Introduction
Multiple myeloma is the third most common form of haematological malignancy after non-Hodgkin’s lymphoma and leukaemia. There are approximately 14600 new cases per annum in the USA and almost 3500 in the UK [1–3]. Certain chemical exposures are reported to increase the risk and an increased incidence is seen in agricultural workers [4]. The condition is rare in Asians but roughly twice as common in Afro-Caribbean ethnic groups compared with Caucasians.
Multiple myeloma is characterised by uncontrolled proliferation of a clone of plasma cells within the bone marrow and should not be confused with the condition known as monoclonal gammopathy of undetermined significance (MGUS). In this disease the serum paraprotein level is <3 g/dl with no evidence of myeloma or a related disorder. The risk of MGUS progressing to myeloma or a related condition is low: 16% at 10 years, 33% at 20 years and 40% at 25 years [5]. A detailed breakdown of the presenting features is given in Table 1.
Table 1.
Presenting features [6]
| Presenting feature | % |
|---|---|
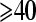 years old years old |
98 |
| Proteinuria | 88 |
| Monoclonal heavy chain on serum | |
| immunoelectrophoresis | 83 |
| Skeletal radiographic abnormalities | 79 |
| Spike on serum protein electrophoresis | 76 |
| Spike on urinary electrophoresis | 75 |
| Bone pain | 68 |
| Anaemia | 62 |
| Men | 61 |
| Renal insufficiency | 55 |
| Bence–Jones proteinuria | 49 |
| Hypercalcaemia | 30 |
| Hepatomegaly | 21 |
Diagnosis is based on laboratory and radiographic findings and depends on three abnormal results:
Bone marrow containing more than 15% plasma cells (normally no more than 4% of the cells in the bone marrow are plasma cells).
Generalised osteopaenia and/or lytic bone deposits on plain film radiography.
Blood serum and/or urine containing an abnormal protein.
Normal blood contains a mixture of immunoglobulins (IgM, IgG, IgA, IgD, IgE). After protein separation (electrophoresis) these show up as various bands or spikes. When most or all of the immunoglobulin present is of one type this will produce a single band on separation. The presence of such a band is called a monoclonal spike or M band.
Chemotherapy is indicated for management of symptomatic myeloma. Autologous transplantation is the treatment of choice for patients aged under 60 years. Median survival with conventional therapy is about 3 years, whilst stem-cell transplant can achieve a median survival of more than 5 years [7]. Overall, the prognosis is poor with the most recent statistics from the USA showing a relative 5-year survival of 29% [2]. Death results from bacterial infection, renal insufficiency and thromboembolism.
Staging
The staging system devised by Durie and Salmon is the most widely used [8] (Table 2). This is based on the serum concentration of haemoglobin, calcium and paraprotein, urinary Bence–Jones protein excretion and the number of skeletal lesions seen on plain radiographs. However, other groups have proposed new systems to more accurately and simply stage and/or classify myeloma patients into prognostic categories. Whilst none has gained universal acceptance two are under active consideration both of which recognise the serum β2 microglobulin level as the core measurement [9, 10].
Table 2.
Durie–Salmon staging system [8]
| Stage | Criteria | Cell mass |
|---|---|---|
| I | All of the following: | Low |
| Haemoglobin > 10 g / 100 ml | <0.6×1012 cells / mm 2 | |
| Normal serum calcium < 12 mg / 100 ml | ||
| Normal bone structure or solitary bone lesion only on radiography | ||
| Low M component production rates | ||
| IgG < 5 g / 100 ml | ||
| IgA < 3 g / 100 ml | ||
| Urine light chain M component on electrophoresis < 4 g / 24 h | ||
| II | Fitting neither stage I nor stage III | Intermediate |
| III | One or more of the following: | High |
| Haemoglobin < 8.5 g / 100 ml | >1.2×1012 cells / mm 2 | |
| Serum calcium > 12 mg / 100 ml | ||
| Advanced lytic bone lesion | ||
| High M component production rates | ||
| IgG > 7 g / 100 ml | ||
| IgA > 5 g / 100 ml | ||
| Urine light chain M component on electrophoresis > 12 g / 24 h | ||
Subclassifications: A = relatively normal renal function (serum creatinine value < 20 mg/100 ml [175 mmol/l]); B = abnormal renal function (serum creatinine value > 20 mg/100 ml [175 mmol/l]).
Radiology
Radiology plays an important role in staging, monitoring treatment response, detection of relapse and assessing complications. The various imaging techniques employed and their associated findings are described more fully below.
Plain film radiography
Almost 80% of patients with multiple myeloma will have radiological evidence of skeletal involvement and the skeletal survey remains the best method of identifying lytic deposits within bone (see Table 3) [6, 11]. The most common sites include the vertebrae (66% of patients), ribs (45%), skull (40%), pelvis (30%) whereas involvement of the distal bones is unusual (Fig. 1).
Table 3.
Multiple myeloma vs. bone metastases
| Radiological | Multiple | Bone |
|---|---|---|
| features | myeloma | metastases |
| Involvement of intervertebral discs | Yes | No |
| Involvement of mandible | Yes | No |
| Involvement of vertebral pedicles | No | Yes |
| Associated paraspinal soft tissue mass | Yes | No |
| Isotope bone scan | Frequently | Frequently |
| negative | positive | |
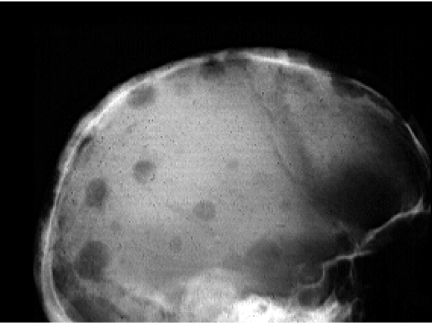
Figure 1.
Plain radiograph of skull (lateral view) demonstrating multiple lytic deposits.
Myeloma lesions are sharply defined, small lytic areas (average size 20 mm) of bone destruction with no reactive bone formation. Although myeloma arises within the medulla, disease progression may produce infiltration of the cortex, invasion of the periosteum and large extraosseous soft tissue masses. The pattern of destruction may be geographic, moth eaten or permeated. Pathological fractures are common [12].
Generalised osteopaenia may be the only bone manifestation of myeloma in up to 15% of patients. Vertebral body collapse is the usual manifestation of this subtype which should not be confused with non-myelomatous osteoporosis which occurs in many older patients. Normal bone surveys are noted in 10% of myeloma patients though this has not always been associated with improved survival [13].
Radionuclide imaging
In multiple myeloma the osteoblastic response to bone destruction is negligible. The bone scan is often therefore normal or may show areas of decreased uptake (photopaenia). Most studies have shown that the sensitivity of skeletal scintigraphy for detecting individual deposits ranges from 40 to 60% [14, 15]. However, skeletal scintigraphy may be helpful in evaluating areas not well visualised on plain film radiographs such as the ribs and the sternum.
99mTechnetium methoxyisobutylisonitrile (99mTc-MIBI) has been shown to be superior to plain film radiography and skeletal scintigraphy in detecting bone and bone marrow involvement [16–19]. Different patterns of 99mTc-MIBI uptake have been described with multiple myeloma (negative, diffuse, focal, combined focal and diffuse) and semiquantitative evaluation of these patterns showed a significant correlation with clinical status and stage of the disease [20]. A negative scan in a patient with multiple myeloma indicates early stage disease or post-treatment remission while the presence of focal uptake and/or intense diffuse bone marrow uptake suggests an advanced stage of active disease. A subsequent follow-up study involving 22 patients showed a significant correlation between the scintigraphic findings and clinical status post chemotherapy [21].
Thallium-201 has also been described in multiple myeloma, but due to limitations of the isotope its use has not been widespread nor has it been shown to be superior to 99mTc-MIBI [22, 23].
Positron emission tomography using the glucose analogue fluorine-18 fluorodeoxyglucose (FDG–PET) has also proved useful. In one series comprising 28 patients, PET was true positive in almost 93% of the radiographically documented osteolytic deposits and demonstrated a greater extent of disease than plain film radiography in 61% of patients [24]. Other studies have demonstrated its reliability in detecting active myeloma both within bone and at extramedullary sites and its ability to differentiate between new active disease and inactive (treated) sites [25–27]. Interestingly, a study just published comparing MIBI with PET indicated that MIBI identified more disease sites than PET [28]. No study has been published to date using integrated PET–CT (computed tomography) imaging.
Cross-sectional imaging
A wide range of findings have been described in CT of myeloma. These include sharp, lytic foci of small and relatively homogeneous size with no sclerotic rim, diffuse faint osteolysis, an angioma-like appearance due to the presence of thickened vertical trabeculae and expansile deposits [29, 30]. CT can accurately depict the extent of associated soft tissue masses and can direct needle biopsy for histological diagnosis. Myelomatous marrow often shows an abnormally high attenuation value compared with normal marrow. Discrete interruption of the cortical contour may be seen.
Recent advances in X-ray tube technology with high heat storage capacities enable examination of the whole spine to be undertaken in less than 1 min. A recent study using multidetector CT (MDCT) in patients with stage III myeloma provided more detailed information on the risk of vertebral fractures compared with plain film radiography and magnetic resonance imaging (MRI). Upward stage migration occurred in 17% of patients [31]. It is likely that there will be an increasing role for this technique in patients who are severely disabled or who are unable to undergo MRI examination.
Magnetic resonance imaging
Bone deposits have been shown by MRI in about 50% of asymptomatic myeloma patients with normal plain radiographs [32]. Nonetheless, despite the increased availability of MRI, plain film radiography retains an important role in myeloma and remains on the list of recommended investigations [3].
Sagittal studies of the spine enable screening of a high proportion of haematopoietic marrow and detection of any potential threat to the spinal cord. Additional coronal images of the pelvis and proximal femora enable evaluation of about an extra one-third of red marrow in an adult. These images may enable detection of deposits potentially at risk of fracture. The clinical benefit of total body MRI has not yet been fully evaluated in myeloma.
The imaging patterns in multiple myeloma can be classified as normal, focal, diffuse and variegated [33, 34]. Normal marrow is present on MRI at diagnosis in 50–75% of patients with early untreated (stage I) myeloma and in about 20% of patients with advanced and treated (stage III) disease.
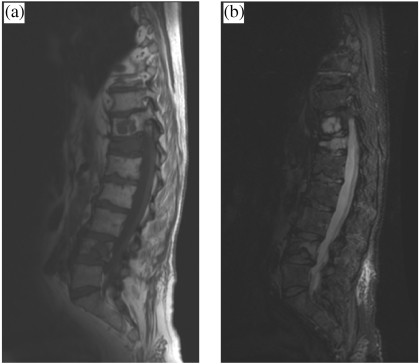
The focal pattern consists of localised areas of decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images (Fig. 2). Myelomatous deposits are generally sharply demarcated on a background of an otherwise normal appearing bone marrow. Homogeneous enhancement occurs on T1-weighted images following intravenous contrast injection.
Figure 2.
(a) Sagittal T1-weighted and (b) short inversion time inversion recovery (STIR) MR images of lower thoracic and lumbar spine showing focal and diffuse myelomatous infiltration affecting multiple vertebrae with marked compression affecting T12.
The diffuse pattern is characterised by a diffuse and homogeneous decrease in marrow signal intensity which becomes identical to or lower than that of adjacent inter-vertebral discs on a T1-weighted image and on a T2-weighted image by a diffuse or patchy increase in signal intensity. Marked enhancement is usually seen on T1-weighted images following intravenous contrast [35].
The variegated pattern is characterised by the presence of multiple foci of low signal intensity on T1-weighted images, intermediate to high signal intensity on T2-weighted images and enhancement following intravenous contrast T1-weighted images. This pattern is seen almost exclusively in an early disease [36].
These patterns of marrow involvement do not seem to correlate with the interstitial, nodular and diffuse patterns of marrow infiltration seen at microscopy. However, they show a positive correlation with some laboratory parameters. Patients with the normal and variegated patterns tend to have a lower tumour burden than those with the focal and diffuse marrow involvement patterns. Higher cellularity, higher plasmacytosis and more severe signs of bone failure are usually found in patients with the diffuse pattern [37].
The lack of specificity of the MRI patterns should be noted. The focal and diffuse patterns may be observed in both metastatic disease from primary solid tumours and in other haematological malignancies, especially lymphoma and leukaemia. Differentiation between red marrow hyperplasia secondary to anaemia, infection, malignant or treated marrow infiltration can be extremely difficult.
Compression fractures in multiple myeloma
Several criteria exist for differentiating benign from malignant vertebral body compression fractures (Table 4). However, these should be applied with caution to patients with multiple myeloma as normal signal intensity within a compressed vertebral body on spinal MR images does not preclude the diagnosis of multiple myeloma. In a study of 224 vertebral fractures in patients with known multiple myeloma, Lecouvet et al. found that 67% appeared benign on MRI and 38% of their 37 patients had benign fractures only at diagnosis [38].
Table 4.
MRI criteria for the differential diagnosis of benign vs. malignant vertebral fractures [41]
| Osteoporotic fractures | Malignant fractures | |
|---|---|---|
| Marrow signal | Normal on all sequences (old fracture) | Diffusely low on T1-weighted images |
| Band like low SI adjacent to fracture (acute) | High or heterogeneous on T2-weighted images | |
| Normal SI preserved opposite the fractured end plate | Round or irregular foci of marrow replacement | |
| Posterior elements involved | ||
| Soft tissues/epidural involvement | ||
| Contrast enhancement | Homogeneous ‘return to normal’ SI after injection | High or heterogeneous |
| Vertebral contours | Retropulsion of a posterior bone fragment (often postero-superior) | Convex posterior cortex |
In patients with osteoporotic or post-traumatic vertebral compression of recent onset, MRI will usually show signal alteration that parallels one of the end plates, involves less than half of the vertebral body, does not extend to the pedicles and enhances homogeneously following intravenous contrast. Diffusion-weighted MRI may also prove to be a useful method to apply to the differential diagnosis of compression fractures [39].
Patients being treated for multiple myeloma may suffer acute back pain secondary to vertebral body collapse even after effective chemotherapy due to resolution of the tumour mass that was supporting the bony cortex. Thirty-five new vertebral compression fractures were discovered on post-treatment MR images of 29 patients with multiple myeloma in remission [40]. In another study, 131 vertebral compression fractures appeared in 37 patients with multiple myeloma after the onset of therapy [38]. Conversely, progression of disease may also be responsible for a new compression fracture and MRI may be useful in differentiating between these two clinical settings.
Assessment of response to treatment
The role of radiology in the assessment of treatment response is limited, and sequential quantification of biological markers of disease (monoclonal protein levels and bone marrow plasmacytosis) are sufficient to assess response to chemotherapy.
Plain film radiography and scintigraphy
On plain film radiography, shrinking or sclerosing deposits indicate a response to therapy. Persistence of radiological abnormalities should not be considered evidence of active disease, since they may represent residual osteolysis in the absence of plasma cell proliferation. Although conventional skeletal scintigraphy is not routinely performed, the presence of abnormal uptake has been shown to indicate residual activity [15]. A more recent study demonstrated conversion from a positive to negative isotope scan using 99mTc-MIBI in successfully treated patients [19]. FDG–PET can also differentiate between active and treated sites of disease [25].
Computed tomography
Disappearance of soft tissue masses and reappearance of a continuous cortical contour and of a fatty marrow content may be observed in treated lytic lesions.
Magnetic resonance imaging
Interpretation of post-treatment MRI changes can be difficult as there is a wide spectrum of possible treatment-induced changes on MRI depending on the pattern of bone marrow infiltration. There has also been little long-term follow-up of these patients. The lack of lesion enhancement or only a peripheral rim enhancement seen after treatment can be indicative of responsive deposits. Focal marrow lesions may remain identical or decrease in size [42–44]. Local radiation therapy of focal complex deposits induces a rapid decrease in the soft tissue extension and appearance of necrotic central areas within the deposit followed by a decrease in lesion size [44].
In diffuse marrow abnormalities, increased marrow signal is usually observed on post-treatment T1-weighted images due to reappearance of fat cells within more hydrated cellular components. Conversion of a diffuse to a focal or variegated pattern is also frequent [40].
After bone marrow transplantation, bone marrow generally has a high signal on T1-weighted images but focal residual deposits are frequent [45]. The prognostic significance of these abnormalities is uncertain as patients with these residual abnormalities did not have a poorer outcome than those with normal post-transplantation MRI scans [46]. Increased marrow cellularity due to marrow-stimulating factors and decreased signal due to marrow haemosiderosis resulting from repeated transfusions may also be present on post-transplantation MR images.
Relationship of radiology to laboratory values and prognosis
Plain film radiographs retain a key role for staging patients with newly diagnosed myeloma. Patients with at least two lytic foci are classified in advanced disease subgroups and aggressive systemic treatment is usually indicated. Although absence of lytic deposits on skeletal radiography is associated with a lower stage and improved survival, Smith et al. found that only 11% of these patients were alive at 3 years [13].
In early asymptomatic stages of the disease with no or only one lytic deposit on plain film radiographs, patients with relevant abnormalities at MR imaging have a significantly shorter time lag before the onset of more aggressive disease than those with normal-appearing marrow at MR imaging [32, 36, 47]. Patients with the normal and variegated patterns tend to have a lower tumour burden than those with the focal and diffuse marrow involvement patterns. Higher cellularity, higher plasmacytosis and more severe signs of bone failure are usually found in patients with the diffuse pattern [37]. In patients with advanced disease stages treated with conventional chemotherapy, patients with normal MR findings at diagnosis have better response to treatment and a longer survival than those with focal or diffuse marrow abnormalities at MR imaging [35].
In patients with a solitary bone plasmacytoma magnetic resonance screening of the spine and pelvis will usually reveal radiographically unsuspected deposits in up to 80% of patients, thus suggesting true myeloma from the outset. This finding is associated with a poor response to localised radiotherapy and an earlier development of systemic disease than in patients with a negative MRI survey [48].
High levels of serum β2 microglobulin correlate with a poor prognosis and remain the single most powerful determinant of outcome [49]. No correlation between this finding and appearances on MRI has yet been demonstrated.
Complications
The complications of multiple myeloma can be summarised as
spinal cord compression
pathological fractures
secondary amyloidosis
renal impairment
predilection for recurrent pneumonia due to leucopaenia
thromoboembolism.
A pathological fracture affects about 50% of patients at some time with many of the fractures affecting the vertebral bodies. Spinal cord compression resulting from vertebral body fracture may occur in up to 25% of patients and has been described as the presenting feature in 12% of patients [50–52]. Magnetic resonance is the imaging investigation of choice. Fractures of the tubular bones heal readily with normal amounts of callus but extensive fractures may require insertion of intramedullary nails. Patients with myeloma have a predilection for recurrent pneumonia due to associated leucopaenia. These patients can be assessed using plain chest radiography and thin-section CT.
Uncommon variants of myeloma
Clinical manifestations of extraosseous myeloma are rare, occurring in less than 5% of patients with multiple myeloma. Primary sclerotic manifestations are rare and occur only in 3% of patients. It may take the form of diffuse osteosclerosis, patchy sclerotic areas throughout the skeleton or very small numbers of focal sclerotic lesions [53].
Summary
Several differential points help to distinguish (a) lytic deposits of multiple myeloma from bone metastases and (b) benign from malignant vertebral compression fractures. Multidetector CT is a realistic alternative for spinal imaging in severely disabled patients or those unable to have an MRI scan. Several different patterns of marrow infiltration appear on MRI which are not specific and do not correlate with microscopic patterns of infiltration. The role of radiology in the assessment of treatment response is limited; sequential analysis of biological markers is preferred.
References
- 1.CancerStats incidence—UK, April 2003. Cancer Research UK, 2003
- 2.Cancer facts and figures 2003. American Cancer Society Inc., 2003
- 3.UK Myeloma Forum The diagnosis and management of multiple myeloma. Br J Haematol. 2001;115:522–40. doi: 10.1046/j.1365-2141.2001.03206.x. [DOI] [PubMed] [Google Scholar]
- 4.Nanni O, Falcini F, Buiatti E, Bucchi L, Naldoni M, Amadori D. Multiple myeloma and work in agriculture: results of a case-control study in Forli, Italy. Cancer Causes Control. 1998;9:277–83. doi: 10.1023/a:1008821119851. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA. Multiple myeloma, macroglobulinaemia and the monoclonal gammopathies. Curr Pract Med. 1999;2:1131–7. [Google Scholar]
- 6.Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975;50:29–40. [PubMed] [Google Scholar]
- 7.Zaidi AA, Vesole DH. Multiple myeloma: an old disease with new hope for the future. CA Cancer J Clin. 2001;51:273–85. doi: 10.3322/canjclin.51.5.273. [DOI] [PubMed] [Google Scholar]
- 8.Durie BGM, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer. 1975;36:842–54. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Facon T, Avel-Loiseau H, Guillarin G, et al. Intergroupe Francophone du Myelome. Chromosome 13 abnormalities identified by FISH analysis and serum β2 microglobulin produce a powerful myeloma staging system for patients receiving high dose therapy. Blood. 2001;97:1566–71. doi: 10.1182/blood.v97.6.1566. [DOI] [PubMed] [Google Scholar]
- 10.Bataille R, Boccadoro M, Klein B, Durie B, Pileri A. C reactive protein and β2 microglobulin produce a simple and powerful myeloma staging system. Blood. 1992;80:733–7. [PubMed] [Google Scholar]
- 11.LeCouvet FE, Malghem J, Michaux L, et al. Skeletal survey in multiple myeloma: radiographic versus MR imaging survey. Br J Haematol. 1999;106:35–9. doi: 10.1046/j.1365-2141.1999.01529.x. [DOI] [PubMed] [Google Scholar]
- 12.Kapadia SB. Multiple myeloma: a clinicopathologic study of 62 consecutively autopsied cases. Medicine. 1980;59:380–92. [PubMed] [Google Scholar]
- 13.Smith DB, Scarffe JH, Eddleston B. The prognostic significance of X-ray changes at presentation and reassessment in patients with multiple myeloma. Haematol Oncol. 1988;6:1–6. doi: 10.1002/hon.2900060102. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig H, Kupman W, Sinzinger H. Radiography and bone scintigraphy in multiple myeloma: a comparative analysis. Br J Radiol. 1982;55:173–81. doi: 10.1259/0007-1285-55-651-173. [DOI] [PubMed] [Google Scholar]
- 15.Bataille R, Chevalier J, Rossi M, Sany J. Bone scintigraphy in plasma cell myeloma. A prospective study of 70 patients. Radiology. 1982;145:801–4. doi: 10.1148/radiology.145.3.6292996. [DOI] [PubMed] [Google Scholar]
- 16.Catalano L, Pace L, Califano C, et al. Detection of focal myeloma lesions by Tc-99m sestaMIBI scintigraphy. Haematologica. 1999;84:119–24. [PubMed] [Google Scholar]
- 17.Alexandrakis MG, Kyriakou DS, Passam F, Koukouraki S, Karkavitas N. Value of Tc-99m sestamibi scintigraphy in the detection of bone lesions in multiple myeloma: comparison with Tc99m methylene diphosphonate. Ann Haematol. 2001;80:349–63. doi: 10.1007/s002770100302. [DOI] [PubMed] [Google Scholar]
- 18.Alper E, Gurel M, Evrensel T, Ozkocaman V, Akbunar T, Demiray M. 99mTc-MIBI scintigraphy in untreated stage III multiple myeloma: comparison with X-ray skeletal survey and bone scintigraphy. Nucl Med Commun. 2003;24:537–42. doi: 10.1097/00006231-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Fonti R, Del Vecchio S, Zannetti A, et al. Bone marrow uptake of Tc-99m MIBI in patients with multiple myeloma. Eur J Nucl Med. 2001;28:214–20. doi: 10.1007/s002590000434. [DOI] [PubMed] [Google Scholar]
- 20.Pace L, Catalano L, Pinto AM , et al. Different patterns of technetium 99m-sestamibi uptake in multiple myeloma. Eur J Nucl Med. 1998;25:714–20. doi: 10.1007/s002590050274. [DOI] [PubMed] [Google Scholar]
- 21.Pace L, Catalano L, Del Vecchio S, et al. Predictive value of technetium-99m sestamibi in patients with multiple myeloma and potential role in follow-up. Eur J Nucl Med. 2001;28:304–12. doi: 10.1007/s002590000440. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi M, Nonoshita M, Uchida M. Bone marrow uptake of thallium-201 before and after therapy in multiple myeloma. J Nucl Med. 1998;39:473–6. [PubMed] [Google Scholar]
- 23.Chun KA, Cho IH, Won KC , et al. Comparison of Tc99m MIBI and Tl-201 uptake in multiple myeloma. Clin Nucl Med. 2001;26:212–5. doi: 10.1097/00003072-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Schirrmeister H, Bommer L, Buck AK , et al. Initial results in the assessment of multiple myeloma using 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:361–6. doi: 10.1007/s00259-001-0711-3. [DOI] [PubMed] [Google Scholar]
- 25.Durie BGM, Waxman AD, D’agnolo A, William CM. Whole body 18F-FDG PET identifies high risk myeloma. J Nucl Med. 2002;43:1457–63. [PubMed] [Google Scholar]
- 26.Orchard K, Barrington S, Buscombe J, Hilson A, Grant Prentice H, Mehta A. Fluoro-deoxyglucose positron emission tomography imaging for the detection of occult disease in multiple myeloma. Br J Haematol. 2002;117:133–5. doi: 10.1046/j.1365-2141.2002.03407.x. [DOI] [PubMed] [Google Scholar]
- 27.Jadvar H, Conti PS. Diagnostic utility of FDG PET in multiple myeloma. Skeletal Radiol. 2002;31:690–4. doi: 10.1007/s00256-002-0580-2. [DOI] [PubMed] [Google Scholar]
- 28.Mileshkin L, Blum R, Seymour JF, Patrikeos A, Hicks RJ, Prince HM. A comparison of fluorine-18 fluorodeoxyglucose PET and technetium-99m sestamibi in assessing patients with multiple myeloma. Eur J Haematol. 2004;72:32–7. doi: 10.1046/j.0902-4441.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 29.Helms CA, Genant HK. Computed tomography in the early detection of skeletal involvement with multiple myeloma. J Am Med Assoc. 1982;248:2886–7. [PubMed] [Google Scholar]
- 30.Laroche M, Assoun J, Sixou L, Attal M. Comparison of MRI and computed tomography in the various stages of plasma cell disorders: correlations with biological and histological findings. Clin Exp Rheumatol. 1996;14:171–6. [PubMed] [Google Scholar]
- 31.Mahnken AH, Wildberger JE, Gehbauer G, et al. Multidetector CT of the spine in multiple myeloma: comparison with MR imaging and radiography. Am J Roentgenol. 2002;178:1429–36. doi: 10.2214/ajr.178.6.1781429. [DOI] [PubMed] [Google Scholar]
- 32.Moulopoulos LA, Dimopoulos MA, Smith TL. Prognostic significance of magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 1995;13:251–6. doi: 10.1200/JCO.1995.13.1.251. [DOI] [PubMed] [Google Scholar]
- 33.Dimopoulos MA, Moulopoulos LA, Datseris I, et al. Imaging of myeloma bone disease. Acta Oncol. 2000;39:823–7. doi: 10.1080/028418600750063578. [DOI] [PubMed] [Google Scholar]
- 34.Lecouvet FE, Vande Berg BC, Malghem J, Maldague BE. Magnetic resonance and computed tomography imaging in multiple myeloma. Semin Musculoskelet Radiol. 2001;5:43–55. doi: 10.1055/s-2001-12920. [DOI] [PubMed] [Google Scholar]
- 35.Lecouvet FE, VandeBerg BC, Michaux L, et al. Stage III multiple myeloma: clinical and prognostic value of spinal bone marrow MR imaging. Radiology. 1998;209:653–60. doi: 10.1148/radiology.209.3.9844655. [DOI] [PubMed] [Google Scholar]
- 36.Vande Berg BC, Lecouvet FE, Michaux L, et al. Stage I multiple myeloma: value of MR imaging of the bone marrow in the determination of prognosis. Radiology. 1996;201:243–6. doi: 10.1148/radiology.201.1.8816551. [DOI] [PubMed] [Google Scholar]
- 37.Lecouvet FE, Malghem J, Michaux L, et al. Vertebral compression fractures in multiple myeloma. Part II. Assessment of fracture risk with MR imaging of spinal bone marrow. Radiology. 1997;204:201–5. doi: 10.1148/radiology.204.1.9205247. [DOI] [PubMed] [Google Scholar]
- 38.Lecouvet FE, VandeBerg BC, Maldage BE , et al. Vertebral compression fractures in multiple myeloma. Part I. Distribution and appearance at MR imaging. Radiology. 1997;204:195–9. doi: 10.1148/radiology.204.1.9205246. [DOI] [PubMed] [Google Scholar]
- 39.Baur A, Stabler A, Bruning R, et al. Diffusion weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology. 1998;207:349–56. doi: 10.1148/radiology.207.2.9577479. [DOI] [PubMed] [Google Scholar]
- 40.Moulopoulos LA, Dimopoulos MA, Alexanian R, Leeds NE, Libshitz HI. MR patterns of response to treatment. Radiology. 1994;193:441–6. doi: 10.1148/radiology.193.2.7972760. [DOI] [PubMed] [Google Scholar]
- 41.Cuenod CA, Laredo JD, Chevret S, et al. Acute vertebral collapse due to osteoporosis or malignancy: appearances on unenhanced and gadolinium-enhanced MR images. Radiology. 1996;199:541–9. doi: 10.1148/radiology.199.2.8668809. [DOI] [PubMed] [Google Scholar]
- 42.Rahmouni A, Divine M, Mathieu D, et al. MR appearance of multiple myeloma of the spine before and after treatment. Am J Roentgenol. 1993;160:1053–7. doi: 10.2214/ajr.160.5.8470575. [DOI] [PubMed] [Google Scholar]
- 43.Lecouvet FE, De Nayer P, Garber C, et al. Treated plasma cell lesions of bone with MRI signs of response to treatment: unexpected pathological findings. Skeletal Radiol. 1998;27:692–5. doi: 10.1007/s002560050461. [DOI] [PubMed] [Google Scholar]
- 44.Lecouvet FE, Richard F, VandeBerg BC , et al. Long term effects of localised spinal radiation therapy on vertebral fractures and focal lesions appearance in patients with multiple myeloma. Br J Haematol. 1997;96:743–5. doi: 10.1046/j.1365-2141.1997.d01-2108.x. [DOI] [PubMed] [Google Scholar]
- 45.Agren B, Reidberg U, Isberg B, Svensson L, Aspelin P. MR imaging of multiple myeloma patients with bone marrow transplants. Acta Radiol. 1998;39:36–42. doi: 10.1080/02841859809172146. [DOI] [PubMed] [Google Scholar]
- 46.Lecouvet FE, Dechambre S, Malghem J, Ferrant A, Vande Berg BC, Maldague B. Bone marrow transplantation in patients with multiple myeloma: prognostic significance of MR imaging. Am J Roentgenol. 2001;176:91–6. doi: 10.2214/ajr.176.1.1760091. [DOI] [PubMed] [Google Scholar]
- 47.Weber DM, Dimopoulos MA, Moulopoulos LA, Delasalle KB, Smith T, Alexanian R. Prognostic features of asymptomatic multiple myeloma. Br J Haematol. 1997;97:810–4. doi: 10.1046/j.1365-2141.1997.1122939.x. [DOI] [PubMed] [Google Scholar]
- 48.Moulopoulos LA, Dimopoulos MA, Weber D, Fuller L, Libshitz HI, Alexanian R. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol. 1993;11:1311–5. doi: 10.1200/JCO.1993.11.7.1311. [DOI] [PubMed] [Google Scholar]
- 49.Sezer O, Niemoller K, Jakob C, et al. Relationship between bone marrow angiogenesis and plasma cell infiltration and serum beta 2 microglobulin levels in patients with multiple myeloma. Ann Haematol. 2001;80:598–601. doi: 10.1007/s002770100361. [DOI] [PubMed] [Google Scholar]
- 50.Woo E, Yu YL, Ng M. Spinal cord compression in multiple myeloma. Who gets it? Aust N Z J Med. 1986;16:671–5. doi: 10.1111/j.1445-5994.1986.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 51.Speiss JL, Adelstein DJ, Hines F. Multiple myeloma presenting with spinal cord compression. Oncology. 1988;45:88–92. doi: 10.1159/000226539. [DOI] [PubMed] [Google Scholar]
- 52.Loughrey GJ, Collins CD, Todd SM, Brown NM, Johnson RJ. MRI in the management of suspected spinal canal disease in patients with known malignancy. Clin Radiol. 2000;55:849–55. doi: 10.1053/crad.2000.0547. [DOI] [PubMed] [Google Scholar]
- 53.Grover SB, Dhar A. Imaging spectrum in sclerotic myelomas. Eur Radiol. 2000;10:1828–31. doi: 10.1007/s003300000499. [DOI] [PubMed] [Google Scholar]