Summary
The PAS and HAMP domain superfamilies are signal transduction modules found in all kingdoms of life. The Aer receptor, which contains both domains, initiates rapid behavioural responses to oxygen (aerotaxis) and other electron acceptors, guiding Escherichia coli to niches where it can generate optimal cellular energy. We used intragenic complementation to investigate the signal transduction pathway from the Aer PAS domain to the signalling domain. These studies showed that the HAMP domain of one monomer in the Aer dimer stabilized FAD binding to the PAS domain of the cognate monomer. In contrast, the signal transduction pathway was intra-subunit, involving the PAS and signalling domains from the same monomer. The minimal requirements for signalling were investigated in heterodimers containing a full-length and truncated monomer. Either the PAS or signalling domains could be deleted from the non-signalling subunit of the heterodimer, but removing 16 residues from the C-terminus of the signalling subunit abolished aerotaxis. Although both HAMP domains were required for aerotaxis, signalling was not disrupted by missense mutations in the HAMP domain from the signalling subunit. Possible models for Aer signal transduction are compared.
Introduction
Escherichia coli monitors the concentration of attractants and repellents in the environment using membrane-bound chemoreceptors that sense stimuli outside the cell and transduce a signal that is processed inside the cell (reviewed in Wadhams and Armitage, 2004). The aerotaxis receptor Aer, on the other hand, is an intracellular sensor that responds to oxygen concentration and other parameters affecting cellular energy levels (Bibikov et al., 1997; Rebbapragada et al., 1997; Taylor and Zhulin, 1999; Taylor et al., 1999; 2003). The input module of Aer is a PAS domain that non-covalently binds FAD [Fig. 1 (Bibikov et al., 1997; 2000; Rebbapragada et al., 1997; Taylor and Zhulin, 1999; Repik et al., 2000)]. Currently, over 4900 PAS domains have been identified, constituting a superfamily of sensory input modules that are wide-spread in the three kingdoms of life (SMART, http://smart.embl-heidelberg.de/). In bacteria, PAS domains sense light, oxygen, redox potential, energy and voltage (Taylor and Zhulin, 1999). Aer also contains a HAMP domain, a member of a superfamily of sensory transduction domains identified through bioinformatic analysis (Aravind and Ponting, 1999; Williams and Stewart, 1999; Ma et al., 2005). Although PAS and HAMP domains are frequently recurring motifs in sensory proteins, PAS/HAMP relationships have not been defined for a signal transduction pathway. In this study, we investigate these relationships during signal transduction in Aer.
Fig. 1.
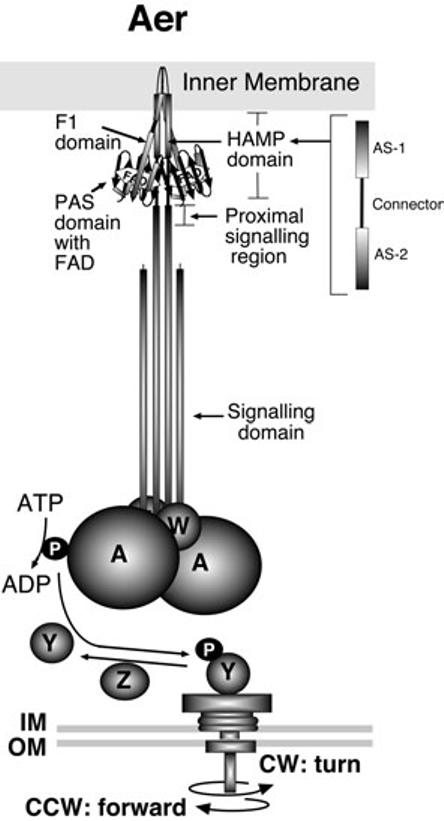
Cartoon of the Aer receptor and aerotaxis pathway in E. coli. The redox state of FAD bound to the dimeric Aer protein regulates autophosphorylation of the histidine kinase CheA. Phospho-CheA trans-phosphorylates the response regulator CheY, which in turn binds to the flagella motor, changing the direction of rotation from counterclockwise to clockwise. This results in a brief tumbling response. IM, inner membrane; OM, outer membrane; A, CheA protein; W, CheW docking protein; Y, CheY protein; Z, CheZ phosphatase; P, phosphate; CW, clockwise; CCW, counterclockwise; AS, amphipathic sequence.
The putative arrangement of domains in the dimeric Aer receptor is shown in Fig. 1. A membrane anchor separates the N-terminal PAS and F1 regions from the C-terminal HAMP and signalling domains. The signalling domain of Aer is homologous to the signalling domain of the chemoreceptors (Tsr, Tar, Trg and Tap), particularly at the highly conserved domain (HCD) (Bibikov et al., 1997; Rebbapragada et al., 1997). The histidine kinase CheA is coupled to the signalling domain by the CheW protein to form a stable ternary complex (Borkovich et al., 1989; McNally and Matsumura, 1991; Gegner et al., 1992). These interactions provide a point of convergence within the chemotaxis pathway, integrating signals from disparate chemoreceptors, and allowing co-ordinated control of the flagellar motors via a shared protein phosphorelay (as shown in Fig. 1).
The Tar HAMP domain from Salmonella enterica serovar Typhimurium consists of two α-helical amphipathic sequences, AS-1 and AS-2, connected by a region of undefined structure (Butler and Falke, 1998). The Aer HAMP domain appears to have a similar structure, except AS-2 contains additional hydrophobic residues and is predicted to be buried (Ma et al., 2005). FAD binding to the Aer-PAS domain requires an intact HAMP domain (Bibikov et al., 2000; Herrmann et al., 2004). This is supported by data showing that missense mutations in AS-2 eliminate FAD binding (Bibikov et al., 2000; Watts et al., 2004; Ma et al., 2005), and can be functionally suppressed by mutations in the PAS domain (Watts et al., 2004). Missense mutations in the proximal signalling region, on the other hand, disrupt signalling to the output domain, without influencing FAD binding, and are generally not resurrected by PAS suppressors (Bibikov et al., 2000; Watts et al., 2004; Ma et al., 2005). The cumulative evidence supports a model in which a contact domain between the HAMP AS-2 region and the Aer-PAS domain stabilizes FAD binding. It is likely that the aerotaxis signal is transmitted directly from the FAD-PAS domain to the HAMP or proximal signalling region, and then to the HCD.
Given the homodimeric nature of the Aer receptor (Ma et al., 2004), domain interactions that stabilize FAD binding or transduce the aerotaxis signal could feasibly occur in cis (within one subunit), or in trans (between cognate subunits). In the present study, we used intragenic complementation to address these questions. We identified inter-subunit (trans) interactions between the PAS and HAMP domains that stabilize FAD binding, but show that signal transduction from the N-terminal PAS input domain to the C-terminal output domain (the HCD) occurs in cis within a single Aer monomer. Further, we employed truncated Aer proteins to confirm these results, and identified the minimal Aer peptides required for aerotaxis signalling.
Results
Aer dimers signal with one functional PAS and HAMP domain
The multidomain structure of the Aer protein makes it a candidate for intragenic complementation analysis. In previous genetic studies, aer mutations that abolished aerotaxis were identified in the coding regions of the PAS (codons 1–119), HAMP (207–253), proximal signalling (254–271), and signalling (272–506) domains (Bibikov et al., 2000; Repik et al., 2000; Watts et al., 2004; Ma et al., 2005). We selected mutations from those studies (Fig. 2) and, using compatible plasmids, coexpressed two Aer proteins with missense mutations in different domains. Mutant Aer proteins were expressed in the aerotaxis- and recombination-deficient background of BT3400 (aer, tsr, recA). The expectation was that mutant Aer subunits would combine to form inactive homodimers, as well as a heterodimer containing one subunit from each mutant Aer protein. Transformants were screened on succinate semi-soft agar to identify heterodimers that recovered aerotaxis through intragenic complementation.
Fig. 2.
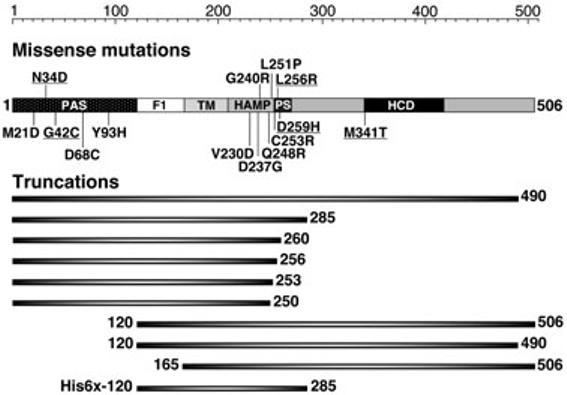
Aer domain organization summarizing relevant mutations and truncations. Missense mutations that had no effect on FAD binding are underlined to differentiate them from FAD-disrupting mutations. The Aer[120–285] truncation has an additional N-terminal six-histidine residue tag (His6x). TM, transmembrane domain; PS, proximal signalling region; HCD, highly conserved domain.
The utility of this technique was evident from initial experiments in which Aer-PAS and Aer-HAMP mutant proteins were coexpressed from compatible pACYC184- and pTrc99A-derived constructs. We designate mutant Aer proteins with an M1 (monomer 1) subscript if they are expressed from a pACYC184-derived plasmid, and an M2 (monomer 2) subscript if they are expressed from a pTrc99A-derived plasmid. Both M1 and M2 constructs were under the control of an isopropyl-β-D-thiogalactopyranoside (IPTG)-inducible, ptrc promoter. Heterodimers of the coexpressed Aer-PASM1 and Aer-HAMPM2 mutants recovered aerotaxis on succinate semi-soft agar plates, indicating Aer can function with one wild-type (WT) PAS and one WT HAMP sequence per dimer. For example, when the PAS mutant Aer-N34DM1 was coexpressed with the HAMP mutant Aer-V230DM2, or Aer-D68CM1 was coexpressed with Aer-G240RM2, aerotaxis was restored to BT3400 (Fig. 3). Following the success of these initial experiments, we designed a series of specific PAS, HAMP, signalling domain and truncation mutants to investigate the mechanics of signalling in Aer by intragenic complementation.
Fig. 3.
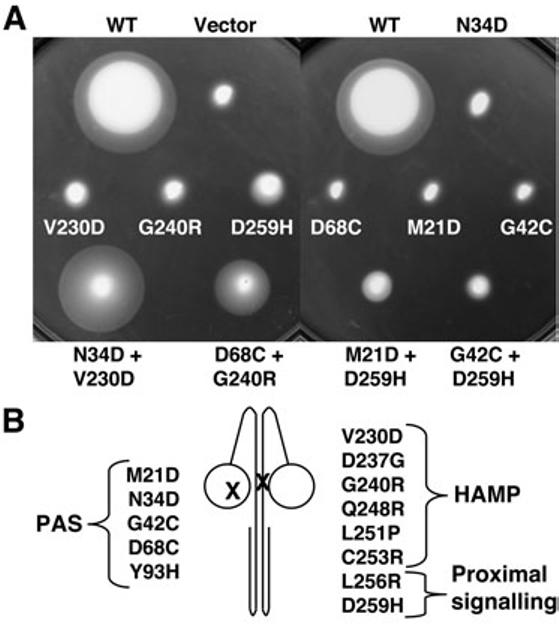
Intragenic complementation between mutant Aer proteins. A. Mutant Aer-PASM1 + Aer-HAMPM2 or Aer-proximal signallingM2 proteins were coexpressed from compatible plasmids in E. coli BT3400 (aer, tsr, recA). To test for aerotaxis, transformants were transferred to 30 mM succinate semi-soft agar and incubated at 30°C for 29 h. Representative examples are shown in which intragenic complementation restored (Aer-N34DM1 + Aer-V230DM2, and Aer-D68CM1 + Aer-G240RM2), partially restored (Aer-M21DM1 + Aer-D259HM2), or did not restore (Aer-G42CM1 + Aer-D259HM2) aerotaxis. The mutant Aer proteins formed non-aerotactic homodimers in BT3400 as shown. B. Cartoon of an Aer heterodimer summarizing missense mutations (X) in the PAS, HAMP and proximal signalling regions.
The HAMP domain stabilizes FAD binding to the cognate PAS domain
In Aer, missense mutations in the HAMP AS-2 region eliminate FAD binding to the PAS domain (Bibikov et al., 2000; Watts et al., 2004; Ma et al., 2005), and are functionally suppressed by missense mutations in the PAS domain (Watts et al., 2004). Whether the HAMP domain stabilizes FAD binding to the PAS domain of the same monomer, or to the cognate PAS domain, is not known. We extended our initial study, and specifically addressed this question by expressing binary combinations of five Aer-PASM1 mutants (Bibikov et al., 2000; Repik et al., 2000; Watts et al., 2004) and four FAD− Aer-HAMPM2 mutants [located between residues V230 and Q248 (Watts et al., 2004; Ma et al., 2005)] (Fig. 2). Non-functional PAS mutants were included that either abolished or retained FAD binding (Table 1). Aer-PAS residues M21, N34 and G42 have predicted locations in the PAS core. Residues D68 and Y93 have predicted locations in the helical connector and the β-scaffold of the PAS domain respectively (Taylor and Zhulin, 1999; Repik et al., 2000). We assume the Aer-PASM1 mutants disrupt FAD binding and/or inactivate signalling to their same PAS domain, because flavin binds directly to the homologous LOV and NifL PAS domains (Crosson and Moffat, 2001; Hefti et al., 2001).
Table 1.
Phenotypes of Aer heterodimers formed from PAS + HAMP or proximal signalling (PS) mutantsa.
| PASM1 |
||||||
|---|---|---|---|---|---|---|
| FAD+ |
FAD− |
|||||
| HAMP/PSM2 | N34D | G42C | D68C | Y93H | M21D | |
| FAD− | V230D | +b | + | + | + | + |
| D237G | + | + | + | + | +/−c | |
| G240R | + | + | + | + | + | |
| Q248R | + | + | + | + | + | |
| L251P | + | +/− | − | +/− | − | |
| C253R | +/− | +/− | +/− | +/− | +/− | |
| FAD+ | L256R | + | − | − | − | − |
| D259H | + | − | − | +/− | +/− | |
Complementation assays were performed as described in the legend to Fig. 3.
Phenotypes are designated as aerotactic (+), partially aerotactic (+/−) and non-aerotactic (−) as described in the text.
The diameter of partially aerotactic swarms was approximately 50% the diameter of aerotactic swarms. The rings of partially aerotactic swarms were also less well defined.
Each of the Aer-PASM1 + Aer-HAMPM2 heterodimers were aerotactic, promoting ring formation on succinate semi-soft agar (Table 1, Fig. 3). We interpret this to mean that the Aer-HAMPM2 mutants prevent FAD binding to the cognate Aer-PASM1 domain but not to the PASM2 domain. Otherwise neither PAS domain in the heterodimer would be functional. This was particularly evident where both the PAS and HAMP mutants disrupted FAD binding, because neither PAS domain in the heterodimer would bind FAD. Therefore, PAS/HAMP interactions must be inter-subunit, resulting in aerotactic heterodimers containing one functional PAS domain, and a functional cognate HAMP domain. The heterodimers continued to support aerotaxis when more severe lesions were introduced into the HAMP domain (V230D/G240R, V230D/D237G and V230D/Q248R), although some of the responses were weaker (Table S1).
The complementation experiments were repeated using FAD− mutants with mutations at the C-terminus of the HAMP domain [L251P and C253R (Watts et al., 2004)] and FAD+ mutants with mutations in the proximal signalling region [L256R and D259H (Ma et al., 2005)] (Fig. 2). Overall, these mutants were less successful than the previous HAMP mutants in complementing the various Aer-PASM1 mutants; resultant swarms ranged from non-functional to partially functional, and aerotactic (−, +/− and + respectively, in Table 1; also see Fig. 3). Partially aerotactic heterodimer swarms were smaller than their aerotactic counterparts, and contained less-defined rings (see example in Fig. 3). It is possible that these residues (251–259) occur at a critical juncture, and play different roles in signalling and in stabilizing FAD binding than the preceding HAMP residues (230–248). Poor complementation could also reflect lower cellular levels of the Aer-PASM1 mutants expressed from the pACYC184 plasmid (Fig. S1), which has a lower copy number than pTrc99A. We previously reported that Aer mutants are often found at lower concentrations in the cell, even though they have normal mRNA levels (Ma et al., 2005). This suggests a higher rate of post-translational turnover. The success of Aer-N34DM1 in complementing mutations in this region (residues 230–259, Table 1) may be related to its higher expression compared with the other Aer-PASM1 mutants (Fig. S1).
The only M2 mutant not efficiently complemented by Aer-N34DM1 was Aer-C253RM2 (Table 1). This finding was unexpected because N34D is an allele-specific suppressor for C253R (Watts et al., 2004), and expression differences do not appear to explain the result. As an alternative explanation for poor complementation, we tested for phenotypic dominance by coexpressing the C-terminal HAMPM2 and proximal signallingM2 mutants with a WT AerM1 construct where WT expression was induced from a tightly regulated pnahG promoter. Titrations were performed with sodium salicylate (the WT M1 construct) and IPTG (M2 mutant constructs) to determine induction levels resulting in an M1/M2 expression ratio of approximately 1:1. Under these conditions, Aer-C253R and Aer-D259H were dominant over WT Aer and prevented aero-taxis, while Aer-L251P and Aer-L256R were recessive to WT function (Fig. S2). The dominant behaviour of Aer-C253R and Aer-D259H help explain the inferior complementation observed using these mutants (Table 1). In addition, dominant Aer-C253R homodimers could be expected to inhibit signalling by Aer-N34DM1 + Aer-C253RM2 heterodimers, resulting in the partially aerotactic phenotype observed.
Aer signals through one subunit
After finding that PAS–HAMP interactions stabilize FAD binding asymmetrically, we wanted to determine whether signals sensed by the PAS domain are transduced to the signalling region of the same monomer, or to the cognate monomer. To distinguish between these possibilities, an M341T missense mutation (Bibikov et al., 2000) was added to the signalling domain. As shown in Fig. 4, heterodimers were only aerotactic when functional PAS and signalling domains were located on the same monomer (Aer-N34D/M341TM1 + Aer-Q248RM2), arguing for signalling within one subunit of the Aer dimer. An Aer-Q248RM1 + Aer-M341TM2 heterodimer was similarly aerotactic (Fig. 5). The non-aerotactic Aer-N34DM1 + Aer-Q248R/M341TM2 heterodimer, which simulated an inter-subunit signalling pathway (Fig. 4), was created from constructs with comparable, stable, expression levels. Therefore expression differences did not contribute to the loss of aerotaxis when M341T was swapped from the N34DM1 to the Q248RM2 monomer (data not shown).
Fig. 4.
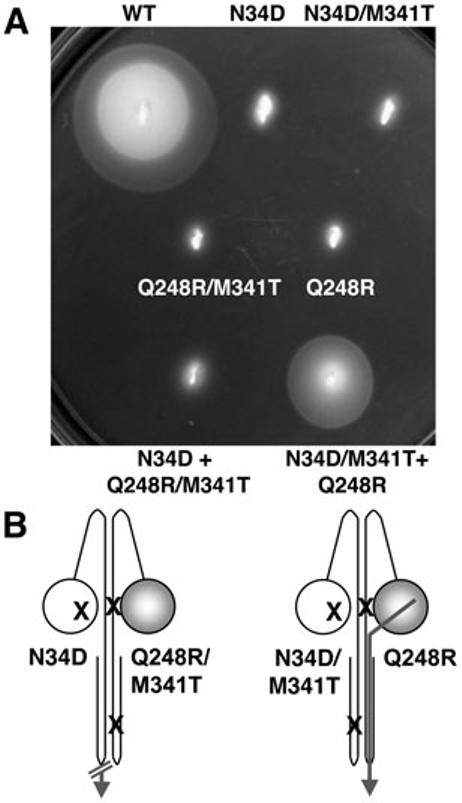
Differentiating between intra- and inter-subunit signal transduction. A. Complementation assays were performed as described in the legend to Fig. 3, except swarm plates were incubated for 24 h. The Aer-N34DM1 + Aer-Q248R/M341TM2 heterodimer and the Aer-N34D/M341TM1 + Aer-Q248RM2 heterodimer were non-aerotactic and aerotactic respectively, supporting a signalling pathway within one subunit. B. Cartoons of Aer heterodimers showing missense mutations (X). An intra-subunit signalling pathway is indicated by a continuous arrow, and a defective signalling pathway by a blocked arrow. A shaded PAS domain (circle) denotes FAD binding.
Fig. 5.
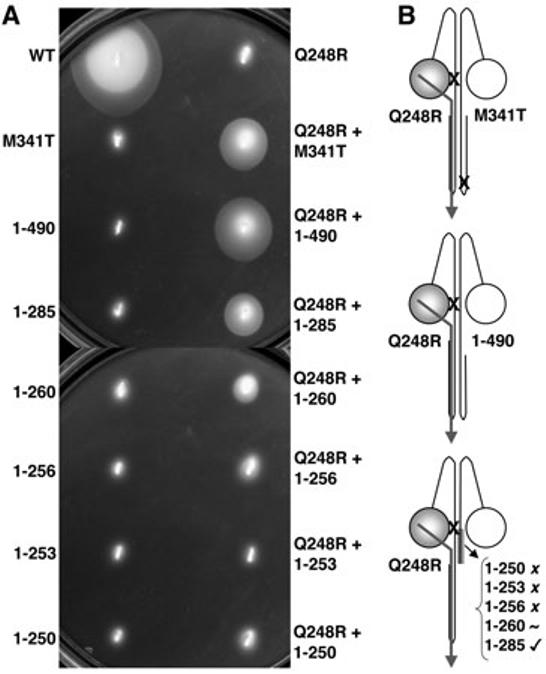
Effect of C-terminal truncations on intragenic complementation by Aer-Q248RM1. A. Complementation assays were performed as described in the legend to Fig. 4. B. Cartoons of Aer heterodimers showing missense mutations (X) and C-terminal truncations. Aer-Q248RM1 + either Aer-M341T or Aer[1–490] were aerotactic. A shaded box on the lower cartoon represents residues 250–285. The activity of heterodimers with truncations from Aer[1–250] to Aer[1–285] is indicated as non-aerotactic (X), partially aerotactic (∼) or aerotactic ([unk]). An intra-subunit signalling pathway is indicated by an arrow and a shaded PAS domain (circle) denotes FAD binding.
To confirm the intra-subunit signalling pathway hypothesis, we created C-terminal Aer truncations and coexpressed them with full-length Aer-Q248RM1 (1–506). The idea was to disrupt FAD binding to the PASM2 domain of each truncated monomer, so that the M1 subunit would become the signalling monomer. Truncations of the Aer C-terminus were made at residues 490 and 285 in the signalling domain (Δ491–506 and Δ286–506 respectively), at residues 260 and 256 in the proximal signalling domain (Δ261–506 and Δ257–506 respectively), at residue 253 on the HAMP/proximal signalling domain boundary (Δ254–506), and within the HAMP domain at residue 250 (Δ251–506) (Fig. 2). None of these truncations were aerotactic as homodimers (Fig. 5A).
Truncations that deleted the terminal 16 residues (Aer[1–490]M2), or most of the signalling domain (Aer[1–285]M2), from the non-signalling subunit, did not disrupt aerotaxis of heterodimers with Aer-Q248RM1 (Fig. 5). This indicates that the M2 signalling domain is not necessary for aerotaxis, and the M1 signalling domain is sufficient. The smaller swarm size seen with the Aer[1–285]M2 heterodimer (Fig. 5) may be due to the loss of CheA/W interaction sites from Aer[1–285], or due to high expression of the mutant protein (approximately threefold greater than WT AerM2). Aer[1–490]M2 contains CheA/W interaction sites and had similar expression to WT AerM2. Two complete HAMP domains were required for aerotaxis in the truncation heterodimers, because function was lost with truncations between residues 260 and 256. The Aer-Q248RM1 + Aer[1–260]M2 heterodimer retained some aerotactic capacity, whereas the Aer[1–256]M2, Aer[1–253]M2 and Aer[1–250]M2 truncation heterodimers were non-aerotactic (Fig. 5). This was not related to expression differences, because Aer[1–260]M2 and Aer[1–256]M2 expression was similar to Aer-WTM2.
To determine if residues deleted from the signalling monomer would affect aerotaxis, Aer[1–490]M2 was coexpressed with full-length Aer-N34DM1. FAD should bind to the Aer[1–490] PASM2 domain of this heterodimer, and the truncated M2 subunit becomes the putative signalling monomer. Deletion of just 16 residues from the C-terminus of the signalling monomer abolished function in the Aer-N34DM1 + Aer[1–490]M2 heterodimer (Table S1). This is in contrast to deletion of the non-signalling Aer subunit in the Aer-Q248RM1 + Aer-truncationM2 heterodimers above, where approximately 240 residues could be removed, and aerotaxis retained.
Sensing with one PAS domain
When the Aer-PASM1 mutants (N34D, G42C, D68C, Y93H and M21D) were coexpressed with Aer-N34DM2, we observed no complementation between PAS mutations, consistent with a requirement for one functional PAS domain per dimer (Table S1). To confirm that aerotaxis requires only one PAS domain, N-terminal Aer-PASM2 truncations were constructed (Fig. 2) and coexpressed with Aer-Q248RM1 (Fig. 6). Heterodimers with Aer[120–506]M2 (with the PASM2 domain deleted), or Aer[120–490]M2 (with the PASM2 domain and part of the C-terminalM2 signalling domain deleted), produced aerotactic swarms, although they were smaller than most other aerotactic heterodimer swarms (see enlargement in Fig. 6B). Aer can therefore sense and signal with one PAS domain per dimer, extending our previous findings in which aerotaxis was supported with one functional and one inactive PAS domain (Table 1 and Fig. 3). The PAS truncation heterodimers also provide evidence that Q248RM1 disrupts FAD binding to the cognate PASM2 domain, as posited previously. Aer-Q248RM1 could not disrupt FAD binding to the PASM1 domain in cis, because it was the only PAS domain present, and the heterodimers retained aerotactic function.
Fig. 6.
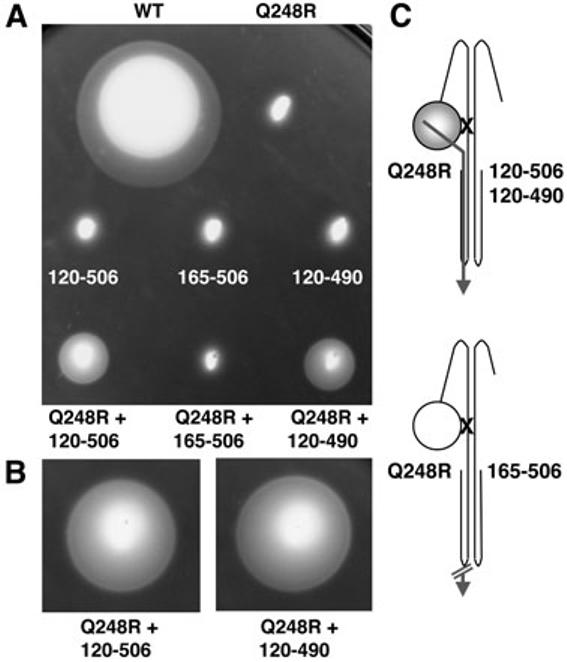
The minimal sequence required for aerotaxis in a heterodimer with Aer-Q248RM1. A. Complementation assays were performed as described in the legend to Fig. 3. The Aer-Q248RM1 + Aer[120–506]M2, and Aer-Q248RM1 + Aer[120–490]M2 heterodimers were aerotactic, whereas the Aer-Q248RM1 + Aer[165–506]M2 heterodimer was non-aerotactic. B. A 34 h incubation and enlargement of the Aer-Q248RM1 + Aer[120–506]M2, and Aer-Q248RM1 + Aer[120–490]M2 swarms. C. Cartoons of Aer heterodimers showing Q248R (X) and truncations. An intra-subunit signalling pathway is designated by a continuous arrow, and a defective signalling pathway by a blocked arrow. A shaded PAS domain (circle) denotes FAD binding.
An Aer construct lacking both the PAS and F1 domains (Aer[165–506]M2) was also assayed for its ability to support aerotaxis in a heterodimer with Aer-Q248RM1. Although Aer[165–506]M2 had similar expression levels to Aer[120–506]M2, a heterodimer between Aer[165–506]M2 and Aer-Q248RM1 was non-aerotactic (Fig. 6). One interpretation is that the F1 domain is needed to stabilize FAD binding (Bibikov et al., 2000) through inter-subunit interactions (in a similar manner to the HAMP domain). Alternatively, the F1 region may be necessary for proper folding or for correct membrane insertion. An Aer peptide lacking the PAS and C-terminal signalling domains (Aer[120–285]M2) was also non-aerotactic when coexpressed with Aer-Q248RM1 (Table S1). Although not tested, this short Aer peptide may not fold correctly, or dimerize with full-length Aer.
Discussion
The presence of both a PAS and HAMP domain in the Aer receptor enabled us to investigate PAS/HAMP interrelationships. We determined that FAD binding was stabilized by PAS and HAMP domains from cognate subunits (Fig. 3 and Table 1), but that the signal transduction pathway proceeded from the PAS domain to the signalling domain of the same subunit (Figs 4-6, and Table S1). The asymmetric role of two Aer subunits in FAD binding was demonstrated by intragenic complementation between PAS mutants and HAMP mutants (residues 230–248 in AS-2, Table 1). Substitutions in residues 251–259 showed incomplete complementation, raising the possibility that these residues have a different role from residues 230–248. We considered an alternative possibility that the WT HAMP domain exerted its effects indirectly by correcting malformations in the mutant HAMP domain, which itself could resurrect FAD binding and aerotaxis. However, this is unlikely, given the range of HAMP defects that were compensated in heterodimers. Although it was reported that symmetric signalling through both HAMP domains is necessary in Tar/EnvZ chimeras (Zhu and Inouye, 2004), the Aer protein required only one WT HAMP sequence for signalling.
Previous studies support a model in which the native fold of the Aer-PAS domain is stabilized by protein/protein interactions with the HAMP domain (Herrmann et al., 2004; Watts et al., 2004). The results of the present study suggest the PAS/HAMP contact domain is between cog-nate Aer subunits (see Fig. 7A). Although there is a crossover between the PAS and HAMP domains, the signalling pathway does not continue down the cognate subunit (Figs 4-6). This finding raises questions about the role of the HAMP domain in signalling. We propose three possible models for signalling in Aer (Fig. 7B-D). In the first model (Fig. 7B), the signalling pathway from a PAS domain is via the HAMP domain on the same monomer. The model assumes that signalling though the HAMP domain is not disrupted by HAMP mutations such as those in Table 1. In the second model (Fig. 7C), signalling crosses over from the PASM1 domain to the cognate HAMPM2 domain, and returns to the signallingM1 domain. The cognate HAMP domain had a WT sequence in all aerotactic heterodimers tested in this study. In the third model (Fig. 7D), the signal is transmitted directly from the PAS domain to the proximal signalling domain of the same subunit. By bypassing either HAMP domain, potential difficulties in propagating signals despite HAMP defects are avoided. Irrespective of a specific model, PAS signals are apparently transduced to the proximal signalling domain before residue 260, because Aer/Tsr chimeras incorporating the first 260 residues from Aer are fully functional (Repik et al., 2000). The model may also account for the different complementation pattern observed for residues 251–259 in Table 1.
Fig. 7.
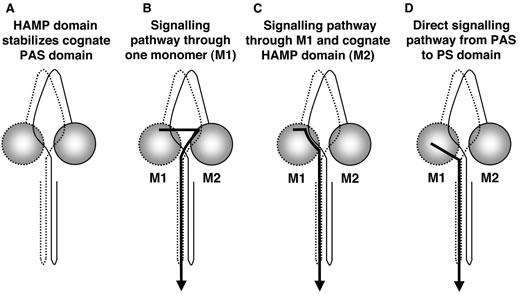
Signalling models for the Aer protein. A. Cartoon of Aer with the HAMP domain from one subunit stabilizing FAD binding to the PAS domain (circle) of the cognate subunit through protein–protein interaction. B–D. Three possible signalling pathways in which (B) The signal is transduced through one monomer (M1) including the HAMP domain (C) The signal is transduced through the PAS and signalling domains of one monomer (M1), but the HAMP domain of the cognate monomer (M2), and (D) The signal is transduced directly from the PAS domain to the proximal signalling (PS) domain of the same monomer, bypassing both HAMP domains. Although either PAS domain can initiate signalling in an Aer dimer, signals initiated by just one PAS domain are shown.
Heterodimers between full-length and truncated Aer monomers were used to investigate the minimum requirements for the non-signalling subunit. Deletion of the PAS (Fig. 6) or signalling (Fig. 5) domain did not prevent aerotaxis in heterodimers. However, deletion of part of the HAMP domain abolished signalling (Fig. 5), probably by disrupting FAD binding to the signalling PAS domain. The F1 domain (Fig. 1) was also required for signalling (Fig. 6). Similar C-terminal truncations of the non-signalling subunit in the Tar chemoreceptor have been reported, except that part of the HAMP domain could also be deleted without inactivating signalling (Gardina and Manson, 1996; Tatsuno et al., 1996). As chemoreceptors form trimers of dimers (Kim et al., 1999; 2002; Ames et al., 2002), receptors with a truncated signalling domain likely create dimeric signalling domains by swapping domains (Gardina and Manson, 1996) within a trimer of dimers.
The full-length signalling subunit was required for aerotaxis by Aer. Deletion of 16 residues from the C-terminus abolished signalling by both homodimers and heterodimers (Table S1). This differs from Tar, which activates CheA normally when 35–37 residues are removed from the C-terminus (Russo and Koshland, 1983; Francis et al., 2004). However, the C-terminus of Tar extends 39 residues beyond that of Aer. By analogy to other chemoreceptors (Le Moual and Koshland, 1996; Kim et al., 1999), it is likely that the C-terminal segment of Aer forms an anti-parallel coiled-coil with the proximal signalling domain. If so, C-terminal truncations could destabilize the proximal signalling region and abolish aerotaxis.
In this study we established that the HAMP domain stabilizes FAD binding asymmetrically to the cognate PAS domain, but that the signalling pathway proceeds in cis, within one Aer subunit. The role of the HAMP and proximal signalling domains is intriguing. Although both HAMP domains are required for function, missense mutations in the HAMP domain are tolerated by the signalling subunit. Further studies are required to determine whether the Aer-HAMP domain has a direct role in signalling, in addition to its role in stabilizing the native fold of the cognate PAS domain.
Experimental procedures
Bacterial strains and plasmids
The recA gene was inactivated in E. coli strain BT3312 [aer, tsr (Repik et al., 2000)] by P1 transduction of recA::cat from BW10724 (Wanner and Boline, 1990; Metcalf and Wanner, 1993), creating BT3400 (aer, tsr, recA). RecA inactivation was confirmed by UV sensitivity (Fletcher et al., 1997). Bacteria were grown at 30°C in Luria–Bertani (LB) medium (Davis et al., 1980) supplemented with 0.5 μg ml−1 thiamine (all strains) and 25 μg ml−1 chloramphenicol (BW10724 and BT3400).
Parental plasmids included pACYC184 (Chang and Cohen, 1978), pTrc99A (Pharmacia, discontinued) and pProEX™HTa (Invitrogen, Carlsbad, CA). The pACYC184 plasmid contains a p15A replication origin, allowing coexpression of genes with pTrc99A or pProEX™HTa plasmids, both of which are members of the ColE1 compatibility group. Relevant strain and plasmid properties are given in Table S2.
Construction of Aer mutants
A pTrc99A derivative expressing WT aer under the control of an IPTG-inducible ptrc promoter, pKW1, and several pKW1 derivatives expressing Aer missense mutants were constructed previously (Watts et al., 2004). Constructs expressing Aer-M341T (Bibikov et al., 2000) or Aer-L256R were constructed by site-directed mutagenesis using pKW1 as template, PfuTurbo® (Stratagene, La Jolla, CA), and primers specific for the introduction of each mutation. Primers were designed as outlined in the QuikChange® site-directed mutagenesis kit (Stratagene). Aer-L256R was identified as a null FAD+ aerotaxis mutant by site-specific mutagenesis of residues in proximity to C253. To create the double HAMP mutants V230D/D237G, V230D/G240R and V230D/Q248R, plasmids pKW2 (Aer-D237G), pKW3 (Aer-G240R) and pKW11 (Aer-Q248R) were amplified with PfuTurbo® using V230D-specific primers. A construct expressing Aer-Q248R/M341T was made by site-directed mutagenesis using pKW11 (Aer-Q248R) and M341T-specific primers.
Constructs expressing Aer-D68C (Repik et al., 2000), Aer-G42C (Repik et al., 2000) and Aer-Y93H (Bibikov et al., 2000) were made by site-directed mutagenesis using pKW1 as template with mutation-specific primers. To transfer DNA encoding Aer-D68C (pKW86) and Aer-N34D (pKW74) into pACYC184, the plasmid region encompassing the ptrc promoter, mutated aer, and lacIq was removed with SphI, treated with Klenow, and ligated into the ScaI site of pACYC184. The pACYC184/aer mutant pKW89 (Aer-N34D) was then used as a template to clone different aer mutants into pACYC184. Plasmids encoding Aer-M21D (pKS1), Aer-Q248R (pKW11), Aer-G42C (pKW87) and Aer-Y93H (pKW88) were digested with HpaI and PstI to remove a 2 kb fragment comprising aer and a 500 bp fragment of pTrc99A. These fragments were ligated into pKW89 with the corresponding HpaI–PstI fragment (including the region encoding N34D) removed. A construct expressing Aer-N34D/M341T from pACYC184 was made by digesting pKW89 with KpnI and PstI, and swapping the 1.2 kb WT aer fragment with the KpnI–PstI fragment from pKW81 (Aer-M341T).
C-terminal Aer truncations were created by polymerase chain reaction (PCR) of pKW1 using a primer complementary to the SphI site at pTrc99A nucleotide 2962 (pTrcSphIF). This was paired with individual primers containing stop codons after Aer residues 490, 285, 260, 256, 253 and 250, followed by an engineered SphI site (AerSphIR primers). PCR products were digested with SphI and ligated to the 2.64 kb SphI fragment of pTrc99A.
N-terminal Aer truncations, Δ1–119 and Δ1–164, were created by PCR of pKW1 using primers complementary to the NcoI site at pTrc99A nucleotide 265 (Aer120NcoIF and Aer165NcoIF), each paired with a primer containing an engineered SalI site (Aer506SalIR). The Aer120NcoIF and Aer165NcoIF primers incorporated a start codon, followed by the codon for residue 120 or 165, while the Aer506SalIR primer included the codon for residue 506 followed by the normal stop codon of Aer. PCR products were digested with NcoI and SalI and ligated to the 4.14 kb NcoI–SalI fragment of pTrc99A. An Aer Δ[1–119/491–506] construct was made by the same method using Aer120NcoIF and Aer490SalIR primers. An Aer Δ1–119/Δ286–506 construct was made by a similar method using Aer120NcoIF and Aer285SalIR primers, except the PCR product was ligated into the NcoI and SalI sites of pProEX™Hta, which encodes an N-terminal His6x tag. Primer sequences for the construction of all truncations are provided in Table S2. The expected mutations and/or truncations were confirmed for all constructs by DNA sequencing. The expression of full-length and truncated Aer proteins were verified by chemiluminescent Western blotting using Anti-Aer2–166 antisera (Repik et al., 2000), except for Aer[120–285], where expression was verified using an INDIA™ HisProbe (Pierce, Rockford, IL).
Complementation assays
BT3400 was transformed with individual pTrc99A/aer or pACYC184/aer derivatives to create Aer homodimers, or simultaneously with both plasmids to create Aer heterodimers. Transformants were selected on LB thiamine (0.5 μg ml−1) agar plates containing 100 μg ml−1 ampicillin (pTrc99A constructs), 15 μg ml−1 tetracycline (pACYC184 constructs), or both ampicillin and tetracycline (pTrc99A and pACYC184 constructs). To analyse aerotaxis phenotypes, individual BT3400 transformant colonies were transferred with sterile toothpicks into 30 mM succinate semi-soft agar swarm plates containing 0.28% agar and either 50 μg ml−1 ampicillin, 7.5 μg ml−1 tetracycline, or both antibiotics. A minimum of four clones were tested on semi-soft agar, and each experiment was repeated two to four times. Initially, we used BT3400/pKW1 as a positive aerotaxis control, and BT3400/pTrc99A as a negative aerotaxis control in swarm plates containing ampicillin. Heterodimers containing Aer-N34D and Aer-V230D were used as positive aerotaxis controls on all swarm plates after initial experiments with these mutants showed intragenic complementation. Swarm plates were incubated for 24–48 h at 30°C and analyses were made at 12, 24 and 48 h. Appropriate homodimer controls were carried out simultaneously with every heterodimer experiment.
Dominance tests
To test for dominant behaviour, mutant Aer constructs were co-transformed with pDS7, which expresses WT Aer from pACYC184 using a tightly regulated sodium salicylateinducible promoter (nahG). The pDS7 plasmid was constructed from pKG117 (kindly provided by K. Gosink) by adding the tetracycline-resistance gene back to the pACYC184 portion of the plasmid with ClaI. Dominance tests were performed the same way as the heterodimer assays outlined above, except 0.5 μM or 1 μM sodium salicylate, and IPTG concentrations between 0 and 0.6 mM were included in the swarm plates in a series of titrations. Induction levels producing approximately 1:1 expression ratios were determined by Western blot.
Supplementary Material
Acknowledgements
We are grateful to Sheena Fry and Nathan Abraham for technical assistance, to Asharie Campbell for constructing pAJC1, to Daniel Salcedo for constructing pDS7, and to Kirsten Sommer for providing pKS1. We would like to thank Barry Wanner for providing BW10724, Khoosheh Gosink and John S. Parkinson for providing pKG117 before publication, and Michael Manson and J.S. Parkinson for helpful discussions. This work was supported by a grant from the National Institute of General Medical Sciences (GM29481) to B.L. Taylor, and a Loma Linda University School of Medicine Research Seed Grant to M.S. Johnson.
Footnotes
Supplementary material
This material is available as part of the online article from http://www.blackwell-synergy.com
References
- Ames P, Studdert CA, Reiser RH, Parkinson JS. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc Natl Acad Sci USA. 2002;99:7060–7065. doi: 10.1073/pnas.092071899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Ponting CP. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol Lett. 1999;176:111–116. doi: 10.1111/j.1574-6968.1999.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Bibikov SI, Biran R, Rudd KE, Parkinson JS. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov SI, Barnes LA, Gitin Y, Parkinson JS. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5830–5835. doi: 10.1073/pnas.100118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Kaplan N, Hess JF, Simon MI. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci USA. 1989;86:1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Falke JJ. Cysteine and disulfide scanning reveals two amphiphilic helices in the linker region of the aspartate chemoreceptor. Biochemistry. 1998;37:10746–10756. doi: 10.1021/bi980607g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AC, Cohen SN. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson S, Moffat K. Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc Natl Acad Sci USA. 2001;98:2995–3000. doi: 10.1073/pnas.051520298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RW, Botstein D, Roth JR. Advanced Bacterial Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1980. [Google Scholar]
- Fletcher HM, Morgan RM, Macrina FL. Nucleotide sequence of the Porphyromonas gingivalis W83 recA homolog and construction of a recA-deficient mutant. Infect Immun. 1997;65:4592–4597. doi: 10.1128/iai.65.11.4592-4597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NR, Wolanin PM, Stock JB, Derosier DJ, Thomas DR. Three-dimensional structure and organization of a receptor/signaling complex. Proc Natl Acad Sci USA. 2004;101:17480–17485. doi: 10.1073/pnas.0407826101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardina PJ, Manson MD. Attractant signaling by an aspartate chemoreceptor dimer with a single cytoplasmic domain. Science. 1996;274:425–426. doi: 10.1126/science.274.5286.425. [DOI] [PubMed] [Google Scholar]
- Gegner JA, Graham DR, Roth AF, Dahlquist FW. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992;70:975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Hefti M, Hendle J, Enroth C, Vervoort J, Tucker PA. Crystallization and preliminary crystallographic data of the PAS domain of the NifL protein from Azotobacter vinelandii. Acta Crystallogr D Biol Crystallogr. 2001;57:1895–1896. doi: 10.1107/s0907444901015657. [DOI] [PubMed] [Google Scholar]
- Herrmann S, Ma Q, Johnson MS, Repik AV, Taylor BL. PAS domain of the Aer redox sensor requires C-terminal residues for native-fold formation and flavin adenine dinucleotide binding. J Bacteriol. 2004;186:6782–6791. doi: 10.1128/JB.186.20.6782-6791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Yokota H, Kim SH. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature. 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- Kim SH, Wang W, Kim KK. Dynamic and clustering model of bacterial chemotaxis receptors: structural basis for signaling and high sensitivity. Proc Natl Acad Sci USA. 2002;99:11611–11615. doi: 10.1073/pnas.132376499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moual H, Koshland DE., Jr. Molecular evolution of the C-terminal cytoplasmic domain of a super-family of bacterial receptors involved in taxis. J Mol Biol. 1996;261:568–585. doi: 10.1006/jmbi.1996.0483. [DOI] [PubMed] [Google Scholar]
- Ma Q, Roy F, Herrmann S, Taylor BL, Johnson MS. The Aer protein of Escherichia coli forms a homodimer independent of the signaling domain and FAD binding. J Bacteriol. 2004;186:7456–7459. doi: 10.1128/JB.186.21.7456-7459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Johnson MS, Taylor BL. Genetic analysis of the HAMP domain of the Aer aerotaxis sensor localizes flavin adenine dinucleotide-binding determinants to the AS-2 helix. J Bacteriol. 2005;187:193–201. doi: 10.1128/JB.187.1.193-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally DF, Matsumura P. Bacterial chemotaxis signaling complexes: formation of a CheA/CheW complex enhances autophosphorylation and affinity for CheY. Proc Natl Acad Sci USA. 1991;88:6269–6273. doi: 10.1073/pnas.88.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf WW, Wanner BL. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene. 1993;129:27–32. doi: 10.1016/0378-1119(93)90692-v. [DOI] [PubMed] [Google Scholar]
- Rebbapragada A, Johnson MS, Harding GP, Zuccarelli AJ, Fletcher HM, Zhulin IB, Taylor BL. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc Natl Acad Sci USA. 1997;94:10541–10546. doi: 10.1073/pnas.94.20.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik A, Rebbapragada A, Johnson MS, Haznedar JO, Zhulin IB, Taylor BL. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol Microbiol. 2000;36:806–816. doi: 10.1046/j.1365-2958.2000.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AF, Koshland DE., Jr. Separation of signal transduction and adaptation functions of the aspartate receptor in bacterial sensing. Science. 1983;220:1016–1020. doi: 10.1126/science.6302843. [DOI] [PubMed] [Google Scholar]
- Tatsuno I, Homma M, Oosawa K, Kawagishi I. Signaling by the Escherichia coli aspartate chemoreceptor Tar with a single cytoplasmic domain per dimer. Science. 1996;274:423–425. doi: 10.1126/science.274.5286.423. [DOI] [PubMed] [Google Scholar]
- Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Zhulin IB, Johnson MS. Aerotaxis and other energy-sensing behavior in bacteria. Annu Rev Microbiol. 1999;53:103–128. doi: 10.1146/annurev.micro.53.1.103. [DOI] [PubMed] [Google Scholar]
- Taylor BL, Johnson MS, Watts KJ. Signal transduction in prokaryotic PAS domains. In: Crews S, editor. PAS Proteins: Regulators and Sensors of Development and Physiology. Kluwer Academic Publisher; Norwell, MA: 2003. pp. 15–50. [Google Scholar]
- Wadhams GH, Armitage JP. Making sense of it all: bacterial chemotaxis. Nat Rev Mol Cell Biol. 2004;5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- Wanner BL, Boline JA. Mapping and molecular cloning of the phn (psiD) locus for phosphonate utilization in Escherichia coli. J Bacteriol. 1990;172:1186–1196. doi: 10.1128/jb.172.3.1186-1196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KJ, Ma Q, Johnson MS, Taylor BL. Interactions between the PAS and HAMP domains of the Escherichia coli aerotaxis receptor Aer. J Bacteriol. 2004;186:7440–7449. doi: 10.1128/JB.186.21.7440-7449.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Stewart V. Functional similarities among two-component sensors and methyl-accepting chemotaxis proteins suggest a role for linker region amphipathic helices in transmembrane signal transduction. Mol Microbiol. 1999;33:1093–1102. doi: 10.1046/j.1365-2958.1999.01562.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Inouye M. The HAMP linker in histidine kinase dimeric receptors is critical for symmetric transmembrane signal transduction. J Biol Chem. 2004;279:48152–48158. doi: 10.1074/jbc.M401024200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


