Summary
Cytolethal distending toxin (CDT) is a multicomponent bacterial holotoxin that targets most eukarytotic cells causing distension and cell cycle arrest. A number of diverse pathogenic bacterial species associated with diarrhoea, chancroid, chronic hepatitis and periodontal disease produce a CDT. Synthesis of the holotoxin is directed by the expression of three genes, cdtA, cdtB and cdtC. Although the product of the CdtB gene was previously identified as a type I deoxyribonuclease, the functions of the cdtA and cdtC products have not been characterized. Using the periodontal pathogen, Actinobacillus actinomycetemcomitans, we demonstrate that the recombinant product of the CdtA gene binds to the surface of Chinese hamster ovary (CHO) cells. This protein did not induce distension or cytotoxicity when introduced into the cytosol using a lipid-based protein delivery system. Recombinant CdtB and CdtC proteins failed to bind to CHO cells. However, the delivery of either CdtB or CdtC into the cytosol resulted in the characteristic pattern of distension followed by cell death. Based on these results, it appears that the CdtA protein subunit alone is responsible for anchoring the holotoxin to the cell surface. The CdtC subunit, in concert with CdtB, contributes to the cytotoxic activities of the holotoxin. The specific mechanism of CdtC cytotoxicity is currently unknown.
Introduction
The facultative Gram-negative bacterium Actinobacillus actinomycetemcomitans has served as a model system for studying the contribution of pathogenic bacterial species and their products to the initiation and progression of oral infectious diseases. This bacterium has a strong historical association with localized aggressive periodontitis (LAP; formerly localized juvenile periodontitis) and, to some extent, adult forms of periodontitis. A novel property of specific strains of A. actinomycetemcomitans is the expression of several complex multigene toxin systems not found in other recognized periodontal pathogens. These multigene toxin systems include a leucotoxin (for a review, see Lally et al., 1999) and the more recently identified cytolethal distending toxin (CDT) (Sugai et al., 1998; Mayer et al., 1999; Shenker et al., 1999).
Although the genetic organization of the leucotoxin locus and the biological activities of its associated gene products have been well studied, the functions and interactions of the CDT genes and gene products are just beginning to be deciphered. The cdt locus in A. actinomycetemcomitans Y4 is composed of three genes (cdtA–C), which appear to form a polycistronic operon (Mayer et al., 1999; Shenker et al., 2000). The gene products for the cdtA, cdtB and cdtC genes are ≈ 27, 30 and 20 kDa, respectively, and it is apparent that expression of all three genes is required for cytotoxicity in vivo (Mayer et al., 1999). The deduced amino acid sequences derived from the three cdt genes are ≈ 25–50% similar (Mayer et al., 1999) to those from Escherichia coli (Pickett et al., 1994; Scott and Kaper, 1994), Shigella dysenteriae (Okuda et al., 1995) and Campylobacter jejuni (Pickett et al., 1996) and > 90% similar to those from Haemophilus ducreyi (Cope et al., 1997).
The CDT from E. coli, C. jejuni, H. ducreyi, A. actinomycetemcomitans and, more recently, Helicobacter hepaticus irreversibly block the cell cycle at the G2 phase of growth in a wide range of host cells (Comayras et al., 1997; Pérès et al., 1997; Sugai et al., 1998; Whitehouse et al., 1998; Cortes-Bratti et al., 1999; Shenker et al., 1999; Young et al., 2000). The inhibition is relatively rapid, usually occurring within 48–72 h of exposure of the cells to bacterial extracts. The cells continue to grow, as protein synthesis is not disrupted, but cannot divide, thus producing the characteristic enlarged or distended cell morphology. It has been proposed that the CDT may inhibit the dephosphorylation of Cdc2 protein kinase by Cdc25 (Comayras et al., 1997; Whitehouse et al., 1998; Cortes-Bratti et al., 1999; 2001; Shenker et al., 1999). This dephosphorylation step is believed to trigger mitosis in normal cells by activation of a Cdc2–cyclin-B1 complex (Pickett and Whitehouse, 1999; Elwell and Dreyfus, 2000).
Lara-Tejero and Galán (2001) used purified recombinant Cdt proteins, from C. jejuni, in glutathione-S-transferase (GST) pull-down, co-immunoprecipitation and gel filtration assays, the results of which supported the possibility that CdtA, CdtB and CdtC combine to form a tripartite complex to create an active holotoxin.
The products of the cdtB genes of E. coli (Elwell and Dreyfus, 2000) and C. jejuni (Lara-Tejero and Galán, 2000) exhibit a type I deoxyribonuclease (DNase I)-like activity that disrupts the chromatin structure of the cell. It is now clear that CdtB belongs to a large enzyme superfamily composed of sphingomyelinases (nSMases), exonucleases, endonucleases and inositol polyphosphate 5-phosphatases (Hofmann et al., 2000). CdtB resides in a subfamily most closely related to mammalian DNase I-type secreted nucleases.
The biological functions of the cdtA and cdtC genes have not yet been identified. Hofman et al. (2000) proposed a similarity between CdtA and the B chain of the plant toxin ricin based on an undisclosed computer alignment algorithm. Ricin is composed of an A subunit that inactivates 80S ribosomes once carried into the cell and a B subunit that attaches to carbohydrate receptors on the cell surface to stimulate uptake of the toxin (Rutenber et al., 1987). The B subunit is a 31 400 kDa polypeptide that binds two galactosides non-co-operatively. This theoretical functional similarity between ricin-like proteins and CdtA has not been substantiated experimentally. No information is available, from either computer or empirical analyses, for elucidating the specific function of CdtC.
In the present study, we report the use of recombinant Cdt proteins, originating from A. actinomycetemcomitans, to examine the biological activities of the individual subunits of the holotoxin. The activities of CdtA and CdtC were assessed using immunofluorescence detection techniques, a protein delivery system and cytotoxicity assays in a Chinese hamster ovary (CHO) cell model system. The recombinant CdtA protein was the only one of the three Cdt proteins that bound to the cells under the conditions used. Both recombinant CdtB and CdtC proteins induced CHO cell distension and cell death when artificially delivered, independently, into actively growing cells. These data are the first to demonstrate that CdtC, completely free from contamination with CdtA and CdtB, has cytotoxic effects.
Results
Cloning, expression and purification of cdtA, cdtB and cdtC
As the level of expression of the cdt genes in A. actinomycetemcomitans precluded the isolation of significant quantities of the gene products for functional studies, the three cdt genes were cloned independently, and in several combinations, in E. coli. Descriptions of the expression clones and their phenotypes relative to the known activities of the CDT are provided in Table 1.
Table 1.
CDT expression clonesa.
| Plasmid | Construction | PCR primers used to make construct (5' to 3') | Gene product(s) | Calculated MW | Cell cycle arrest | DNase activity |
|---|---|---|---|---|---|---|
| pET15bcdt | 2800-bp XhoI/BamHI fragment from pCRAA-14 ligated to BamHI site of pET15b | None | CdtA, CdtB and CdtC | + | + | |
| pET15bcdtA | 691-bp PCR product using primers cdtA1, cdtA2 and DNA from pCRAA-14 ligated to XhoI/BamHI sites of pET15b | GCGCTCGAGAGGTACAATGA GCGGGATCCAGCTTAATT | His6-tagged CdtA | 27,364 | − | − |
| pET15bcdtB | 880-bp PCR product using primers cdtB1', cdtB2 and DNA from pCRAA-14 ligated to BamHI site of pET15b | GCGGGATCCAGTTTATATGCA GATGGATCCTCCTTAGCG | His6-tagged CdtB | 34,505 | − | + |
| pET15bcdtC | 587-bp PCR product using primers cdtC1', cdtC2 and DNA from pCRAA-14 ligated to BamHI site of pET15b | GCGGGATCCGAATACTATGA GATGGATCCATTAGCTACCCT | His6-tagged CdtC | 23,671 | − | − |
| pET15bcdtAB | 1557-bp PCR product using primers cdtA1, cdtB2 and DNA from pCRAA-14 ligated to XhoI/BamHI sites of pET15b | GCGCTCGAGAGGTACAATGA GATGGATCCTCCTTAGCG | His6-tagged CdtA and CdtB | − | + | |
| pET15bcdtBC | 1449-bp PCR product using primers cdtB1', cdtC2 and DNA from pCRAA-14 ligated to BamHI site of pET15b | GCGGGATCCAGTTTATATGCA GATGGATCCATTAGCTACCCT | His6-tagged CdtB and CdtC | − | + |
All plasmids were transformed into E. coli BL21(DE3).
The expression and isolation of the individual recombinant cdt genes is shown in Fig. 1. Total-cell lysates from the clones were examined on SDS–PAGE and stained with Coomassie brilliant blue (CBB). E. coli BL21(DE3) containing pET15bcdtA, pET15bcdtB and pET15bcdtC produced significantly elevated quantities of His6-tagged CdtA, CdtB and CdtC, respectively, relative to the amounts of the native proteins that could be detected in A. actinomycetemcomitans. Even though whole-cell (sonic) extracts of A. actinomycetemcomitans contained cytotoxic activity (Mayer et al., 1999), the gene products were undetectable by staining in SDS–PAGE. The His6-tagged proteins were recovered in significant quantities by affinity chromatography after induction of the bacterial genes with IPTG and solubilization of released inclusion bodies with urea. The presence of the His6-tag provided a convenient and specific method for detecting the recombinant proteins. As shown in Fig. 2, the recombinant His6-tagged CdtA–C proteins were readily detected on Western blots using an anti-His monoclonal antibody. This antibody was also used to confirm that CdtA made by clones E. coli BL21(DE3) (pET15bcdt) and E. coli BL21(DE3) (pET15bcdtAB) and CdtB made by E. coli BL21(DE3) (pET15bcdtBC) contained the His6 tag (data not shown). No immunopositive bands were detected with the anti-His monoclonal antibody on Western blots using sonic extract from E. coli BL21(DE3) (pET15b).
Fig. 1.
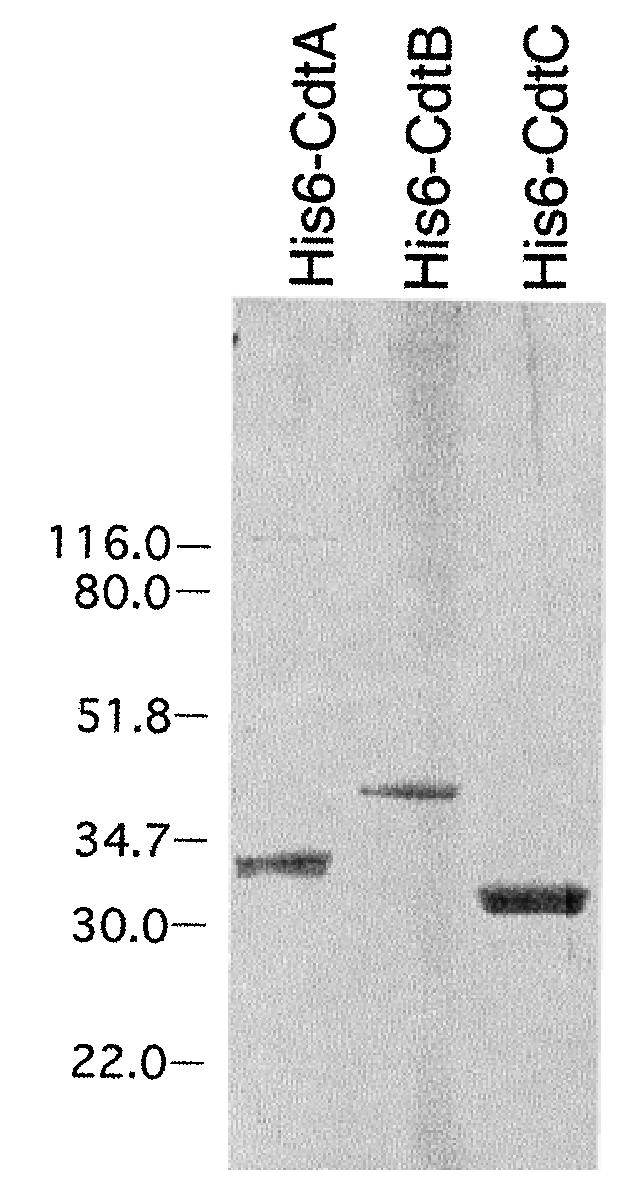
SDS–PAGE of purified recombinant His6-tagged Cdt proteins. Proteins were isolated as described in Experimental procedures, and 6 μg of each protein sample was applied to the gel. The gel was stained with CBB. Molecular weight markers, in kDa, are shown to the left of the gel.
Fig. 2.

Application of anti-His monoclonal antibody for the detection of His6-tagged recombinant Cdt proteins by Western blotting. Whole-cell lysates (25 μg of protein) from the recombinant clones pET15bcdtA, pET15bcdtB and pET15bcdtC were applied to the gel. The blot was probed with His•Tag monoclonal antibody at a 1:3000 dilution and horseradish peroxidase-conjugated anti-mouse IgG diluted 1:3000. Immunopositive bands were detected by chemiluminescence. Molecular weight markers, in kDa, are shown to the left of the film.
The distension and growth arrest of CHO cells in tissue culture was observed only with sonic lysates and spent culture medium from the recombinant clone [E. coli BL21(DE3) (pET15bcdt)] that expressed all three cdt genes. These results were expected, as expression of all three genes is required to observe cytotoxicity in the cell assays. Sonic extracts and spent culture medium from clones expressing the cdtA/cdtB and cdtB/cdtC genes, as single transcriptional units, were devoid of toxic activity.
Whole-cell lysates and spent cell medium from recombinant clones E. coli BL21(DE3) (pET15bcdt), E. coli BL21(DE3) (pET15bcdtB) and E. coli BL21(DE3) (pET15bcdtAB) expressed DNase I-like activity when assayed with supercoiled plasmid DNA as the substrate. In some experiments, limited, but detectable, background DNA nicking activity was evident as a result of undefined nuclease activity in lysates from the E. coli host. Various host modification- and restriction-defective strains of E. coli, including E. coli JM109 and E. coli HB101, were tested to try to minimize this extraneous nuclease background. This approach was unsuccessful. However, the DNase activity of purified His6-tagged CdtB from E. coli BL21(DE3) (pET15bcdtB) was free from this intrinsic background and readily measured by a conversion of supercoiled (S) form plasmid DNA to the relaxed (R) form (Fig. 3). Furthermore, the purified His6-tagged CdtB was biologically active in spite of having a 27-amino-acid extension (MGSSHHHHHHSSGLVPRGSHMLEDPVY) at the amino-terminal end of the protein. Neither His6-tagged CdtA nor His6-tagged CdtC exhibited nuclease nicking activity in the mobility shift assay.
Fig. 3.

DNase activity of recombinant His6-tagged CdtB. Purified His6-tagged CdtB (0–15 μg) was incubated for 1 h at 37°C with supercoiled plasmid DNA. The contents of the reaction tubes were applied to a 1% agarose gel. The gel was stained with ethidium bromide. S, supercoiled form of the plasmid DNA; R, relaxed form of the plasmid DNA.
Reconstitution of biologically active recombinant holotoxin
Various concentrations of purified His6-tagged CdtA, His6-tagged CdtB and His6-tagged CdtC were added to CHO cell cultures alone and in combination to determine which proteins were required to establish a toxic phenotype. These experiments were also performed to determine whether a biologically active holotoxin could be assembled in vitro from the recombinant proteins. Each of the purified His6-tagged proteins alone (3–30 mg of protein per 240 cells) had no effect on the morphology and growth of the CHO cells after 6–7 days in culture (Fig. 4). Likewise, preincubated mixtures of CdtA/CdtB (15 μg/1.5 μg of protein per 240 cells) and CdtB/CdtC (1.5 μg/15 μg of protein per 240 cells) did not affect the growth or survival of CHO cells. Mixtures of all three recombinant proteins, in various ratios, caused a reduction in the colony-forming units (cfu) of the CHO cell cultures. A ratio of 30 μg of His6-tagged CdtA:3 μg of His6-tagged CdtB:30 μg of His6-tagged CdtC/240 cells reduced the survival of the CHO cells in culture to 0 cfu.
Fig. 4.

In vitro reconstitution of biologically active CDT. Purified His6-tagged CdtA, His6-tagged CdtB, His6-tagged CdtC or pairs of proteins (unshaded bars) or premixed combinations of all three proteins (shaded bars) were added to CHO cell cultures (240 cells), and cfu were counted after growth for 6 days. The amount of each protein, in μg, is shown on the x-axis.
Functional studies of the CdtA and CdtC proteins
A conserved domain (CD) database search, using an RPS (reverse position specific)-BLAST algorithm (http://www.ncbi.nlm.nih.gov/), revealed that a portion of the deduced amino acid sequence of the recombinant A. actinomycetemcomitans cdtA gene, representing amino acids 123–211, has a match to the amino-terminal end (residues 1–83) of the B chain of ricin. The CD alignment length was 125 residues with 66.4% aligned. The score was 33.5 bits with an E-value of 0.001. These findings support the possibility that the CdtA protein works by a lectin-like interaction to bind the holotoxin to the eukaryotic cell surface (Lara-Tejero and Galán, 2001). To test this hypothesis, His6-tagged CdtA protein, as well as His6-tagged CdtB and His6-tagged CdtC as controls, were incubated with CHO cells, and immunofluorescence was used to document binding to the cell surface. As shown in Fig. 5A, the His6-tagged CdtA-treated cells contained bound fluorescent-labelled antibody with localized regions of intense fluorescence. Neither His6-tagged CdtB nor His6-tagged CdtC proteins were detected on the CHO cells (Fig. 5B and C respectively). Fluorescence of low intensity was observed when the CHO cells were incubated with a preincubated mixture of all three recombinant proteins (Fig. 5D). As in the case of recombinant CdtB, biological function was observed in spite of the amino-terminal modification (MGSSHHHHHHSSGLVPRGSHMLERGT) of His6-tagged CdtA.
Fig. 5.

Binding of recombinant Cdt proteins to the surface of CHO cells. The proteins or combination of proteins were incubated with CHO cells on chamber slides for 30 min at 37°C. Bound protein was detected with His•Tag monoclonal antibody (1:1000 dilution) and fluoresceinconjugated second antibody (1:1000 dilution). The slides were examined under a fluorescent microscope and images recorded with a digital camera. A. His6-tagged CdtA. B. His6-tagged CdtB. C. His6-tagged CdtC. D. Preincubated mixture of His6-tagged CdtA, His6-tagged CdtB and His6-tagged CdtC. E. Cells incubated only with HisTag monoclonal antibody and second antibody. F. Cells incubated only with second antibody.
To determine the contribution and extent of the individual His6-tagged Cdt proteins to the cytotoxic effects of the holotoxin, a lipid-based protein delivery system (BioPorter protein delivery reagent; Gene Therapy Systems) was used to put each protein, and mixtures of proteins, inside the cells. Increasing concentrations, ranging from 7 to 1000 ng, of His6-tagged CdtA, His6-tagged CdtB and His6-tagged CdtC were introduced into CHO cells in tissue culture, and the effects of the proteins on cell survival were observed (Fig. 6A). Up to 1 mg of His6-tagged CdtA/5 × 103 CHO cells had no effect on growth or cell viability. A decrease in cell viability was observed with 250 ng of His6-tagged CdtB/5 × 103 cells, which continued in a dose-dependent fashion with up to 1 μg of the recombinant protein. A similar reduction in cell viability was obtained with His6-tagged CdtC. A decrease in cell number was observed with as little as 125 ng of protein/5 × 103 CHO cells. His6-tagged CdtC contained the extra sequence, MGSSHHHHHHSSGLVPRGSHMLEDPNT, at the amino-terminal end of the protein.
Fig. 6.

Effect of recombinant Cdt proteins delivered to the cell cytosol. CHO cells were incubated with the various Cdt proteins complexed with a lipid-based protein delivery reagent. A. Increasing concentrations of His6-tagged CdtA (grey bars), His6-tagged CdtB (diagonal filled bars) and His6-tagged CdtC (white bars) were delivered separately to CHO cells in culture. Cultures were incubated for 48 h at 37°C. The number of surviving cells was determined indirectly by measuring the A492 of the culture after the addition of a vital dye staining reagent. B. Various concentrations of His6-tagged CdtA, His6-tagged CdtB, His6-tagged CdtC or mixtures of these proteins were incubated with CHO cells using the same conditions as those described for the experiment in (A). Proteins were added to CHO cell cultures with (white bars) and without (diagonal filled bars) the protein delivery reagent. The numbers below the bars in (A) and (B) represent the amount (ng) of each protein added.
The recombinant Cdt proteins were also introduced into the CHO cells in mixtures containing pairs of the proteins as well as all three proteins (Fig. 6B). No significant reduction in cell survival was observed when the individual proteins, without the BioPorter reagent, were incubated with the CHO cells in culture. A slight reduction in viability was apparent when a mixture of all three proteins, in the absence of the BioPorter reagent, was added to cell cultures. The effect was not as dramatic as that observed in the reconstitution experiment shown in Fig. 4, because significantly smaller amounts of protein (170 ng of each His6-tagged protein) and larger numbers of cells (5 × 103 cells) were used in the BioPorter experiments. There did not seem to be a significant reduction in viability when up to 500 ng of His6-tagged CdtA was introduced into the cells using the delivery system. However, the uptake of 170–500 ng of recombinant His6-tagged CdtB and His6-tagged CdtC alone, and in combination with the other proteins, resulted in a dramatic effect on cell survival. There appeared to be an additive detrimental effect on cell survival when His6-tagged CdtB and His6-tagged CdtC were delivered together into the cytosol.
Discussion
CDT is a complex holotoxin made by a number of taxonomically distinct Gram-negative bacterial species including E. coli (Johnson and Lior, 1987a; Scott and Kaper, 1994), Shigella dysenteriae (Johnson and Lior, 1987b; Okuda et al., 1995; Okuda et al., 1997), C. jejuni (Johnson and Lior, 1988; Pickett et al., 1996), H. ducreyi (Cope et al., 1997), A. actinomycetemcomitans (Sugai et al., 1998; Mayer et al., 1999; Shenker et al., 1999) and H. hepaticus (Young et al., 2000). All these bacteria can be categorized as pathogenic species in diarrhoeal disease, chancroid, chronic hepatitis or periodontal disease. In general, the CDT arrests the growth of most types of eukaryotic cells in vitro (Comayras et al., 1997; Pérès et al., 1997; Oguchi et al., 1998; Cortes-Bratti et al., 1999; Shenker et al., 1999; Escalas et al., 2000). A multitude of studies has focused on the cell pathways that are affected by the CDT to induce cell cycle arrest. However, very little information has been published to date to establish the functional roles of the individual components of the CDT.
Those bacterial species that exhibit toxic activity have proved to be recalcitrant to the isolation and purification of the individual Cdt proteins. One of the major difficulties in Cdt protein purification from the native bacterial species is a consequence of the synthesis of very small quantities of highly active gene products. For example, we have found that a TD50, for CHO cells, was obtained with as little as 400 ng of total soluble protein from A. actinomycetemcomitans (Mayer et al., 1999). Stringently controlled functional studies are also hampered by the apparent cross-contamination of individual Cdt protein preparations. To overcome these obstacles, studies of the composition and organization of the CDT holotoxin have been performed using recombinant proteins isolated from clones that contain single cdt genes. Dissecting the biological contributions of the individual holotoxin components is complicated further by the fact that cells in culture are killed by bacterial lysates or spent culture medium only when all three cdt genes are expressed (Pickett et al., 1994; Scott and Kaper, 1994; Lewis et al., 2001). No cytotoxic effects were observed when individual recombinant C. jejuni Cdt proteins were added to cultures of Henle-407 intestinal epithelial cells (Lara-Tejero and Galán, 2001). However, when the three proteins were mixed and added to cells, a full complement of cytotoxic activity was detected. In the present study, we were able to duplicate this finding using purified His6-tagged recombinant Cdt proteins from A. actinomycetemcomitans and CHO cells. But, unlike the observations reported by several other groups (Purven et al., 1997; Shenker et al., 1999), the addition of purified recombinant CdtC or CdtB, respectively, to cell cultures did not result in cellular distension or cytotoxic effects.
Based on the initial report by Hofmann et al. (2000), it was found, using amino acid sequence threading, that the region encompassing amino acids 160–220 of the CdtA of C. jejuni is similar to a lectin fold present in the B chains of ricin and abrin (Lara-Tejero and Galán, 2001; Hassane et al., 2001). The B chain of ricin binds galactosides and is thought to promote the uptake of the A chain by endocytosis (Rutenber et al., 1987). Therefore, it was proposed that CdtA, perhaps in combination with CdtC, may have a similar cell receptor-binding function in the holotoxin (Lara-Tejero and Galán, 2001). However, no experimental evidence has been reported to substantiate this possibility. We found a similar relationship between the deduced amino acid sequence of the recombinant A. actinomycetemcomitans CdtA using an RPS-blast search (Altschul et al., 1997). Binding studies were performed based on the results of this analysis. His6-tagged CdtA, bound to the surface of CHO cells, was confirmed by immunofluorescence detection. This appeared to be a specific interaction, as neither His6-tagged CdtB nor His6-tagged CdtC was detected on the CHO cells under identical experimental conditions. Binding of the CDT holotoxin to the cell surface was evident but not conclusive because of significantly reduced fluorescence intensity compared with that obtained with His6-tagged CdtA. There are a number of reasons why it was difficult to demonstrate binding of the holotoxin. For instance, the epitopes for the anti-His monoclonal antibody used to detect the protein on the cell surface may be masked in the holotoxin complex but exposed on free His6-tagged CdtA. Furthermore, binding of the holotoxin may be more transient than that of the His6-tagged CdtA protein because of the size of the complex and the presumed endocytosis of CdtB or a CdtB–CdtC complex. Interestingly, the addition of various galactosides and mannosides to the cell cultures failed to inhibit the binding of His6-tagged CdtA. These observations suggest that the mechanism of binding may be distinct from that of the B chain of ricin.
Several groups have shown recently that CdtB is a mammalian-like DNase I that introduces chromatin damage within the cell nucleus (Elwell and Dreyfus, 2000; Lara-Tejero and Galán, 2000). Either microinjection (Lara-Tejero and Galán, 2000) or electroporation (Elwell et al., 2001) of recombinant CdtB alone into cells results in cytotoxicity similar to that observed with the native holotoxin. We have demonstrated a cytotoxic effect using recombinant His6-tagged CdtB from A. actinomycetemcomitans (see Fig. 4). Cytotoxic activity was observed in spite of the fact that the protein contained extra amino acids at the amino-terminus. A commercial protein delivery system was used to introduce the Cdt proteins into the CHO cells. The proteins are captured by interaction with a charged lipid carrier, and uptake occurs by fusion with the plasma membrane or endocytosis. The use of this type of delivery technology may be more advantageous than those methods used previously because it is considerably simpler, requiring no specialized equipment, and probably less traumatic to the cells than microinjection or electroporation.
No effect was observed when the recombinant His6-tagged CdtB alone was simply added to cell cultures. It was established that the purified His6-tagged CdtB had DNase-like activity in vitro and was active when cells were treated with a mixture containing all three His6-tagged Cdt proteins. This is in contrast to the previously reported results in which purified CdtB from A. actinomycetemcomitans (Shenker et al., 1999) or CdtC from H. ducreyi (Purven et al., 1997; Akifusa et al., 2001) was sufficient to arrest cells in culture. In our hands, mixtures of His6-tagged CdtA/His6-tagged CdtB and His6-tagged CdtB/His6-tagged CdtC were also not sufficient to distend or kill CHO cells as reported by Lara-Tejero and Galán, 2001). It appears that the presence of small amounts of CdtA is required to promote the internalization of CdtB.
The precise role of the CdtC protein in the mechanism of action of the holotoxin is unknown at the present time. The fluorescence binding experiments did not show any evidence of His6-tagged CdtC on the surface of the CHO cells when added either alone or in combination with His6-tagged CdtA. Therefore, His6-tagged CdtC was examined for the ability to affect the growth of the CHO cells. The expected result was that CdtC would complement or enhance the ability of CdtB to reduce the survival of the CHO cells. It was surprising to find that delivery of purified recombinant His6-tagged CdtC into the CHO cells resulted in cellular distension and eventual death. The results were similar to those obtained with His6-tagged CdtB, such that cytotoxicity was observed only when the protein was actively transported into the cytosol. The activities of internalized His6-tagged CdtB and His6-tagged CdtC appeared to be specific because no effects were observed when purified His6-tagged CdtA was taken up by the cells, and neither protein affected the cells when added to cultures in the absence of the delivery reagent. These data supported the results of antibody inhibition studies indicating that CdtC contributed to the expression of a toxic phenotype (Cope et al., 1997; Mayer et al., 1999). The cytotoxic effects of His6-tagged CdtC were distinct from those attributed to His6-tagged CdtB because the former protein failed to exhibit nuclease activity in vitro. Attempts to locate His6-tagged CdtB and purified His6-tagged CdtC inside the CHO cells by immunofluorescence were unsuccessful. Lara-Tejero and Galán (2000) were able to stain CdtB that had been microinjected into COS-1 cells. One difference between our results and those reported by Lara-Tejero and Galán (2000) may be explained by the difference in methods used to deliver the proteins into the cytosol. Considerably smaller amounts of protein were used in the protein delivery technology relative to those used in microinjection. Experiments are in progress to devise a protocol to localize the His6-tagged CdtC protein in the CHO cells and to determine whether pathways, similar to those affected by CdtB, are also triggered by CdtC.
The new findings reported in this study expand the biological model for the CDT by establishing a binding function for CdtA and a cytotoxic function for CdtC. The most favourable interpretation of the data is that the CdtA protein works alone to bind the holotoxin to the cell surface. Both CdtB and CdtC proteins are internalized where the CdtC protein contributes, in an additive fashion with CdtB, to the cytotoxic effects of the holotoxin.
Experimental procedures
Plasmid construction
The synthetic oligonucleotide primer pairs shown in Table 1 were used to amplify the various cdt gene sequences for cloning. The primer sequences were based on the sequence of the cdt locus in A. actinomycetemcomitans Y4 (GenBank accession no. AF006830). The amplified products were cloned into pET15b to make the constructs shown in Table 1. Amplified gene sequences cdtB, cdtC and cdtBC were cloned into the BamHI site of pET15b. Amplified gene sequences cdtA and cdtAB were cloned into the XhoI–BamHI sites of pET15b. All constructs were first transformed into E. coli JM109 {endA1 gyrA96 hsdR17(rk− mk−) mcrB+ recA1 relA1 supE44 thi-1 Δ(lac-proAB F′[traD36 proAB lacIqZΔM15])} competent cells (Promega) for long-term storage and as a stable source of plasmid DNA for restriction endonuclease mapping and DNA sequencing. Transformants were selected on LB agar containing 75 μg ml−1 ampicillin. Restriction endonuclease mapping was used to confirm that the DNA fragments were ligated in the proper orientation. Plasmid DNA was then isolated from each of the clones and transformed into E. coli BL21(DE3) [F− ompT hsdSB (rB− mB−) gal dcm (DE3)] competent cells (Novagen) primarily for recombinant protein isolation.
Escherichia coli BL21(DE3) (pET15bcdt) was constructed by digesting plasmid DNA from E. coli DH5a(pCRAA-14) (Mayer et al., 1999) with XhoII and BamHI. The 2.8 kb XhoII–BamHI DNA fragment containing the three cdt genes was ligated to the BamHI site of pET15b and transformed into competent cells of E. coli BL21(DE3).
Isolation of recombinant CdtA, CdtB and CdtC proteins
Single colonies of E. coli BL-21(DE3) (pET15bcdtA), E. coli BL-21(DE3) (pET15bcdtB) and E. coli BL-21(DE3) (pET15bcdtC) were inoculated separately into 10 ml of LB medium containing 50 μg ml−1 ampicillin and incubated overnight at 37°C. The overnight culture was added to 100 ml of the same medium and grown, with vigorous shaking, at 37°C until late log phase (OD600 = 0.8–1.0) was reached. IPTG was added to a final concentration of 1 mM, and the cells were grown for an additional 4–5 h. Expression of target genes was assessed by analysis of total-cell protein on a 10–20% polyacrylamide Tris-HCl Ready Gel (Bio-Rad Laboratories). Total-cell protein was obtained by boiling an aliquot of the bacterial culture in gel loading buffer (2% SDS, 0.05 M Tris-HCl, pH 6.8, 10% glycerol) for 5 min. Molecular weight standards were from Bio-Rad Laboratories.
Bacteria were harvested by centrifugation at 6000 g for 10 min, washed and suspended in 10 ml of 1× binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl, pH 7.9). The suspension was sonicated for three 30 s bursts on ice. The sonicates were centrifuged at 6000 g for 15 min to collect the inclusion bodies. This procedure was repeated, and the final inclusion body pellets were suspended in 10 ml of 1× binding buffer containing 6 M urea. After incubation on ice for 1 h to dissolve the protein completely, the suspension was centrifuged at 12 000 g for 30 min. The supernatant fluid was then passed through a 0.45 micron filter.
The bacterial extracts were applied to a 1.0 ml Ni-IDA (nickel–iminodiacetic acid; Novagen,) column that had previously been equilibrated in 1× binding buffer containing 6 M urea. The column was washed with the same buffer, followed by wash buffer I (20 mM Tris-HCl, 0.5 M NaCl, 20 mM imidazole and 6 M urea) and wash buffer II (20 mM Tris-HCl, 0.5 M NaCl, 50 mM imidazole and 6 M urea). Bound protein was eluted with buffer containing 300 mM imidazole. The eluted proteins were dialysed against 10 mM Tris-HCl (pH 7.9), 100 mM NaCl, 5 mM MgCl2 to promote refolding. On average, the yield of recombinant His6-tagged CdtA, His6-tagged CdtB and His6-tagged CdtC was 3, 4 and 3 μg of protein ml−1 bacterial culture respectively.
Western blot analysis
Bacteria collected from 1 ml of IPTG-induced culture were mixed with 100 μl of gel loading buffer and heated in a boiling water bath for 5 min. Samples were applied to a 10–20% polyacrylamide gel (25 μg of protein per lane). After electrophoresis, protein bands were transferred to a nitrocellulose membrane. The membrane was blocked with 3% BSA in TBS buffer (20 mM Tris-HCl, pH 7.6, 0.8% NaCl) for 1 h at room temperature with shaking. The membrane was washed and then incubated with His·Tag monoclonal antibody (Novagen), diluted 1:3000 in 3% BSA–TBS, for 1 h at room temperature with shaking. The filter was washed and incubated for 1 h with horseradish peroxidase-conjugated anti-mouse IgG (Novagen) diluted 1:3000 in 10% skim milk. Immunopositive protein bands were detected with an Enhanced Chemiluminescence Western blotting detection kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Prestained molecular weight standards were obtained from New England Biolabs.
Tissue culture
Chinese hamster ovary (CHO-K1) cells were grown routinely in T25 flasks (Nunc) in Ham's F12 medium containing 5% fetal calf serum (FCS) as described previously (Mayer et al., 1999). Cells were detached from the plates after growth for 3–4 days by treatment with 0.5 ml of trypsin (1 mg ml−1) in PBS for 1–3 min at 37°C and suspended in the same medium containing 1% FCS for passage or dilution for cell assays.
CDT activity assay
CDT activity of bacterial sonicates and spent culture medium was determined as described previously (Mayer et al., 1999). Briefly, CHO cell suspensions were counted in a haemocytometer and adjusted to 1 × 104 cells ml−1 medium. Twenty-four microlitres of cell suspension and 3 ml of fresh medium were added per well in six-well plates. Bacterial sonicate or purified recombinant protein was added to the wells at various concentrations appropriate for each experiment. Protein was quantified with the Micro BCA protein assay kit (Pierce Chemical). Plates were incubated for 6–7 days to allow colonies to form. The medium was decanted, and the colonies were fixed in 10% formalin. After incubation for 5 min, the colonies were stained with crystal violet for 10 min, the plates air dried and the colonies counted. The number of colonies per well was expressed as colony-forming units (cfu). All samples were run in triplicate wells.
DNase assay
DNase activity was assessed as described by Elwell and Dreyfus (2000). Supercoiled pET15b plasmid DNA (1 μg per reaction) was incubated with various amounts of sonic extract or spent culture medium or purified His6-tagged Cdt proteins (0–15 μg of protein per reaction) in a buffer consisting of 25 mM HEPES (pH 7.0), 10 mM MgCl2 and 5 mM CaCl2. After a 1 h incubation at 37°C, the reaction was stopped by the addition of 10 mM EDTA, 6% glycerol and bromophenol blue. The samples were loaded directly on a 1% agarose gel and subjected to electrophoresis in TBE buffer. Gels were stained with ethidium bromide to view any changes in the electrophoretic mobility of the supercoiled plasmid DNA band.
Toxin reconstitution assay
The recombinant His6-tagged proteins were added either individually or in various combinations with gentle rocking for 1 h at 4°C to CHO cells seeded in six-well plates (Costar). Each well received 240 cells and 3.0 ml of Ham's F12 medium containing 5% FCS. Samples containing more than one Cdt protein were premixed in various ratios as shown in the Fig. 4. The Cdt proteins were added at the time the CHO cells were seeded on the plates. The plates were incubated for 6 days at 37°C in an atmosphere containing 5% CO2. The medium was removed at the end of the growth period, and 4% formalin was added to fix the colonies. Colonies were counted after staining with crystal violet as described previously (Mayer et al., 1999). Data are reported as colony-forming units (cfu). All samples were run in triplicate wells.
CHO cell binding assay
CHO cells, suspended in growth medium, were added to each well (1 × 104 cells per well) of an eight-well chamber slide (Nunc) and incubated for 48 h at 37°C in a moist atmosphere containing 5% CO2. The medium was removed and the cells washed once with cold PBS. The purified His6-tagged proteins were suspended in 200 μl of cold PBS and added to the wells (1–5 μg per well). The slides were then incubated on ice for 15–30 min, washed twice with PBS and fixed in 10% formalin for 5 min at room temperature. The slides were washed three times with PBS, and free sites were blocked with 200 μl per well of 3% BSA in PBS for 30 min at room temperature. The slides were washed twice with PBS and incubated with 200 μl per well of His·Tag monoclonal antibody diluted 1:1000 in 3% BSA–PBS for 1 h at room temperature. Unbound antibody was removed by washing the slides three times with PBS. Two hundred microlitres of a 1:1000 dilution of Alexa Fluor 488 goat anti-mouse IgG (heavy and light chain) conjugate (Molecular Probes) in 3% BSA–PBS was then added to each well. The slides were washed three times, and 5 μl of mounting solution was added per well. Coverslips were placed on the slides, which were viewed under a Nikon Eclipse E600 fluorescent microscope with a fluorescein isothiocyanate (FITC)-HYQ filter (excitation wavelength 460–500 nm). Binding experiments were repeated several times to confirm reproducibility.
CHO cell uptake assay
BioPorter protein delivery reagent (Gene Therapy Systems) was dissolved in 250 μl of chloroform. The dissolved reagent (1 μl) was added to Eppendorf tubes and dried under a hood for 4 h at room temperature. Recombinant His6-tagged Cdt proteins were diluted in 10 mM Tris, 150 mM NaCl (pH 7.0). Protein solutions (1 μg of protein in 50 μl of Tris buffer) were added to the BioPorter reagent tubes and incubated for 5 min at room temperature. The samples were then gently vortexed for 3–5 s at low speed. Serial dilutions were made, and serum-free Ham's F12 medium was added to bring the final volume to 100 μl per well. CHO cells were washed once and suspended in serum-free medium so that the concentration was 5000 cells per 90 μl. This cell suspension (90 μl) was added to the BioPorter–Cdt protein mixture dilutions and transferred to 96-well plates. The cultures were incubated for 3–4 h at 37°C; then 100 μl of Ham's F12 medium containing 5% FCS was added to each well. The cultures were incubated for an additional 48 h. The CellTiter96 aqueous non-radioactive cell proliferation assay kit (Promega) was used to quantify changes in cell viability. The absorbance of each well was measured at 492 nm. A reduction in cell viability was represented as a decrease in the A492. All samples were run in triplicate. A standard curve of absorbance versus CHO cell number prepared previously showed a linear increase in the A492 of a CHO cell culture over the range of 500–5000 cells.
Acknowledgements
This study was supported by National Institutes of Health grant DE12593. We thank Dr J. Korostoff for critical reading of the manuscript.
References
- Akifusa S, Poole S, Lewthwaite J, Henderson B, Nair SP. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect Immun. 2001;69:5925–5930. doi: 10.1128/IAI.69.9.5925-5930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comayras C, Tasca C, Pérès SY, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope LD, Lumbley S, Latimer JL, Klesney-Tait J, Stevens MK, Johnson LS, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Invest. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Bratti X, Karlsson C, Lagergard T, Thelestam M, Frisan T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J Biol Chem. 2001;276:5269–5302. doi: 10.1074/jbc.M008527200. [DOI] [PubMed] [Google Scholar]
- Elwell CA, Dreyfus LA. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- Elwell C, Chao K, Patel K, Dreyfus L. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect Immun. 2001;69:3418–3422. doi: 10.1128/IAI.69.5.3418-3422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalas N, Davezac N, De Rycke J, Baldin V, Mazars R, Ducommun B. Study of the cytolethal distending toxin-induced cell cycle arrest in HeLa cells: involvement of the CDC25 phosphatase. Exp Cell Res. 2000;257:206–212. doi: 10.1006/excr.2000.4878. [DOI] [PubMed] [Google Scholar]
- Hassane DC, Lee RB, Mendenhall MD, Pickett C. Cytolethal distending toxin demonstrates genotoxic activity in a yeast model. Infect Immun. 2001;69:5752–5759. doi: 10.1128/IAI.69.9.5752-5759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci USA. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WM, Lior H. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol Lett. 1987a;43:19–23. [Google Scholar]
- Johnson WM, Lior H. Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microbiol Lett. 1987b;48:235–238. [Google Scholar]
- Johnson WM, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- Lara-Tejero M, Galán JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- Lara-Tejero M, Galán JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun. 2001;69:4358–4365. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Stevens MK, Latimer JO, Ward CK, Deng K, Blick R, et al. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vivo and in vitro systems. Infect Immun. 2001;69:5626–5634. doi: 10.1128/IAI.69.9.5626-5634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MPA, Bueno LC, Hansen EJ, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi M, Ishisaki A, Okahashi N, Koide M, Koseki T, Yamato K, et al. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in the G2/M phase and apoptosis. Infect Immun. 1998;66:5980–5987. doi: 10.1128/iai.66.12.5980-5987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathol. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérès SY, Marchès O, Daigle F, Nougayrède J-P, Hérault F, Tasca C, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Whitehouse CA. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purven M, Frisk A, Lonnroth I, Lagergard T. Purification and identification of Haemophilus ducreyi cytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326:624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- Scott DA, Kaper JB. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, McKay TL, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- Shenker BJ, Hoffmaster RH, McKay TL, Demuth DR. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- Sugai M, Kawamoto T, Pérès SY, Ueno Y, Komatsuzawa H, Fujiwara T, et al. Cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL. Camphylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


