Abstract
Transplanted adult bone marrow-derived cells (BMDCs) have been shown to adopt the phenotype and function of several nonhematopoietic cell lineages and promote tumorigenesis. Beyond its cancer enhancing potential, cell fusion has recently emerged as an explanation of how BMDCs regenerate diseased heptocytes, contribute to Purkinje neurons and skeletal and cardiac muscle cells, and participate in skin and heart regeneration. Although bone marrow-derived epithelial cells also have been observed in the intestine, fusion as a mechanism has not been investigated. Here, we show that transplanted BMDCs fuse with both normal and neoplastic intestinal epithelium. Long-term repopulation by donor-derived cells was detected in all principal intestinal epithelial lineages including enterocytes, goblet cells, Paneth cells, and enteroendocrine cells, suggesting that the fusion partners of the BMDCs are long-lived intestinal progenitors or stem cells. Fusion of BMDCs with neoplastic epithelium did not result in tumor initiation. Our findings suggest an unexpected role for BMDCs in both regeneration and tumorigenesis of the intestine.
Keywords: intestine, tumorigenesis, cell fusion, tissue regeneration
The use of adult stem cells for therapeutic treatment of human disease has great potential. Recently, adult stem cells were successfully used for gene therapy in a mouse model of Type 1 tyrosinema (1) and demonstrated to accelerate wound healing in a human burn patient (2). Although the biological significance and potential clinical applications of the incorporation of bone marrow-derived cells (BMDCs) are unquestionably important (2–5), the underlying mechanism of cell incorporation into other organs remains unclear. The mechanism of cellular fusion of BMDCs with host cells in organs such as the liver has been elegantly illustrated by using serially transplanted cells and extensive karyotyping of engrafted cells (6, 7). Other studies, however, suggest that BMDCs incorporate into organs through mechanisms whereby the transplanted cells transdifferentiate into tissue-specific stem cells (8–10). The means of BMDC incorporation in the intestine, however, have not been fully defined. Recent reports illustrate that BMDCs incorporate into the pericryptal fibroblast region of the small intestine (11) and contribute to the intestinal epithelium of human gender-mismatched bone marrow transplants (12). However, neither of these observations has been attributed to a cellular fusion mechanism. To understand the role of BMDC contribution in the progression of disease or the regeneration of damaged tissue, the underlying mechanism and cellular participants must be identified.
Although it may be possible that BMDCs participate in intestinal epithelial regeneration or repair through a variety of different mechanisms, it has been hypothesized that circulating BMDCs could fuse with transformed intestinal epithelium to confer phenotypic and genotypic diversity to the tumor (13) and participate in tumorigenesis (14–16). It is intriguing to hypothesize that these rare fusion events may lead to a cell type that possesses greater metastatic characteristics. Currently, in vitro studies support this hypothesis (17), although in vivo occurrences of this phenomenon have been difficult to prove. In this study, we show that transplanted BMDCs can incorporate into the intestinal epithelium of gamma-irradiated mice through a fusion mechanism and provide evidence that the intestinal stem cell is the fusion partner. Finally, we provide in vivo evidence that strongly supports the hypothesis that fusion between circulating hematopoietic cells and transformed intestinal epithelium is involved in intestinal tumorigenesis.
Results and Discussion
To determine the fate of BMDCs in intestinal epithelium, we transplanted whole bone marrow or hematopoietic stem cells (HSCs) from female EGFP- or β-gal-expressing mice into lethally irradiated male recipients. As early as 2 weeks and for as long as 14 months after transplant, donor-derived cells were detected in both crypt and villus epithelium, and lamina propria of the small intestine at a sustained level (Fig. 1A–C). EGFP- and β-gal-expressing crypts identified by whole-mount analysis were confirmed with antibody staining of intestinal tissue sections. Moreover, donor-derived cells were detected readily in the pericryptal mesenchyme as reported in ref. 11. Similar results were observed after transplantation of highly purified HSCs (Fig. 1 D–F). The architecture of crypt-villus units containing EGFP-expressing cells was morphologically indistinguishable from regions devoid of epithelial-derived donor cells. EGFP-expressing cells were present in discontinuous regions throughout the epithelium, a finding that is likely due to variegation of EGFP transgene expression that is also observed within the intestinal epithelium of the transgenic donor mouse (Fig. 7, which is published as supporting information on the PNAS web site). In contrast, β-gal is ubiquitously expressed in the intestinal epithelium of reverse orientation splice acceptor 26 (ROSA26) mice (18), which prompted us to use it as a marker to determine the efficiency of donor cell incorporation. For this calculation, we counted the number of donor-derived intestinal crypts in whole-mount-prepared intestines from transplanted mice (Fig. 1B). Although donor-derived intestinal crypts were present throughout the length of the intestine, they were most frequent in the distal region of the small intestine and represented 0.25 ± 0.05% of total crypts (n = 4 animals). The level of donor cell incorporation into the intestine was similar for both whole bone marrow- and HSC-transplanted intestines, implicating an HSC or its progeny as the donor cell responsible. In these mice, chimerism in the peripheral blood ranged from 80% to 90%. The percentage of donor-derived cells in the intestine is consistent with levels reported in other tissues (19).
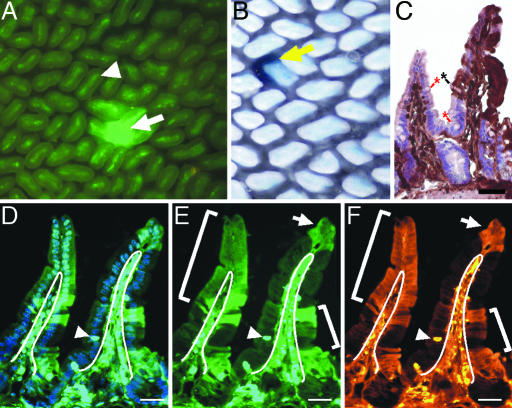
Fig. 1.
BMDCs incorporate into intestinal epithelium. (A) Whole-mount preparation of the distal small intestine of a lethally irradiated (12 Gy) recipient mouse transplanted with 1 × 106 EGFP-expressing BMDCs, 6 months after transplantation. Arrow indicates EGFP-positive epithelial cells populating a crypt and an adjacent villus. Arrowhead indicates EGFP-positive nonepithelial cells that populate the villus core of all villi. (B) Whole-mount intestine from a β-gal-expressing bone marrow-transplanted recipient. Yellow arrow indicates β-gal-positive crypt and villus. (C) Tissue section from a transplanted distal small intestine stained with antibodies to EGFP, detected with DAB (brown), and counterstained with hematoxylin. The brown staining represents EGFP-positive epithelium, whereas the EGFP-negative epithelium appears pink/purple. (Controls for antibody staining are found in, which are published as supporting information on the PNAS web site.) EGFP-positive cells populate the epithelium of the right villus. The left villus is negative for EGFP expression. The black asterisk marks a differentiated goblet cell that is EGFP-positive, and the red asterisks denote EGFP-negative goblet cells. (D–F) Tissue section of the distal small intestine of a recipient mouse transplanted with 500 EGFP-positive HSCs. Section in D is stained with Hoechst dye (blue) and visualized for EGFP expression (green) by direct fluorescence. (E) EGFP detected by direct fluorescence. EGFP-positive cells populate the villus-associated epithelium and are designated with white brackets and an arrow. White lines designate the boundary between the epithelium and lamina propria. Arrowhead indicates an intraepithelial lymphocyte. (F) Identical tissue section in D and E stained with antibodies to EGFP and detected with Cy3-conjugated secondary antibodies (orange). (Scale bars: 25 μm.)
To determine whether cell fusion is the mechanism that facilitates BMDC integration, we assayed for presence of the Y chromosome in donor-derived cells in the intestines of transplanted male mice (Fig. 2A–C). Colocalization of donor-derived EGFP expression and recipient-specific Y chromosome labeling within the same cell indicated that these two cell populations had undergone fusion (Fig. 2 A–C). An average of 51% of the donor-derived cells were positive for both markers in a total of 200 epithelial nuclei analyzed from six mice. Because the villus epithelial compartment is anatomically distinct from the hematopoietic cells that reside within the lamina propria, it is unlikely that cells positive for both the Y chromosome and EGFP represent contaminating lymphocytes or macrophages. This distinction was confirmed by using antibodies to the panhematopoietic marker CD45 (Fig. 8, which is published as supporting information on the PNAS web site). WT male intestinal sections probed with whole Y chromosome paint produced a detectable signal in ≈85% of cells (Fig. 9, which is published as supporting information on the PNAS web site). By using the percentage of detectable Y chromosomes as a correction factor, ≈60% of donor-derived epithelial cells were positive for both donor and recipient cell markers.
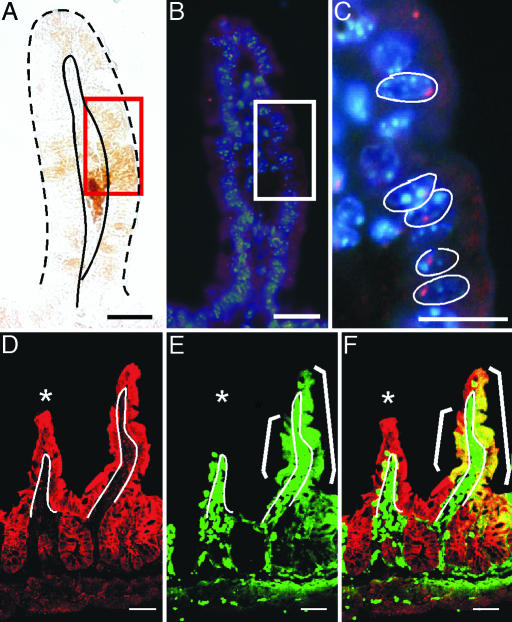
Fig. 2.
Fusion between BMDCs and intestinal epithelium. Intestine from a male recipient transplanted with female donor EGFP-expressing BMDCs analyzed for EGFP expression and Y chromosome. (A) EGFP-stained intestinal epithelium detected with EGFP antibodies and DAB staining (brown). Red rectangle represents a region containing EGFP-positive cells. Epithelium is demarcated with solid and dashed black lines. (B) Y chromosome probe (red) and Hoechst-stained nuclei (blue) detected on the same tissue section in A. EGFP-expressing region is denoted by a white rectangle. The boxed region is magnified in C. Nuclei in C are outlined in white. (D–F) Intestinal tissue section from a β-gal-expressing recipient transplanted with EGFP-positive BMDCs was analyzed for coexpression of β-gal and EGFP by confocal microscopy. White line indicates the boundary between the epithelium and lamina propria. White asterisk denotes a villus lacking EGFP epithelial expression. (D) β-gal (red) is uniformly expressed in the intestinal epithelium as detected with antibodies to β-gal and Cy5-conjugated secondary antibodies. (E) EGFP expression (green) on the same tissue section as D detected by direct fluorescence. (F) Merge of β-gal- and EGFP-stained tissue showing colocalization of markers for both donor and recipient populations (yellow). Epithelial cells expressing β-gal only appear red, and EGFP-positive lamina propria cells are green. (Scale bars: 25 μm.)
A second independent approach for examining fusion in the intestine supported these initial observations. In β-gal-expressing recipient male mice transplanted with female EGFP-expressing BMDCs, both β-gal and EGFP were detected in the same epithelial cell by using confocal microscopy (Fig. 2 D–F). We were unable to identify intestinal regions containing cells expressing EGFP but not β-gal. This finding suggests that using the Y chromosome approach as a host-specific marker may underestimate the extent of cellular fusion.
Importantly, fusion between BMDCs and intestinal epithelial cells resulted in a morphologically normal epithelial population. To address the fate of the initial fusion product, we surveyed hematopoietic gene expression within the donor-derived cells in the intestinal epithelium. Expression of CD45, c-kit, or Sca-1 was present in cells residing within the lamina propria but was absent from all intestinal epithelial cells (Fig. 8). These results suggest that the nuclei of the fused cells have been reprogrammed and no longer express these hematopoietic markers. We found BMDC-expressing markers for all four principal intestinal epithelial lineages for up to 6–14 months after transplantation. Donor-derived absorptive enterocytes were identified by double labeling of intestinal sections with antibodies to EGFP and to cytokeratin (Fig. 3B–D) and the epithelial differentiation marker Fatty acid binding protein (Fabp; data not shown). There was no apparent difference in enterocytic cytokeratin or Fabp expression between EGFP-positive or WT cells, indicating that EGFP-expressing enterocytes possessed normal differentiation profiles. The three secretory lineages also were represented among the donor-derived population. Differentiation of donor-derived cells into enteroendocrine cells was demonstrated with antibodies to serotonin (Fig. 3 E–G). Donor-derived goblet cells were morphologically identified by brightfield detection for EGFP-expressing cells (Fig. 3A, black asterisk). Moreover, both donor-derived goblet cells and Paneth cells also were identified by lectin staining (data not shown).
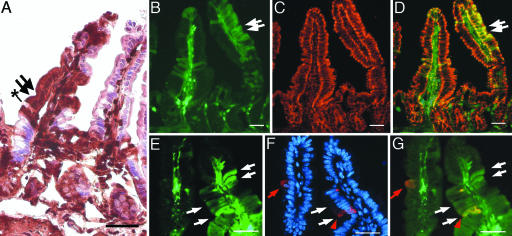
Fig. 3.
EGFP expression is detected in all four principal intestinal epithelial lineages. The intestines from EGFP-expressing BMDC-transplanted recipients were analyzed with markers for intestinal lineages at 6 months after transplantation. (A) Intestine stained with antibodies to EGFP, detected with DAB (brown), and counterstained with hematoxylin. The EGFP-negative epithelium on the right villus appears pink/purple, whereas the EGFP-positive epithelium on the left villus is dark brown. Black asterisk denotes an EGFP-positive goblet cell adjacent to enterocytes (arrows) on the same villus. (B–D) Intestine stained with the enterocyte marker cytokeratin. (C) Cytokeratin staining (red) is uniformly distributed in villus-associated epithelium and is unaltered in EGFP-expressing cells (green; B). (D) Merged image from B and D showing colocalization of EGFP and cytokeratin (yellow). Arrows point to EGFP-expressing epithelium. (E–G) Intestine stained with an enteroendocrine cell marker. (E) EGFP-expressing cells (green). White arrows point to four of the EGFP-expressing cells on the right villus. (F) Enteroendocrine cells identified with antibodies to serotonin (red) in the nuclear Hoechst dye-stained tissue (blue). Red arrow/arrowhead indicate the serotonin-positive cells. (G) Merged image; serotonin expression in EGFP-expressing enteroendocrine cells (yellow cell and red arrowhead) is similar to expression in WT enteroendocrine cells (red cell and red arrow). (Scale bars: 25 μm.)
The presence of donor-derived cells in all four principal epithelial lineages and even more so, the presence of multiple differentiated epithelial lineages in a single villus, indicate that BMDCs fuse with a multipotent intestinal progenitor or stem cell. The multipotent intestinal stem cell has been shown to give rise to all four principal epithelial lineages (20). These epithelial cells migrate in a linear fashion out of the monoclonal crypts onto the adjacent villi (21). Therefore, EGFP-expressing epithelium detected with brightfield methods allows for morphologic identification of mucous-secreting goblet cells interspersed among absorptive enterocytes (Figs. 1C and 3A) and suggests that these cells are clonally derived. Furthermore, long-term detection of EGFP-expressing cells suggests that a long-lived progenitor/stem cell population propagates the EGFP signal, because the differentiated intestinal epithelium is rapidly renewing and turns over every 3–5 days (22). Therefore, if the BMDCs fuse with differentiated cells, donor-derived markers would be absent at the later posttransplantation time points. Lineage-committed progenitor cells are retained in the intestine for ≈100 days (23), whereas long-lived progenitors and stem cells reside for longer periods. Although the persistence of donor-derived epithelium in transplanted mice suggests BMDC fusion with a long-lived progenitor or intestinal stem cell, it does not rule out fusion with lineage progenitor cells or even differentiated cells. It is unlikely that donor-derived intestinal cells of various differentiated lineages are derived from continuously occurring direct fusion events, because EGFP-expressing BMDCs failed to incorporate into the intestinal epithelium of nonirradiated mice (data not shown). This observation suggests that local injury is important for BMDC fusion and that this fusion mechanism participates in epithelial regeneration.
Our data suggest that fusion of BMDCs with WT intestinal epithelial cells occurs in the stem cell niche of the small intestine. This observation led us to ask whether transplanted BMDCs have an increased propensity to incorporate into the highly proliferative epithelium of intestinal tumors or whether they act as tumor stem cells, as has been recently reported in gastric carcinoma (16). Remarkably, we found that EGFP-expressing BMDCs incorporate into tumor intestinal epithelium of mice harboring intestinal adenomas (24) (Min mice) at a higher frequency when compared with WT intestinal epithelium (Fig. 4A). Although EGFP-expressing cells were observed in the majority of intestinal adenomas screened, we did not detect any ubiquitously EGFP-expressing adenomas. This finding, along with the observation that BMDC transplant did not alter overall polyp number (Fig. 10, which is published as supporting information on the PNAS web site), suggests that BMDCs are not involved in tumor initiation in the small intestine. Donor-derived tumor epithelium possessed a normal expression pattern of cytokeratin, a widely expressed epithelial marker (Fig. 4 B and C), but failed to express the intestinal epithelial differentiation marker Fabpl (25); Fig. 4 D and E). Loss of epithelial differentiation markers suggests that EGFP-expressing tumor epithelium fuse to undifferentiated or stem cell-like tumor cells. Alternatively, integrated donor cells may fail to efficiently express tissue-specific differentiation markers as a response to the tumor environment. Similar to donor-derived epithelium in WT recipients, EGFP-expressing tumor epithelium did not express hematopoietic differentiation markers (data not shown).
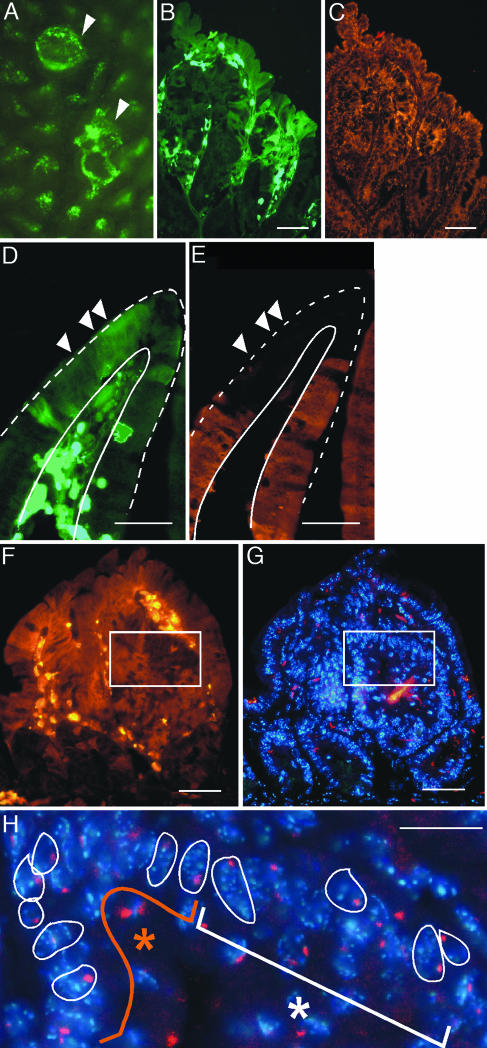
Fig. 4.
BMDCs fuse with tumor epithelium. (A and B) EGFP-expressing BMDCs incorporate into intestinal adenomas (arrowhead) observed by intestinal whole-mount (A) and tissue (B) sections. (B and C) EGFP-expressing regions (green) have a normal expression pattern of the epithelial marker cytokeratin (orange) when compared to adjacent non-donor-derived areas. (D and E) However, donor-derived regions (green) do not express the intestinal epithelial differentiation marker Fabp (orange). White arrowheads mark several EGFP-expressing tumor epithelial cells. Villus epithelium is demarcated with white lines. (F–H) BMDCs fuse with tumor epithelium. (F) Adenoma stained with antibodies for EGFP and Cy3-conjugated secondary antibodies (orange). White box contains both EGFP-positive and WT cells. (G) Adjacent tissue section in F stained with Hoechst nuclear dye (blue) and Y chromosome probe (red). (H) Higher magnification of boxed region. The orange asterisk represents donor-derived population, whereas the white asterisk represents the recipient population. Nuclei are outlined in white. (Scale bars: 25 μm.)
To determine whether transplanted BMDCs incorporate into tumor epithelium by cell fusion, we analyzed EGFP-expressing regions of intestinal adenomas for the presence of the host-specific Y chromosome. Donor-derived cells expressed both EGFP and the Y chromosome, indicating that cell fusion had occurred (Fig. 4 F–H). Because the transplanted BMDCs contain WT copies of the Apc gene, it is unlikely that these cells could acquire mutations in both alleles and give rise to intestinal adenomas within 2 weeks after transplantation. Our data strongly support the notion that BMDCs fuse with tumor epithelium rather than function as a tumor stem cell as reported in gastric cancer (16). However, the previous study did not use a dual marker system for detecting donor and recipient populations, but rather relied on the absence of multiple nuclei to exclude fusion. Therefore, it is possible that unrecognized cell fusion also may underlie BMDC incorporation into tumors of the gastric epithelium.
Although our data directly demonstrate that BMDCs fuse with the intestinal epithelium, we cannot rule out additional mechanisms for BMDC incorporation into the intestinal epithelium (26) because the Y chromosome was undetected in up to 40% of donor-derived epithelial cells. However, using the Y chromosome as a host-specific marker leads to underestimation of fusion events, which suggests that alternative mechanisms such as transdifferentiation might be rare.
Importantly, our findings show that BMDCs can fuse with a long-lived progenitor or intestinal stem cell population of gamma-irradiated damaged intestinal epithelium. Fusion of BMDCs with progenitors or stem cells may play an important role in regeneration of damaged tissue. Currently, the role of BMDC fusion with tumor epithelium is less clear. Although it is apparent that incorporation of these cells into intestinal adenomas does not initiate cancer of the small intestine, Min mice do not survive long enough to evaluate the impact of fusion on tumor progression. Although our data do not support the notion that BMDCs act as tumor stem cells, the fusion between BMDCs and tumor cells may be a common but late event in intestinal tumorigenesis. There is precedence for the involvement of BMDCs in tumorigenesis, as demonstrated by the contribution of these cells to tumor vasculature (15) and in initiation of the angiogenic switch that is critical for tumor progression (14). Further, there is in vitro evidence that fusion between blood-derived cells and tumor cell lines results in a more metastatic cellular product (13). Thus the in vivo fate of these fusogenic cells is clearly important. Whether this fusion event allows cells to acquire metastatic potential or whether they become genetically unstable remains to be investigated. The observation that BMDCs can fuse with tumor epithelium is an important finding, and further examination of this event will enhance our understanding of the biology of tumorigenesis and may provide a novel strategy for the development of anticancer therapies.
Materials and Methods
Bone Marrow Transplantation.
BMDCs were harvested from 5- to 12-week-old female donor mice harboring β-gal (ROSA26; ref. 18) or EGFP transgenes (27) by using standard procedures (28). Harvested bone marrow was filtered to obtain a single-cell suspension and resuspended in modified Hank's balanced salt solution (HBSS). HSCs were isolated as described in ref. 29. An aliquot was diluted in Turk's reagent (Merck, Darmstadt, Germany) to perform a cell count. To prepare recipient mice for transplantation, 8-week-old recipient male C57BL/6 mice or 5-week-old recipient male Min mice (The Jackson Laboratory) were enterally administered acidified water (pH 2.2) for 1 week before whole-body gamma irradiation (12 Gy) in two equal doses, 4 hours apart. The early intestinal epithelial response to 12 Gy is crypt cell apoptotic death; however, clonogenic regeneration of the crypt is observed over the first 4 days after irradiation, and complete healing of the epithelium is apparent by 7 days after irradiation (30). A total of 5 × 106 unfractionated donor bone marrow cells (or 500 HSCs) were then injected into the retro-orbital plexus of the recipient mouse. All recipient mice were administered antibiotic drinking water (neomycin sulfate at 1.1 g/liter and polymixin B sulfate at 167 mg/liter) for 1 month after transplantation to reduce infection. Nonirradiated control recipient mice were transplanted once daily with 2 × 107 bone marrow cells retro-orbitally for 5 sequential days. Mice were analyzed at 5 weeks after transplantation. All procedures were performed in accordance to the Oregon Health & Science University Animal Care and Use Committee.
Analysis of Transplanted Intestine.
BMDC-transplanted mice were analyzed at various times between 2 weeks and 14 months after transplant. Whole-mount analysis was conducted as described in ref. 31, except that intestines were fixed with 4% paraformaldehyde and visualized with fluorescence (EGFP bandpass filter 470/40 nm) for EGFP-incorporated intestines or with X-Gal and light microscopy for ROSA26-incorporated intestines, and images were captured digitally. The number of donor-derived intestinal crypts were quantified in mice transplanted with ROSA26 bone marrow by counting the number of X-Gal-positive crypts in the distal third of the small intestine and was reported as LacZ-positive crypts per cm2. Standard error of the mean and significance were calculated.
After gross analysis of the intestine, frozen blocks were prepared and sectioned as described in ref. 31. Five-micrometer-thick sections were analyzed for EGFP-expressing cells by using polyclonal antibodies (1:250; Molecular Probes) and fluorescent secondary antibodies [indocarbo-cyanine (Cy3)-labeled donkey anti-rabbit antibody; 1:500; Jackson ImmunoResearch] or for brightfield detection by using biotin–avidin secondary antibodies and visualization with 3-3′-diaminobenzidine (DAB) according to the manufacturer's guidelines (Vector Laboratories). EGFP-expressing cells were double-labeled with antibodies to hematopoietic lineage markers (CD45 and c-kit; eBioscience, San Diego) or intestinal epithelial-specific markers (Fabp, a kind gift from J. I. Gordon, Washington University School of Medicine, St. Louis; cytokeratin, Research Diagnostics, Flanders, NJ; and 5α-HT, lysozyme, Peninsula Laboratories). Sections were examined by using a Leica DMR microscope and standard epifluorescence filters for FITC (470/40) and Cy3 (535/50). Digital images were captured.
Analysis of Cell Fusion.
Y chromosome FISH was performed on EGFP-transplanted intestinal paraffin sections. EGFP-expressing cells first were identified by using antigen retrieval (5 mM sodium citrate buffer, pH 6.0), polyclonal EGFP antibodies, and brightfield development with DAB (Vector Laboratories). Adjacent serial sections then were stained as before with EGFP antibodies but with light DAB detection to facilitate subsequent detection of the Y chromosome on the same tissue section. FISH was subsequently performed as previously reported by using Cy3-conjugated Y chromosome paint (Cambio, Cambridge, U.K.) followed by incubation with 2′-(4-hydroxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi(1H-benzimidazole) trihydrochloride (Hoechst 33258; 0.1 μg/ml in PBS for 10 min). The presence of the Y chromosome was localized to the nucleus by using overlay of Hoechst-stained and Cy3-stained digital images and confirmed by confocal microscopy. Tissue sections of EGFP-expressing female or male mice were used as controls.
Analysis of EGFP-transplanted ROSA26 intestines was performed as described above. Intestinal sections of transplanted mice were stained with antibodies for β-gal (1:100; Eppendorf). Secondary antibodies conjugated to Cy5 were used for β-gal detection because the excitation spectrum for Cy5 (590–650 nm) is spectrally separate from the fluorescence emission of EGFP (450–570 nm). Regions coexpressing EGFP and β-gal were identified by using a MRC-1024 confocal laser scanning imaging system (Bio-Rad).
Supplementary Material
Acknowledgments
We thank Scott Stadler for his expert assistance with the confocal microscopy. This research was supported by National Institutes of Health Grants DK068326 (to M.H.W.), HL069133 and HL077818 (to W.H.F.), T32-CA106195 (to P.S.D.), and T32-GM08617 (to A.D.D.).
Abbreviations
- BMDC
bone marrow-derived cell
- DAB
3-3′-diaminobenzidine
- Fabp
Fatty acid binding protein
- HSC
hematopoietic stem cell
- ROSA26
reverse orientation splice acceptor 26.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 2.Rasulov M. F., Vasilchenkov A. V., Onishchenko N. A., Krasheninnikov M. E., Kravchenko V. I., Gorshenin T. L., Pidtsan R. E., Potapov I. V. Bull. Exp. Biol. Med. 2005;139:141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dolado M., Pardal R., Garcia-Verdugo J. M., Fike J. R., Lee H. O., Pfeffer K., Lois C., Morrison S. J., Alvarez-Buylla A. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 4.Laflamme M. A., Myerson D., Saffitz J. E., Murry C. E. Circ. Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 5.Nygren J. M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B. K., Jacobsen S. E. Nat. Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 6.Vassilopoulos G., Wang P. R., Russell D. W. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Willenbring H., Akkari Y., Torimaru Y., Foster M., Al-Dhalimy M., Lagasse E., Finegold M., Olson S., Grompe M. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 9.Ianus A., Holz G. G., Theise N. D., Hussain M. A. J. Clin. Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagers A. J., Sherwood R. I., Christensen J. L., Weissman I. L. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 11.Brittan M., Hunt T., Jeffery R., Poulsom R., Forbes S. J., Hodivala-Dilke K., Goldman J., Alison M. R., Wright N. A. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto T., Okamoto R., Yajima T., Mori T., Okamoto S., Ikeda Y., Mukai M., Yamazaki M., Oshima S., Tsuchiya K., et al. Gastroenterology. 2005;128:1851–1867. doi: 10.1053/j.gastro.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 13.Duelli D., Lazebnik Y. Cancer Cell. 2003;3:445–448. doi: 10.1016/s1535-6108(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 14.Lyden D., Hattori K., Dias S., Costa C., Blaikie P., Butros L., Chadburn A., Heissig B., Marks W., Witte L., et al. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 15.Peters B. A., Diaz L. A., Polyak K., Meszler L., Romans K., Guinan E. C., Antin J. H., Myerson D., Hamilton S. R., Vogelstein B., et al. Nat. Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 16.Houghton J., Stoicov C., Nomura S., Rogers A. B., Carlson J., Li H., Cai X., Fox J. G., Goldenring J. R., Wang T. C. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 17.Kerbel R. S., Lagarde A. E., Dennis J. W., Donaghue T. P. Mol. Cell. Biol. 1983;3:523–538. doi: 10.1128/mcb.3.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano P. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 19.Daley G. Q., Goodell M. A., Snyder E. Y. Hematology (Am. Soc. Hematol. Educ. Program) 2003:398–418. doi: 10.1182/asheducation-2003.1.398. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H., Leblond C. P. Am J. Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt G. H., Winton D. J., Ponder B. A. Development (Cambridge, U.K.) 1988;103:785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- 22.Wright N. A. Int. J. Exp. Pathol. 2000;81:117–143. doi: 10.1046/j.1365-2613.2000.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjerknes M., Cheng H. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 24.Moser A. R., Pitot H. C., Dove W. F. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 25.Carroll S. L., Roth K. A., Gordon J. I. Gastroenterology. 1990;99:1727–1735. doi: 10.1016/0016-5085(90)90480-o. [DOI] [PubMed] [Google Scholar]
- 26.Pomerantz J., Blau H. M. Nat. Cell Biol. 2004;6:810–816. doi: 10.1038/ncb0904-810. [DOI] [PubMed] [Google Scholar]
- 27.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 28.Battaile K. P., Bateman R. L., Mortimer D., Mulcahy J., Rathbun R. K., Bagby G., Fleming W. H., Grompe M. Blood. 1999;94:2151–2158. [PubMed] [Google Scholar]
- 29.Bailey A. S., Jiang S., Afentoulis M., Baumann C. I., Schroeder D. A., Olson S. B., Wong M. H., Fleming W. H. Blood. 2004;103:13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 30.Potten C. S. Int. J. Radiat. Biol. 1990;58:925–973. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- 31.Wong M. H., Rubinfeld B., Gordon J. I. J. Cell Biol. 1998;141:765–777. doi: 10.1083/jcb.141.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.