance of the colon surface hinders endoluminal viewing, making it difficult to scrutinize the scans for small polyps. However, preliminary results have been promising: polyps 1 cm and larger could be identified in 80–100%, using only dilute oral contrast for 48 h as bowel preparation [10, 11]. If clinically available, this subtraction technique may obviate the need for bowel preparation with laxatives, which would dramatically improve patient acceptance and willingness to undergo CTC for screening.
Antispasmodic drugs
In several studies on CTC, antispasmodic agents have been used for better distension of the colon and less discomfort (1 mg of glucagon or 20 mg of Buscopan) [6, 12–14]. Antispasmodic drugs have been successfully used during barium enemas for more than 25 years [15]. These drugs reduce patient discomfort, while relieving colon tonicity and providing better colon distension. However, whether intravenous muscle relaxants are necessary to achieve optimal colon distension is controversial [16]. In a study by Taylor et al., the IV administration of 20 mg of hyoscine butylbromide (Buscopan ®) resulted in better colon distension [17], whereas Bruzzi et al. did not find a beneficial effect for Buscopan, except for patients with sigmoid diverticular disease [18].
In a study by Yee et al., IV administration of glucagon did not improve colonic distension [19]. However, in that study, glucagon was administered immediately prior to colonic insufflation, although it is known that there is a delay of several minutes after IV administration of glucagon before the drug exerts the maximum effect on the colon. Thus, in general, based on personal experience and studies published on the use of pharmacologic agents in barium enemas and CTC, antispasmodic drugs are currently used in many institutions.
CTC technique
After placement of a soft-tip rectal catheter or a Foley bladder catheter (and insufflation of the balloon) in the rectum, patients are placed prone on the CT table. Room air or CO2 is then slowly insufflated. On average, 30 bulb compressions provide good colon distension [19]. Insufflation of CO2 is more expensive, but it has a much higher diffusion coefficient than room air. Therefore, CO2 is more rapidly absorbed, which results in much less crampy discomfort than room air insufflation. In our experience, insufflation of approximately 3 l of CO2 at a flow rate of 600 ml /min provides excellent colonic distension. The scout CT image is acquired to check the adequacy of distension. In general, CTC is performed in the supine and the prone position. During repositioning of the patient between the prone and supine scans, additional room air or CO2 is insufflated to compensate for leakage through the ileocecal valve or the anus. After acquisition of a second scout image, scanning is repeated.
Imaging in the prone and the supine position avoids ‘blind spots’ of collapsed bowel segments and facilitates movement of residual fecal material for better differentiation from sessile polypoid lesions [20]. With the use of both prone and supine position scanning, the rate of scans with adequate colon distension increases from 59 to 87% [6]. Distension of the sigmoid colon and rectum is better in the prone than in the supine position, whereas the supine position provides better visualization of the transverse colon. It has been shown that dual positioning improves the ability to identify colon polyps with a diameter of at least 0.5 cm because of better assessment of bowel segments that may be collapsed at either the prone or the supine position [20]. In a study of 180 patients at the Mayo Clinic, approximately 40% of additional lesions  5 mm in size, and detected with prone imaging, were located in the sigmoid or rectum, where supine imaging often has a blind spot [20] (Fig. 2).
5 mm in size, and detected with prone imaging, were located in the sigmoid or rectum, where supine imaging often has a blind spot [20] (Fig. 2).
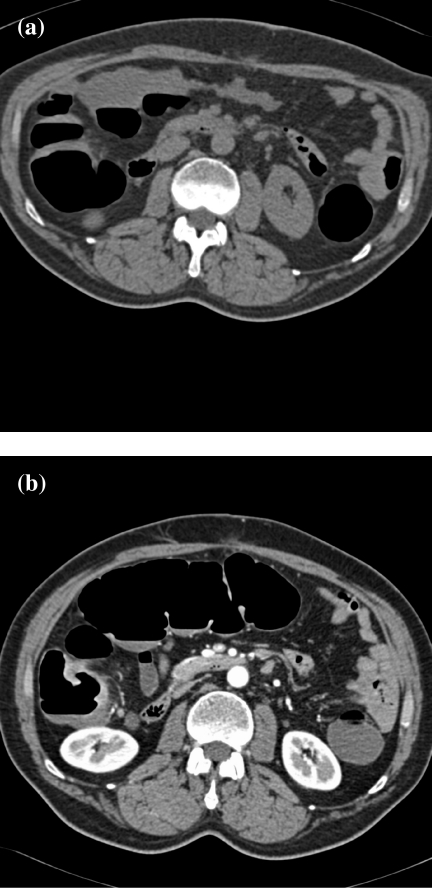
Figure 2.
Distension of the transverse colon: prone vs. supine imaging. (a) In the prone position, the transverse colon is collapsed and cannot be adequately assessed (the image is flipped to facilitate comparison with the scan in the supine position). The ascending and descending colon are adequately distended. (b) In the supine position, there is good distension of the transverse colon to assess the mucosa.
CTC in the supine and the prone position can be performed without IV contrast material [6, 7, 12, 21]. However, if a tumor or an inflammatory stricture is suspected, IV contrast material should be used. In general, the contrast-enhanced scan is performed in the supine position, unless a stricture of the sigmoid colon or rectum is suspected. The use of IV contrast material improves reader confidence in the assessment of bowel wall conspicuity and medium-sized polyps, especially in suboptimally prepared colons [22]. It is mandatory in patients with suspected colorectal cancer for staging of the disease (Fig. 3). The main limitations of the intravenous administration of contrast material are the additional costs and the, albeit small, risk of serious adverse reactions.
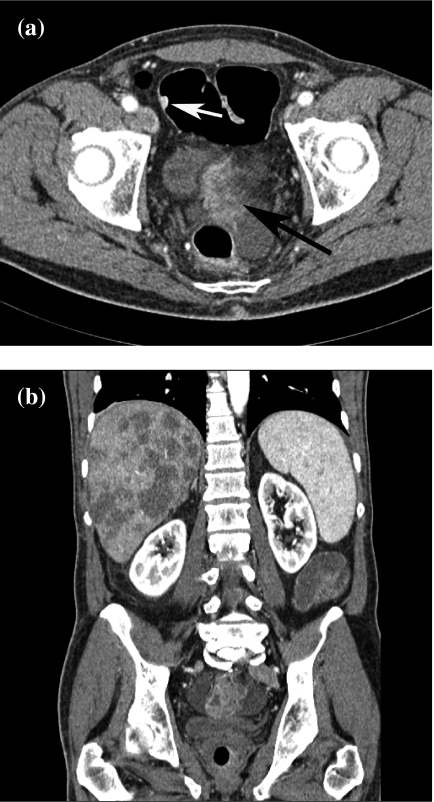
Figure 3.
Rectal cancer with liver metastases, and sigmoid polyp. (a) Source image reveals contrast-enhanced rectal cancer (black arrow). There is a small polyp in the sigmoid colon, which turned out to be an adenoma (white arrow). (b) Coronal MPR reveals multiple liver metastases.
CTC is better performed with a multi-detector CT (MDCT) scanner. The scanning and contrast injection protocol of our MDCT scanner is shown in Table 1. Near-isotropic imaging begins with a 4-row MDCT scanner with a detector configuration of 4×1 mm (minimal slice thickness of 1.25 mm), which allows scanning of the abdomen during a 30 s breath-hold. With a 16-row MDCT scanner and a detector configuration of 16×0.75 mm, scanning is completed in 11–12 s. To avoid breathing artifacts, which are more prominent in the upper abdomen, scanning is performed in a cephalo-caudal direction. Hara et al. demonstrated the superiority of MDCT over single-slice helical CTC [14]. With the use of an MDCT scanner, the rate of suboptimal colon distension decreased from 52 to 19%. Respiratory artifacts were seen in only 16% (vs. 61% in single-slice CT). With the use of thin slices, the analysis of the internal structure of small polypoid lesions is facilitated. It is easier to differentiate between polyps and sessile fecal material (Fig. 4).
Table 1.
CTC protocol for a 16-row MDCT scanner
| Manufacturer | Siemens Somatom Sensation16 | |
|---|---|---|
| Position | Prone | Supine |
| Collimation (mm) | 16×1.5 | 16×0.75 |
| Table feed/rotation (mm) | 30 | 18 |
| Slice thickness (mm) | 5+2 | 5+1 |
| Reconstruction interval (mm) | 4+1 | 4+1 |
| Rotation time (s) | 0.5 | 0.5 |
| kVp | 120 | 120 |
| mAs | 50 | 100 |
| Scan direction | Cranio-caudal | Cranio-caudal |
| Contrast material | Unenhanced | 150 ml |
| Flow rate (ml/s) | — | 4 |
| Scan delay (s) | — | 50 |
| Matrix size | 512×512 | 512×512 |
In case of suspected tumor in the recto-sigmoid colon, the first scan (unenhanced) is performed in the supine position, and the contrast-enhanced scan in the prone position for better distension of recto-sigmoid colon.
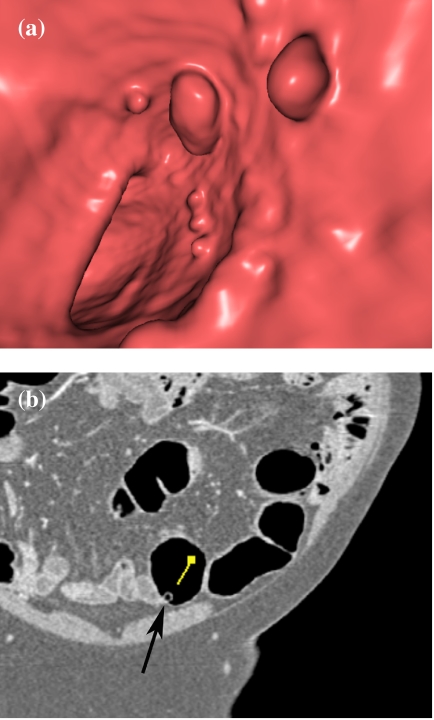
Figure 4.
Analysis of a small polypoid lesion: identification of sessile fecal material by texture analysis. (a) The 3D endoluminal view shows polypoid lesions, suspicious for true polyps. (b) The 2D source image reveals a tiny air bubble in the polypoid lesion (arrow), typical of stool (arrow).
Image analysis
In general, the axial 2D source images generated during prone and supine scanning are used for analysis. Coronal or sagittal multi-planar reformations (MPRs) are helpful to differentiate between true polypoid lesions and bulbous folds. All vendors now offer 3D software, which provides an endoluminal view of the colon. A path is automatically generated to lead the endoluminal ‘camera’ through the colon (Fig. 5). The route through the colon is indicated and the region of polyps can be marked to facilitate the orientation of the colonoscopist. However, there is considerable variance between the clinical applicability of 3D endoluminal view software packages currently available. In a study by Pickhardt [23], endoluminal volume rendering and navigational capabilities were compared. The V3D-Colon software (Viatronix, Stony Brook, USA) provided better polyp conspicuity and endoluminal navigation than other software packages (Navigator, General Electric; Vitrea 2, Vital Images).
Figure 5.
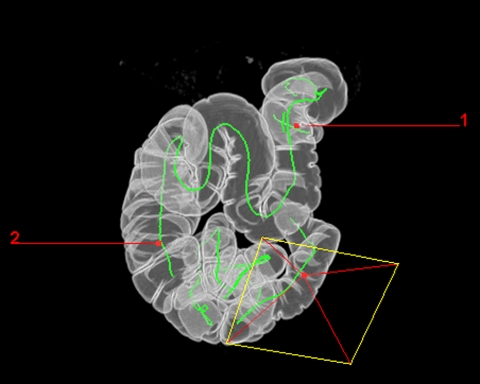
Surface-rendered image of the entire colon. The path of the virtual colonoscopic ‘camera’ is demonstrated. It is possible to mark lesions found to facilitate orientation of the colonoscopist during endoscopic polypectomy.
Radiation exposure
In a survey by van Gelder et al. on radiation exposure during CTC in 13 different centers, the median effective dose of CTC, performed in the supine and the prone position, was 8.8 mSv for standard mAs settings [24]. Radiation exposure with CTC at standard mAs settings for abdominal CT studies is too high to be acceptable for a screening procedure. Recent studies have successfully attempted to reduce radiation exposure by decreasing mAs levels to as low as 50 mAs without decreasing polyp detectability [7]. In one of these studies, the estimated effective dose of dual positioning CTC would be 5.0 mSv for men and 7.8 mSv for women. These results compare favorably with the mean effective dose of 9.3 mSv for barium enema examinations, as reported in a recent multi-center trial in Finland [25]. In the study of van Gelder et al., the feasibility of dose reduction from 100 mAs to 50 mAs and 30 mAs was evaluated [24]. Although image quality decreased significantly with increasing image noise at lower mAs levels, the polyp detection rate was unchanged and the effective radiation dose was reduced to 3.6 mSr.
The issue of radiation exposure is of growing concern to patients. In a much-disputed recent study in the Lancet, 0.6–1.8% of the cumulative risk of cancer to the age of 75 was attributed to diagnostic X-ray exposure [26, 27]. However, the estimated risk of inducing cancer with CTC is approximately 0.02% for a 50-year-old individual and lower for older people [24].
Patient acceptance
To date, most research on CTC has focused on its technical performance to reliably detect and exclude the presence of adenomatous polyps in the colon. However, for CTC to win the ‘competition’ with conventional colonoscopy in colorectal cancer screening, CTC must be more convenient and acceptable to patients than colonoscopy [28]. Patient compliance is inversely related to the unpleasant effects of cathartic bowel preparation prior to the examination, the discomfort during the study, and the fear of what might be found at the study. In a study by Svensson et al. [29], a majority rated colonoscopy as more difficult than CTC (49 vs. 22%). Despite the fact that IV sedation is routinely given during colonoscopy, colonoscopy was rated as more unpleasant by 54% (vs. 22% for CTC).
In a study by Taylor et al. [30], patient acceptance of MDCTC, barium enema, flexible sigmoidoscopy, and colonoscopy was compared. Not surprisingly, patient acceptance of the barium enema was significantly worse than with any other test. CTC was better tolerated than colonoscopy, but overall satisfaction was greater with colonoscopy, possibly because it is perceived as a ‘definitive’ test, which provides diagnostic information and therapy in one setting. Both for CTC and conventional colonoscopy, bowel preparation is rated uncomfortable by approximately 90% of patients. Patients’ willingness to undergo frequent screening was greater for CTC than for colonoscopy [31].
Detection of polyps and cancer
In the past few years, the typical imaging features of colorectal lesions and common pitfalls at CTC have been described in several studies [7, 16, 32]. With CTC, colonic polyps appear as murally-based soft tissue nodules, sometimes with a stalk, on axial 2D source images. During endoluminal viewing, these polyps appear as intraluminal filling defects (Fig. 6). CTC is unable to distinguish between hyperplastic and adenomatous polyps, with only the latter being the target lesions in colorectal cancer screening. CTC can quite reliably distinguish between polyps and fecal residue. Stool moves in position between supine and prone scanning (Fig. 1). Even fecal material adherent to the colonic wall can be identified by CTC, because the internal structure of stool with miniscule air bubbles is diagnostic (Fig. 4). Fecaliths retained within diverticula may protrude into the colon lumen and mimic polyps at endoluminal viewing during CTC. However, 2D source images reveal the presence of diverticular disease and the true nature of the polypoid lesion. Likewise, the nature of lipomas is readily identified when axial images with soft tissue window settings are used. It should be emphasized, however, that endoluminal viewing must always be supplemented by careful analysis of the axial images in lung and soft tissue window settings to avoid false-positive diagnoses.
Figure 6.
Image viewing of a polyp in 2D and 3D. (a) Axial source image reveals the polypoid lesion to be contrast-enhanced, indicative of a true polyp (arrow). (b) Endoluminal viewing shows a polyp.
A number of studies have been performed in which CTC, with single-detector helical and multi-detector CT scanners, and conventional colonoscopy were compared with regard to detection of colorectal polyps and cancer (Table 2). With helical CTC, the sensitivity for detection of polyps at least 10 mm in diameter was 75–91% (Table 2). Sensitivity rates dramatically dropped, to as low as 33%, for polyps 6–9 mm in size. Not surprisingly, polyps smaller than 5 mm (the actual collimation used in most single-slice CTC studies) were rarely detected. With MDCT, the collimation can be reduced from 5 to 1–2 mm, which improves detection rates for small polypoid lesions. With MDCTC, sensitivity rates of more than 90% for polyps 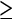 10 mm and more than 70% for polyps 6–9 mm are consistently reported (Table 2). The reasons for the failure to identify lesions at CTC include lesions being perceived as folds and flat adenomas, which are not raised by more than 1 mm from the colonic surface.
10 mm and more than 70% for polyps 6–9 mm are consistently reported (Table 2). The reasons for the failure to identify lesions at CTC include lesions being perceived as folds and flat adenomas, which are not raised by more than 1 mm from the colonic surface.
Table 2.
Performance of CTC for detection of polyps 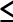 5, 6–9, and
5, 6–9, and 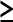 10 mm and carcinomas
10 mm and carcinomas
| Author | Year | Technique | Number of patients | Sensitivity (%) |
|||
|---|---|---|---|---|---|---|---|
Polyps 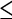 5 mm 5 mm |
Polyps 6–9 mm | Polyps 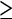 10 mm 10 mm |
Colorectal carcinomas | ||||
| Hara | 1997 | Helical | 70 | 25–27 | 56–69 | 67–73 | NA |
| Dachman | 1998 | Helical | 44 | 15 | 33 + | 83 # | NA |
| Fenlon | 1999 | Helical | 100 | 55 | 83 | 91 | NA |
| Fletcher | 2000 | Helical | 180 | NA | 47* | 75 | NA |
| Kay | 2000 | Helical | 38 | NA | 38* | 91 | NA |
| Yee | 2001 | Helical | 300 | 59 | 80* | 90 | 8/8 (100) |
| Macari | 2002 | Multi-detector | 105 | 12 | 70 | 93 | NA |
| Iannaccone | 2003 | Multi-detector | 158 | 51 | 83 | 100 | 100 |
| Johnson | 2003 | Helical, multi-detector | 703 | NA | 54* | 63 | NA |
| Taylor | 2003 | Multi-detector | 54 | 44 | 75 | 100 | 5/6 (83) |
| Pickhardt | 2003 | Multi-detector | 1233 | NA | NA | 94 | 2/2 (100) |
NA, not applicable. Polyp size category: *5–9 mm; +5–8 mm; #>8 mm.
Recently, a study about the value of CTC for colorectal neoplasia screening was published in the New England Journal of Medicine [21]. In this study, 1233 asymptomatic adults underwent same-day CTC and colonoscopy. Ninety-seven percent of the patients were considered to be at average risk for colorectal cancer. The performance of CTC to detect adenomas was superior to optical colonoscopy for adenomas 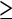 10 mm (92 vs. 88%). Only when all adenomas
10 mm (92 vs. 88%). Only when all adenomas 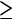 6 mm were included in the analysis did colonoscopy rate better than CTC (90 vs. 86%). There were two colorectal cancers, both of which were found at CTC. One 11 mm malignant polyp was missed by optical colonoscopy and only found at reevaluation. Moreover, five extracolonic cancers (0.4%) were diagnosed by CT.
6 mm were included in the analysis did colonoscopy rate better than CTC (90 vs. 86%). There were two colorectal cancers, both of which were found at CTC. One 11 mm malignant polyp was missed by optical colonoscopy and only found at reevaluation. Moreover, five extracolonic cancers (0.4%) were diagnosed by CT.
In a CTC colorectal cancer screening study by Edwards et al. [33], a participation rate of 28.4% was achieved. CTC yielded a positive predictive value of 0.73 for polyps and 0.55 for adenomas/carcinomas. This means that in 73% with a positive finding at CTC, a polypoid lesion was subsequently detected by colonoscopy, and in 55% of patients with positive CTC, an adenoma/carcinoma was detected.
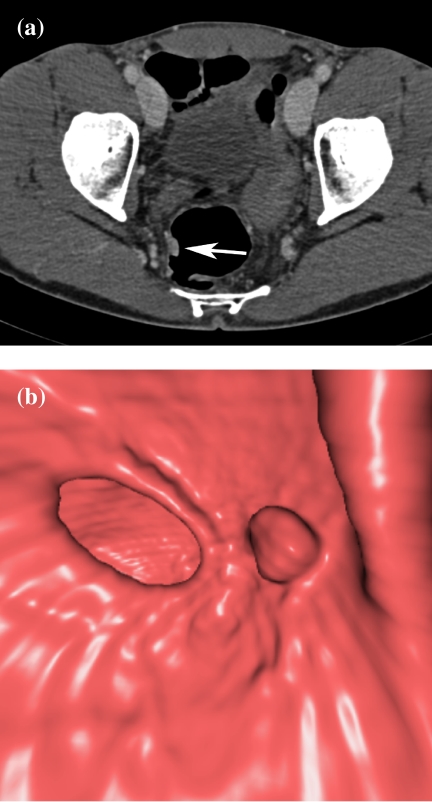
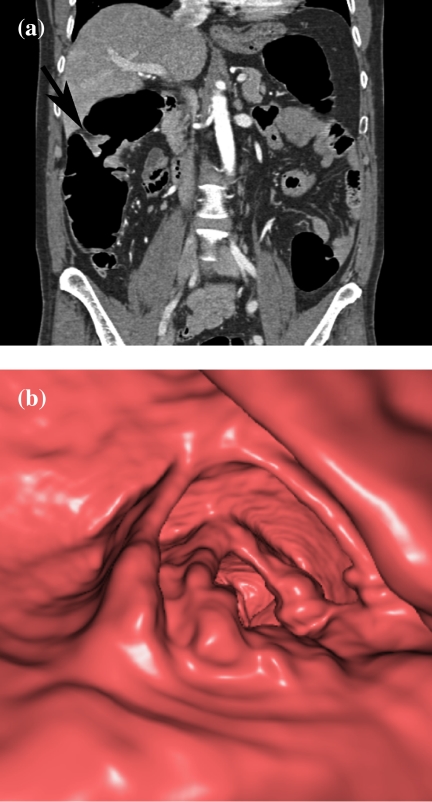
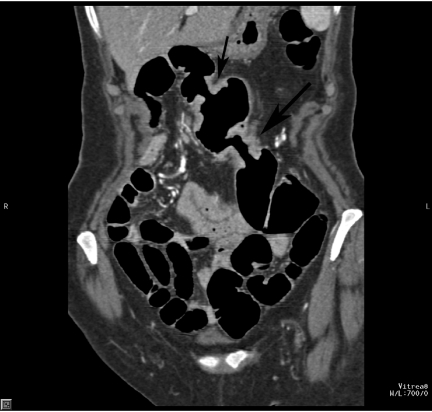
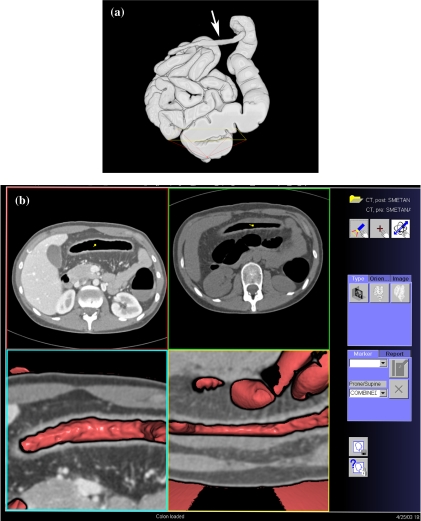
CTC is especially useful in patients with incomplete colonoscopy (Fig. 7). In the studies of Fenlon et al. on 29 patients with distal occlusive cancers, which could not be traversed endoscopically, CTC revealed synchronous proximal cancers in three patients [34] (Fig. 8). Neri et al. assessed 29 patients with incomplete endoscopy, but strong clinical suspicion of colorectal cancer. In their study, CTC detected 10 colorectal cancers and three synchronous cancers missed by colonoscopy [35]. CTC shows the morphology of stricture to a greater detail than barium enema. It not only depicts the mucosal surface, but also mural thickening and the extramural extent of disease, which helps in the differentiation of strictures of other causes (Fig. 9).
Figure 7.
Detection of stenotic cancer in a patient with polyposis. (a) Coronal MPR shows a stenotic cancer of the right flexure with a typical apple-core appearance (arrow). (b) Endoluminal view shows stenotic cancer.
Figure 8.
Detection of synchronous second cancer in a patient with incomplete colonoscopy. The coronal MPR shows a stenotic cancer in the transverse colon (large arrow), which could not be passed during colonoscopy. There is a second tumor in the proximal transverse colon (small arrow).
Figure 9.
Stricture in Crohn’s disease. (a) Surface-rendered image shows long and smooth stricture of the transverse colon (arrow) in a patient with Crohn’s disease. (b) 3D viewing of the surface and morphology of the stricture.
In conclusion, MDCTC is a robust technique and an effective tool for the detection of colorectal adenomas and carcinomas. To win over public opinion and increase patient acceptance of CTC colon cancer screening, the issue of minimizing patient discomfort by bowel preparation and radiation exposure during CTC is of the utmost importance, probably even more important than technical refinements of the study itself.
References
- 1.Weitz J, Schalhorn A, Kadmon M, Eble MJ, Herfarth C. Kolon-und Rektumkarzinom. In: Hiddemann W, Huber H, Bartram C, editors. Die Onkologie, Teil 2. Berlin: Springer; 2004. pp. 875–932. [Google Scholar]
- 2.Rustgi A, Crawford JM eds. Gastrointestinal Cancers. Edinburgh: Saunders; 2003. p. 435. [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN , et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Heiken JP. Colon cancer screening. Cancer Imaging. 2001;2:10–14. [Google Scholar]
- 5.Fenlon HM, Nunes DP, Schroy PC III, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med. 1999;341:1496–503. doi: 10.1056/NEJM199911113412003. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, Lu DSK, Hecht JR, Kadell BM. CT colonography: value of scanning in both the supine and prone positions. Am J Roentgenol. 1999;172:595–9. doi: 10.2214/ajr.172.3.10063842. [DOI] [PubMed] [Google Scholar]
- 7.Macari M, Bini EJ, Xue X, et al. Colorectal neoplasms: prospective comparison of thin-section low-dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology. 2002;224:383–92. doi: 10.1148/radiol.2242011382. [DOI] [PubMed] [Google Scholar]
- 8.Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Van Holsbeck BG. Dietary fecal tagging as a cleansing method before CT colonography: initial results—polyp detection and patient acceptance. Radiology. 2002;224:393–403. doi: 10.1148/radiol.2241011222. [DOI] [PubMed] [Google Scholar]
- 9.Zalis ME, Hahn PF. Digital subtraction bowel cleansing in CT colonography. Am J Roentgenol. 2001;176:646–8. doi: 10.2214/ajr.176.3.1760646. [DOI] [PubMed] [Google Scholar]
- 10.Callstrom MR, Johnson CD, Fletcher JG , et al. CT colonography without cathartic preparation: feasibility study. Radiology. 2001;219:693–8. doi: 10.1148/radiology.219.3.r01jn22693. [DOI] [PubMed] [Google Scholar]
- 11.Zalis ME, Perumpillichira J, Del Frate C, Hahn PF. CT colonography: digital subtraction bowel cleansing with mucosal reconstruction—initial observations. Radiology. 2003;226:911–7. doi: 10.1148/radiol.2263012059. [DOI] [PubMed] [Google Scholar]
- 12.Dachman AH, Kuniyoshi JK, Boyle CM , et al. CT colonography with three-dimensional problem solving for detection of colonic polyps. Am J Roentgenol. 1998;171:989–95. doi: 10.2214/ajr.171.4.9762982. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher JG, Luboldt W. CT colonography and MR colonography: current status, research directions and comparison. Eur Radiol. 2000;10:786–801. doi: 10.1007/s003300051006. [DOI] [PubMed] [Google Scholar]
- 14.Hara AK, Johnson CD, MacCarty RL, Welch TJ, McCollough CH, Harmsen WS. CT colonography: single- versus multi-detector row imaging. Radiology. 2001;219:461–5. doi: 10.1148/radiology.219.2.r01ma28461. [DOI] [PubMed] [Google Scholar]
- 15.Skucas J. The use of antispasmodic drugs during barium enemas. Am J Roentgenol. 1994;162:1323–5. doi: 10.2214/ajr.162.6.8191992. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SA, Halligan S, Bartram CI. CT colonography: methods, pathology and pitfalls. Clin Radiol. 2003;58:170–90. doi: 10.1016/s0009-9260(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SA, Halligan S, Goh V, et al. Optimizing colonic distention for multi-detector row CT colonography: effect of hyoscine butylbromide and rectal balloon catheter. Radiology. 2003;229:99–108. doi: 10.1148/radiol.2291021151. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzi JF, Moss AC, Brennan DD, MacMathuna P, Fenlon HM. Efficacy of IV Buscopan as a muscle relaxant in CT colonography. Eur Radiol. 2003;13:2264–70. doi: 10.1007/s00330-003-2012-7. [DOI] [PubMed] [Google Scholar]
- 19.Yee J, Hung RK, Akerkar GA, Wall SD. The usefulness of glucagon hydrochloride for colonic distension in CT colonography. Am J Roentgenol. 1999;173:169–72. doi: 10.2214/ajr.173.1.10397121. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher JG, Johnson CD, Welch TJ , et al. Optimization of CT colonography technique: prospective trial in 180 patients. Radiology. 2000;216:704–11. doi: 10.1148/radiology.216.3.r00au41704. [DOI] [PubMed] [Google Scholar]
- 21.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 22.Morrin MM, Farrell RJ, Kruskal JB, Reynolds K, McGee JB, Raptopoulos V. Utility of intravenously administered contrast material at CT colonography. Radiology. 2000;217:765–71. doi: 10.1148/radiology.217.3.r00nv42765. [DOI] [PubMed] [Google Scholar]
- 23.Pickhardt PJ. Three-dimensional endoluminal CT colonography (virtual colonoscopy): comparison of three commercially available systems. Am J Roentgenol. 2003;181:1599–606. doi: 10.2214/ajr.181.6.1811599. [DOI] [PubMed] [Google Scholar]
- 24.van Gelder RE, Venema HW, Serlie IWO , et al. CT colonography at different radiation dose levels: feasibility of dose reduction. Radiology. 2002;224:25–33. doi: 10.1148/radiol.2241011126. [DOI] [PubMed] [Google Scholar]
- 25.Lampinen JS, Rannikko S. Patient specific doses used to analyse the optimum dose delivery in barium enema examinations. Br J Radiol. 1999;72:1185–95. doi: 10.1259/bjr.72.864.10703476. [DOI] [PubMed] [Google Scholar]
- 26.Berrington de Gonzalez A, Derby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–51. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 27.Herzog P, Rieger CT. Risk of cancer from diagnostic X-rays. Lancet. 2004;363:340–1. doi: 10.1016/S0140-6736(04)15470-6. [DOI] [PubMed] [Google Scholar]
- 28.Heiken JP. CT colonography (‘virtual colonoscopy’): is it ready for colorectal cancer screening? Cancer Imaging. 2003;3:146–8. [Google Scholar]
- 29.Svensson MH, Svensson E, Lasson A, Hellstrom M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology. 2002;222:337–45. doi: 10.1148/radiol.2222010669. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SA, Halligan S, Saunders BP, Bassett P, Vance M, Bartram CI. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. Am J Roentgenol. 2003;181:913–21. doi: 10.2214/ajr.181.4.1810913. [DOI] [PubMed] [Google Scholar]
- 31.Gluecker TM, Johnson CD, Harmsen WS, Harris AM, Wilson LA, Ahlquist DA. Colorectal cancer screening with CT colonography, colonoscopy, and double-contrast barium enema examination: prospective assessment of patient perceptions and preferences. Radiology. 2003;227:378–84. doi: 10.1148/radiol.2272020293. [DOI] [PubMed] [Google Scholar]
- 32.Johnson CD, Dachman AH. CT colonography: the next colon screening examination? Radiology. 2000;216:331–41. doi: 10.1148/radiology.216.2.r00au47331. [DOI] [PubMed] [Google Scholar]
- 33.Edwards JT, Mendelson RM, Fritschi L, et al. Colorectal neoplasia screening in average-risk asymptomatic subjects: community-based study. Radiology. 2004;230:459–64. doi: 10.1148/radiol.2302021422. [DOI] [PubMed] [Google Scholar]
- 34.Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology. 1999;210:423–8. doi: 10.1148/radiology.210.2.r99fe21423. [DOI] [PubMed] [Google Scholar]
- 35.Neri E, Giusti P, Barttolla L, et al. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology. 2002;223:615–9. doi: 10.1148/radiol.2233010928. [DOI] [PubMed] [Google Scholar]