Abstract
The objective of this study was to quantify the anisotropy of ultrasonic velocity in freshly excised myocardial tissue and to examine the effects of formalin-fixation. Through-transmission radiofrequency-based measurements were performed on ovine and bovine myocardial specimens from 24 different hearts. A total of 81 specimens were obtained from specific locations within each heart to investigate the possibility of regional differences in anisotropy of velocity in the left ventricular wall and septum. No regional differences were observed for either lamb or cow myocardial specimens. In addition, no specific species-dependent differences were observed between ovine and bovine myocardium. Average values of velocity at room temperature for perpendicular and parallel insonification were 1556.9 ± 0.6 m/s and 1565.2 ± 0.7 m/s (mean ± standard error), respectively, for bovine myocardium (N = 45) and 1556.3 ± 0.6 m/s and 1564.7 ± 0.7 m/s for ovine myocardium (N = 36). Immediately after measurements of freshly excised myocardium, ovine specimens were fixed in formalin for at least one month and then measurements were repeated. Formalin-fixation appears to increase the overall velocity at all angles of insonification and to increase the magnitude of anisotropy of velocity.
I. Introduction
The objective of this study was to quantify the anisotropy of ultrasonic velocity in freshly excised myocardial tissue and to examine the effects of formalin-fixation on velocity in myocardium. While some studies have examined insonification perpendicular or parallel to the predominant direction of the myofibers (Akashi et al., 1995; Fei et al., 1987; Goss et al., 1980; Mol and Breddels, 1982; Saijo et al., 1997; Shung and Reid, 1977; Shung and Reid, 1978), direct studies have not been performed on the anisotropy of velocity over a complete range of angles of insonification in freshly excised normal myocardial tissue. The speed of sound is usually taken to be 1540 m/s in medical ultrasonic imaging devices. With the growing number of approaches to ultrasonic tissue characterization, knowledge of the anisotropy of ultrasonic velocity in freshly excised myocardium may be helpful in determining the extent to which angle of insonification relative to direction of the myofibers may or may not influence results. Our approach was to perform broadband, through-transmission radiofrequency measurements in freshly excised ovine and bovine myocardial specimens at frequencies typical of those used in clinical echocardiography.
Previous studies have examined the influence of formalin-fixation on ultrasonic measurements in biological tissues other than myocardium (Bamber and Hill, 1979; Bamber et al., 1979; Sasaki et al., 2003). This study permits a direct examination of changes in the measured anisotropy of velocity resulting from formalin-fixation by quantifying the anisotropy of velocity in freshly excised myocardium and then comparing these results with measurements of the same myocardial specimens after formalin-fixation. We investigated the effects of fixation at a full range of angles of insonification relative to the predominant direction of the myofibers.
II. Methods
A. Preparation of specimens
A total of 36 tissue specimens from 12 freshly excised lamb hearts and 45 tissue specimens from 12 freshly excised cow hearts were investigated in this study. The hearts were obtained within 30 minutes of slaughter from a local commercial slaughterhouse and immersed in 0.9% saline solution prior to specimen coring. From each ovine heart, three cylindrical plugs were cored, two from the left ventricular free wall and one from the septum using a 14.5 mm inner-diameter coring tool. Specimens were cored from endocardium to epicardium such that the axis of symmetry of the cylindrical specimens was oriented orthogonal to the epicardial surface of the heart. The two left ventricular free wall specimens were cored in approximately the same plane perpendicular to the apex-to-base axis of the heart and superior to the anterior and posterior papillary muscles. The septal specimens were cored roughly halfway between the plane of the left v entricular specimens and the apex. Immediately following measurements of freshly excised myocardium, ovine specimens were fixed in formalin for at least one month prior to repeated measurements.
Because of their larger size, a total of four cylindrical plugs were cored from bovine hearts. Three were prepared in an identical manner to that for ovine specimens, and an additional left ventricular specimen was obtained in the same plane as the other left ventricular specimens, in the region between the anterior and posterior papillary muscles. This method of coring provided for a regional comparison of the anisotropy of the myocardial specimens. Epicardial surfaces were trimmed of excessive fat, if deemed necessary, prior to being glued to a plastic (Delrin®) mount of equivalent diameter for data acquisition.
B. Acquisition of ultrasonic quasi-longitudinal velocity data
Each myocardial specimen was cored from an intact left ventricle at room temperature that had been stored in the saline solution to reduce the potential for osmotic effects. Immediately after coring, the specimen was mounted and then immersed in a water bath (also at room temperature) approximately 5 to 10 minutes prior to measurements. The total time from death of the animal to completion of all measurements on that particular heart was approximately three to five hours. Temperature changes during any one data acquisition were no more than 0.1°C. Fig. 1 shows the equipment set up for measurements. The through-transmission radiofrequency data were acquired using a matched pair of 5 MHz focused piezoelectric transducers (Panametrics V309, 1/2 inch diameter, 2 inch focal length; Panametrics, Waltham, MA). The tissue specimens were positioned at the focus of both transducers and the ultrasonic beam was centered on the midmyocardial region of each specimen. Calculations of the beam diameter in the focal zone suggest that the – 6 dB beamwidths were between 1 mm and 2 mm for the frequencies used in this study.
Fig. 1.
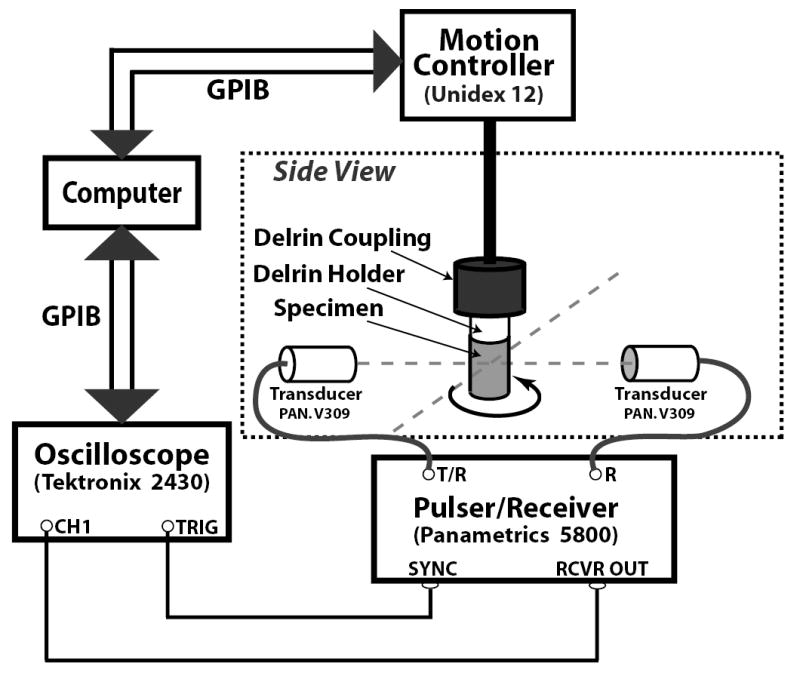
Experimental set-up for performing measurements of the anisotropic properties of ultrasonic velocity in myocardium.
An ultrasonic pulse was generated with a Panametrics 5800 Pulser/Receiver operating in pitch/catch mode and sent to the transmit transducer. The through-transmitted signal was received by the receive transducer, amplified by the Panametrics 5800, and digitized with a Tektronix 2430A (Tektronix, Beaverton, OR) oscilloscope. The pulser/receiver settings were optimized to provide sufficient dynamic range over the usable bandwidth of the measurement system for all angles of insonification relative to the predominant fiber orientation.
For data acquisition, the Delrin mount of each myocardial specimen was friction fit into a cylindrical Delrin coupling that was, in turn, attached to the metal shaft of the rotational axis. A Unidex 12 motion controller (Aerotech, Pittsburgh, PA) was utilized to allow the measurement assembly to have x, y, and z translational movements as well as rotational freedom. The assembly was adjusted to minimize precession of the specimen upon rotation. A computer (Apple Computer, Cupertino, CA) served as a data acquisition system controller and off-line storage device for digitized data. Each myocardial specimen was rotated in 5° increments under computer control. Substitution through-transmission data were obtained by averaging 32 radiofrequency traces at each incremented angle over a complete rotation. In addition, immediately after through-transmission data acquisition, pulse-echo data were acquired for specimen thickness measurements at each incremented angle. Corresponding water path reference data were obtained to within ± 0.1°C of the corresponding specimen data.
C. Description of data analysis for measuring ultrasonic velocity
The determination of velocity was accomplished by separate measurements of specimen thickness and the difference in time-of-flight of an ultrasonic pulse traveling a specific distance through the host medium of known sound speed (in this case water) compared to that of a pulse traveling the same distance in the host medium with the myocardial specimen inserted in the path of insonification. These measurements were performed at each angle of rotation of the myocardial specimens in order to determine the anisotropy of velocity.
The velocity of sound in the specimen (Vspecimen) at angle, θ , is obtained by Eq. (1):
| (1) |
where Vwater is the speed of sound in water, Lspecimen (θ) is the thickness of the myocardial specimen through which the ultrasonic pulse propagates at an angle of rotation, θ , and Δt(θ) is the difference in arrival times between the specimen and water reference through-transmitted radiofrequency traces at angle of rotation, θ. The speed of sound in water was obtained by measurement of the temperature of the water bath and making a calculation based on a fifth-order polynomial expression for the speed as a function of temperature (Del Grosso and Mader, 1972).
D. Ultrasonic measurement of myocardial specimen thickness
In freshly excised myocardium cored with a cylindrical coring tool, specimens often have elliptical rather than perfectly circular cross sections (in the plane perpendicular to the axis of rotation for data acquisition). Ultrasonic measurements of thickness were performed at each angle of rotation for each myocardial specimen. These ultrasonically measured values of thickness showed reasonably good agreement with independent assessments carried out with vernier calipers.
Myocardial specimen thickness can be measured by first determining the distance between the transducers using the known speed of sound in water and the measured time-of-flight of an ultrasonic pulse transmitted through water (without the myocardial specimen). Then with the myocardial specimen inserted, for each angle of rotation, the part of the total path length occupied by water is determined by making time-of-flight measurements of the reflected ultrasound from the transmitting transducer to the front wall and separately from the receiving transducer to the back wall of the tissue. This approach overcomes a difficulty described in previous studies from this laboratory in making time-of-flight measurements of the back wall from pulse/echo through the attenuating specimen in which “attenuation of the signal caused a weakened back wall echo to be returned, thereby making it difficult to distinguish the specular echo surface phenomenon from the backscatter in bulk” (Verdonk et al., 1992). By subtracting the part of the total path length occupied by water from the distance between the two transducers, the thickness of the specimen could then be determined at each angle of rotation. As described, this technique would require separate pulse/echo measurements at each angle of rotation for both the front wall and the back wall of the myocardial specimen. Because measurements can be time-sensitive, the following technique was used, in practice, to eliminate the need for two sets of pulse/echo measurements: Timing mark measurements for front and back wall echoes were made relative to a reference (the cylindrical plastic coupling of known thickness into which the plastic mount of the myocardial specimen was fit). The thickness of the specimen at angle θ then equals the thickness of the reference minus the difference between the front walls of the reference and the specimen at angle θ , minus the difference between the front walls of the reference and specimen at angle θ + 180°. By this relative approach, any slight precession in the measurement assembly would be intrinsically compensated because it would presumably act on both the plastic coupling and the myocardial specimen.
For calculations of the thickness of myocardial specimens using this technique, we used a 31 mm diameter plastic (Delrin) coupling as a reference as shown in Fig. 1. The difference in arrival times between the signal reflected back from the plastic coupling reference and that reflected back from the tissue specimen, (Δ tfront wall (θ)), was calculated for each angle of rotation for the front wall. Measurements of the time differences were repeated upon rotation by 180° which corresponds to measurements of the time differences for the back wall at angle θ (Δ tback wall (θ)). Myocardial specimen thickness as a function of angle of rotation, Lspecimen (θ), was then calculated by
| (2) |
in which Vwater is the speed of sound in water, and Lreference (θ) is the known thickness of the plastic coupling reference which was measured ultrasonically.
Specific timing measurements were made on the envelope of the radiofrequency signal obtained by computing the magnitude of the analytic signal. Our criterion for making timing measurements was based on searching from baseline electronic noise toward the front wall echo for the first sample point with a voltage level that was equal to 15 times the standard deviation of the baseline noise (the value of 15 was chosen empirically). After timing marks were determined for each specimen, curves of timing mark as a function of angle of rotation were generated and smoothed with a 4-point binomial filter for use in the calculation of thickness using Eq. (2).
E. Through-transmission timing measurements
For calculation of the velocity, Eq. (1) requires measurement of the difference in arrival times between the specimen and water reference through-transmitted radiofrequency traces at angle of rotation, θ. This was accomplished by cross-correlation of the specimen and water reference through-transmitted radiofrequency signals. Unique maxima were determined by shifting data that had been sampled at 4 ns/point. In the Appendix, we address some important considerations for making time-of-flight measurements in attenuating media arising from changes in radiofrequency pulse shape resulting from the effects of varying attenuation.
III. Results
The measured average ultrasonic velocities for freshly excised ovine and bovine myocardial specimens are shown in Fig. 2. Average values of velocity for perpendicular and parallel insonification were 1556.9 ± 0.6 m/s and 1565.2 ± 0.7 m/s (mean ± standard error), respectively, for bovine myocardium (N = 45) and 1556.3 ± 0.6 m/s and 1564.7 ± 0.7 m/s for ovine myocardium (N = 36). No significant regional differences were observed for either lamb or cow myocardial specimens between specimens cored from the left ventricle compared to the septum. In addition, no significant regional differences between myocardial specimens cored from the left ventricles were observed for either lamb (specimens cored superior to the anterior and posterior papillary muscles) or for cow (same as lamb, but with an additional specimen cored from the left ventricle between the specimens cored from above the anterior and posterior papillary muscles).
Fig. 2.
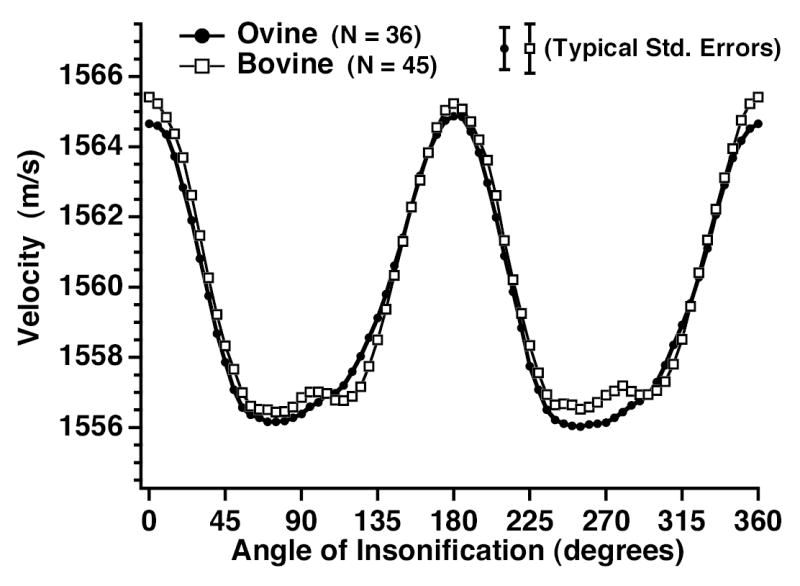
Comparison of ultrasonic velocities versus angle of insonification relative to the predominant direction of the myofibers for freshly excised ovine (N = 36 cylindrical specimens obtained from 12 ovine hearts) and freshly excised bovine (N = 45 cylindrical specimens obtained from 12 bovine hearts) myocardial tissue. Representative standard errors are shown.
Average measured values of velocity for perpendicular and parallel insonification in formalin-fixed ovine myocardium (N = 36) were 1568.4 ± 0.4 m/s and 1580.7 ± 0.7 m/s (mean ± standard error), respectively. Values of average ultrasonic velocity in freshly excised and formalin-fixed ovine myocardial tissue are compared in Fig. 3 (upper panel). A more direct comparison of changes in the angular dependence of velocity resulting from formalin-fixation is seen in Fig. 3 (lower panel) which shows the corresponding velocities with the mean values subtracted. Formalin-fixation appears to increase the overall velocity at all angles of insonification (Fig. 3 upper panel) and to also increase the magnitude of anisotropy of velocity (Fig. 3 lower panel). The average magnitude of anisotropy is 8.4 ± 0.9 m/s for freshly excised myocardium compared to 12.3 ± 0.8 m/s for formalin-fixed myocardium. Again, no significant regional differences were observed in formalin-fixed ovine myocardial tissue (either between septum and left ventricle or among left ventricular specimens).
Fig. 3.
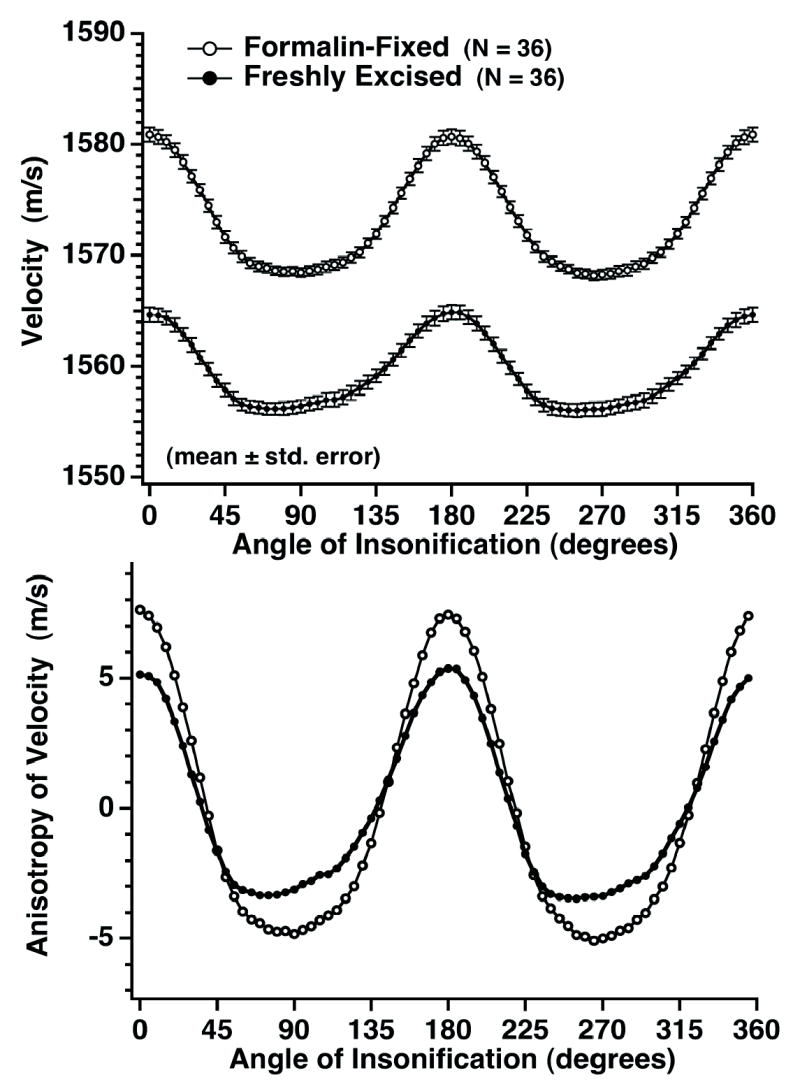
Effects of formalin-fixation on measurements of ultrasonic velocity in ovine myocardial tissue (N = 36 cylindrical specimens obtained from 12 ovine hearts). The top panel represents absolute measurements of ultrasonic velocity and the bottom panel shows the relative anisotropy with mean values subtracted. Formalin-fixation appears to result in an overall increase in ultrasonic velocity at all angles of insonification as well as a greater magnitude of anisotropy of ultrasonic velocity.
For 38 out of 45 freshly excised bovine specimens measured, the temperature was maintained between 19.3 and 20.4°C. For the remaining 7 bovine specimens, temperature extremes were 16.2 to 18.8°C. Mean results excluding these specimens were not significantly different (approximately 0.2 m/s larger and approximately constant with angle of insonification)). For 27 out of 36 freshly excised ovine specimens measured, the temperature was maintained between 19.1 and 20.1°C. For the remaining 9 ovine specimens, temperature extremes were 17.6 to 19.1°C. Mean results excluding these specimens showed only a very modest increase of 0.7 m/s (approximately constant with angle of insonification). In the case of formalin-fixed ovine myocardium, all 36 specimens were measured over a temperature range of 19.0 to 20.1°C.
IV. Discussion
A. Anisotropy of velocity in freshly excised myocardial tissue
Previous measurements of the ultrasonic quasi-longitudinal velocity of freshly excised myocardium have involved insonification strictly perpendicular and parallel to the predominant direction of the myofibers (Akashi et al., 1995; Fei et al., 1987; Mol and Breddels, 1982; Shung and Reid, 1977). A comparison of results from this study with previous results is shown in Table I (all values are reported as mean ± one standard deviation). With the potential for differences in temperature of myocardial specimens upon data acquisition, current results appear to show reasonable agreement with previous results from other laboratories.
Table I.
Comparison of measured ultrasonic myocardial velocities of the current study with previous measurements from other laboratories (mean ± one standard deviation).
| Velocity Parallel to Myofibers (m/s) | Velocity Perpendicular to Myofibers (m/s) | Magnitude of Anisotropy Of Velocity (m/s) | Ratio of Parallel to Perpendicular | |
|---|---|---|---|---|
| Results of Current Study: | ||||
| Freshly Excised Bovine Myocardium | 1565 ± 5 | 1557 ± 4 | 8 ± 3 | 0.51% |
| Freshly Excised Ovine Myocardium | 1565 ± 4 | 1556 ± 4 | 9 ± 2 | 0.58% |
| Formalin-Fixed Ovine Myocardium | 1581 ± 4 | 1568 ± 3 | 12 ± 3 | 0.83% |
| Results of Previous Studies: | ||||
| Freshly Excised Bovine 23 ± 1°C, 20–40 MHz (Akashi et al. 1995) | 1565 – 1570a | - | - | |
| Freshly Excised Calf 22 ± 1°C, 5 MHz (Shung & Reid 1977 & 1978) | - | 1546 ± 4 | - | - |
| Freshly Excised Bovine 23 ± 0.5°C, 5 MHz (Fei, Shung, & Wilson 1987) | - | 1558 ± 4 | - | - |
| Freshly Excised Canine 20 ± 2°C, 5 MHz (Mol & Breddels 1982) | 1558 ± 6 | 1550 ± 6 | 7 ± 3 | 0.52% |
| Formalin-Fixed Calf 22 ± 1°C, 5 MHz (Shung & Reid 1977) | - | 1559 ± 7 | - | - |
| Formalin-Fixed Human 18.1 ± 1.2°C, 5 MHz (Verdonk, Wickline, Miller 1992) | 1550 ± 5 | 1530 ± 3 | 20 ± 6 (reportedb)
12 (re-analyzed using RF correlationb) |
- |
angle of insonification relative to predominant direction of myofibers not specified
see Appendix
Measurements of ultrasonic velocity in freshly excised bovine heart muscle were performed by Akashi et al. using an ultrasonic transmission comparison method in the frequency range of 20 MHz to 40 MHz (Akashi et al., 1995). They report measured velocities over a temperature range of 22–24°C of 1565 m/s to 1570 m/s, but it is unclear at what angle relative to the predominant direction of the myofibers these measurements were made. Our bovine myocardial measurements were performed at temperatures ranging from 18–20°C and at lower frequencies (approximately 3 MHz to 7 MHz). If the ultrasonic velocity of myocardial tissue were to experience a similar temperature dependence as that of water (3 m/s per degree Celsius), then these values would still fall within the range of values measured in the current study, as seen upon comparison of values in Table I. In addition, it should be noted that myocardium may be expected to exhibit velocity dispersion that increases modestly with increasing frequency. Previous studies in this laboratory have predicted a 2.5 m/s difference for phase velocities at 1 and 9 MHz for perpendicular insonification in freshly excised canine myocardium (O'Donnell et al., 1981; Waters et al., 2003; Waters et al., 2000). Measurements on a limited number of hearts for perpendicular insonification (at 5 MHz and 22 ± 1°C) as reported by Shung and Reid showed a lower average value of ultrasonic velocity of 1546 ± 4 m/s (Shung and Reid, 1977; Shung and Reid, 1978). However, these measurements were reported for calf myocardium. In our experience, calf myocardium appears to be qualitatively much less stiff than cow myocardium, as was measured in this current study. For this reason, measurements in young bovine myocardial specimens might exhibit lower (more water-like) values of ultrasonic velocity. In addition, specimens studied by Shung and Reid were excised randomly from both the ventricles and the atria. In our current study, measurements were performed only on the left ventricle and septum. Subsequent work at 5 MHz center frequency at 23° ± 0.5°C reported by Fei, Shung & Wilson on bovine myocardium shows an average velocity at perpendicular insonification of 1558 ± 4 m/s (Fei and Shung, 1985; Fei et al., 1987).
Measurements of ultrasonic velocity in freshly excised canine myocardium for perpendicular and parallel insonification were reported by Mol and Breddels at 5 MHz at temperatures between 18 and 22°C and were scaled to 20°C assuming a temperature coefficient of 3 m/s per degree Celsius (Mol and Breddels, 1982). That study (which employed a zero crossing method of timing mark detection) showed an average of 1550 ± 6 m/s and 1558 ± 6 m/s for perpendicular and parallel insonification, respectively. These averages are slightly lower than the averages for lamb and cow myocardium reported in our study. However, the magnitude of anisotropy of ultrasonic velocity in canine myocardium, 7 ± 3 m/s, is in agreement with that of lamb and cow myocardium in our study (9 ± 2 m/s and 8 ± 3 m/s, respectively).
Our results extend previous studies by measuring the anisotropy of ultrasonic quasi-longitudinal velocity as a function of angle of insonification over a 360° rotation relative to the predominant direction of the myofibers in freshly excised myocardial tissue (Fig. 2). The ultrasonic velocity shows maxima for angles of insonification parallel to the predominant direction of the myofibers and minima for perpendicular insonification. Results show good agreement between freshly excised ovine and bovine myocardial tissue with ratios of the difference in measured ultrasonic velocities for parallel to perpendicular insonification of 0.58% and 0.51%, respectively. Maxima appear more sharply peaked compared to minima (as can perhaps more clearly be seen by viewing Fig. 2 upside down). This indicates greater sensitivity of measured ultrasonic velocity for slight angular deviations from insonification parallel to the myofibers than for slight angular deviations from perpendicular insonification.
B. Comparison of changes in the anisotropy of ultrasonic velocity from freshly excised to formalin-fixed myocardial tissue
The work by Shung and Reid reported an increase upon formalin-fixation of 13 m/s in the measured ultrasonic velocity compared to values in freshly excised calf myocardial tissue for insonification perpendicular to the predominant direction of the myofibers (Shung and Reid, 1977). As can be seen from Table I, this is very nearly equal to the increase in average measured ultrasonic velocity for formalin-fixed compared to freshly excised lamb myocardial tissue in the current study, which was observed to be 12 m/s for perpendicular insonification.
Previous measurements from this laboratory on formalin-fixed human myocardial tissue (at 5 MHz and 18.1° ± 1.2°C) demonstrated a clear anisotropy of ultrasonic velocity with maxima for insonification parallel to the predominant direction of myofibers and minima for perpendicular insonification (Verdonk et al., 1992). As described in the Appendix, when these data were reanalyzed using the approach of the current study in order to minimize the error in measurement introduced by specimen attenuation on timing mark determination, identical magnitudes of the anisotropy of velocity were determined between the formalin-fixed human myocardium of that study and the formalin-fixed ovine myocardium of the present study. Overall values of ultrasonic velocity for that study were lower than results for the current study and other studies as seen in Table I. We have observed a decrease in the measured speed of sound in formalin-fixed myocardial tissue as a function of time in the water bath before measurements are made. This decrease appears to be on the order of 10 m/s after approximately 3 hours and 20 m/s after approximately 15 hours. Measurements in the current study were made within 5 to 10 minutes of immersion in the water bath. However, if specimens were left in the water for long amounts of time prior to measurement, this may partially explain the lower overall values of velocity in the previous study. In addition, it should be noted that the formalin-fixed human myocardial measurements were performed on seven tissue specimens from six explanted human hearts obtained from patients who had undergone heart transplantation for ischemic cardiomyopathy. “Grossly normal myocardial segments, distant from regions of infarction, were selected for investigation” (Verdonk et al., 1992). A detailed comparison of the results obtained from the seriously compromised hearts of that study with the results of the present study may not be appropriate.
Results of the current study allow a direct comparison of measured ultrasonic velocities for freshly excised and formalin-fixed ovine myocardial tissue as shown in Fig. 3. Formalin-fixation appears to result in an overall increase in ultrasonic velocity at all angles of insonification as well as in a small increase in the magnitude of anisotropy of ultrasonic velocity. (This corresponds to a 1.0% increase in velocity for parallel insonification and a 0.8% increase in velocity for perpendicular insonification.) Results show a ratio of measured ultrasonic velocities for parallel to perpendicular insonification for formalin-fixed ovine myocardial tissue of 0.83% (compared to 0.58% for freshly excised myocardium prior to fixation). Inspection of Fig. 3, reveals that the maxima for near parallel angles of insonification appear more peaked (reflected in the larger magnitude of anisotropy of ultrasonic velocity). This effect may result from a greater stiffening and corresponding increase in ultrasonic velocity along myofibers than between them as a result of the action of formalin-fixation.
C. Observations regarding measurements of ultrasonic velocity across species
In addition to the very good agreement between results for lamb and cow myocardial tissue reported in the current study, there is also good agreement between current measurements of velocity and the magnitude of anisotropy for freshly excised lamb and cow myocardium and the results for freshly excised canine myocardium reported by Mol and Breddels (Mol and Breddels, 1982) as seen in Table I. In formalin-fixed tissue, measurements of the magnitude of anisotropy in lamb myocardium (12 ± 3 m/s) were also in agreement with human myocardium (12 m/s). It has been observed that the ultrasonic velocity of biological tissues is determined by the ultrasonic properties of their constituent protein contents and that relatively small changes in collagen content can have a measurable effect (Goss et al., 1980). The agreement in anisotropy of velocity for lamb, cow, dog, and human myocardial tissue may reflect the similarities in cardiac anatomy for various species of mammals.
D. Observations regarding the nature of uncertainties in measurements
It is also interesting to note the behavior of uncertainties in measurements of the average overall velocity compared to uncertainties in the average anisotropy of velocity. This was examined by comparing standard deviations for the average velocity with those calculated from individual myocardial results whose mean values had been subtracted prior to averaging. By subtracting the mean values from individual myocardial specimens, any contributions to uncertainty that resulted from constant offsets among specimens would be eliminated. Only differences in the relative shapes of curves of velocity as a function of angle of insonification would be reflected in subsequent standard deviations. In the results from freshly excised tissue, when constant offsets are subtracted prior to averaging, average standard deviations fall by roughly a factor of 3 for both ovine myocardial specimens (from 3.6 m/s to 1.1 m/s) and for bovine myocardial specimens (from 4.5 m/s to 1.6 m/s). This suggests that a substantial part of the uncertainty does result from constant offsets rather than from differences in shape between velocity curves as a function of angle of insonification (i.e. the anisotropy of velocity). Such offsets may result from such factors as small differences in temperature at the time of data acquisition, small differences in the time that the myocardial specimens were suspended in the water reference prior to the onset of measurements (potential osmotic effects), or normal biological variation. This same effect was observed for formalin-fixed ovine myocardium, but to a slightly smaller degree, where average standard deviations decreased by a factor of 2 (from 3.1 m/s to 1.5 m/s) for formalin-fixed ovine myocardial specimens. These results might hint at the possibility that formalin-fixed myocardium is less sensitive to factors that cause constant offsets in freshly excised myocardial tissue. This same effect was also observed in the fresh canine myocardial study of Mol and Breddels, as seen in Table I, where reported standard deviations in velocities parallel and perpendicular to the myofibers were ± 6 m/s, but the standard deviations for the magnitude of anisotropy dropped to ± 3 m/s.
V. Summary
This study has quantified the anisotropy of ultrasonic velocity in freshly excised and formalin-fixed myocardium as a function of angle of insonification relative to the predominant direction of the myofibers. Maximum velocities were observed at parallel insonification and minima at perpendicular insonification. Formalin-fixation results in an increase in measured ultrasonic velocity as well as in an increase in the magnitude of the anisotropy of velocity.
Acknowledgments
This work was supported, in part, by NIH R37 HL40302. The authors gratefully acknowledge Star Packing Company, St. Louis, MO for providing ovine and bovine myocardial tissues. These investigations benefited substantially from the very careful studies conducted by Edward Verdonk when he was a member of this Laboratory.
Appendix: Implications of method used to estimate time-of-flight on measurements of velocity in specimens with attenuation that differs significantly from that of the reference media
Previous work from this laboratory has employed a timing mark detection algorithm which searched for a fixed percentage (15%) of the baseline-to-peak voltage levels of the magnitude of the analytic signal of the radiofrequency trace (Verdonk et al., 1992). With measurements using this technique in formalin-fixed human myocardium, the authors reported that “Complications introduced by the anisotropy of attenuation were a potential source of systematic error in our measurements of the anisotropy of velocity. The low-pass filtering effect exhibited by soft tissues can confound timing measurements by changing the pulse shape as the wideband ultrasonic signal propagates through the sample” (Verdonk et al., 1992). While that work reported a clear anisotropy of velocity in myocardial tissue with maximal velocity along the myofibers, they suggested that potential errors in the measured values of velocity introduced by the anisotropy of attenuation (with that technique) would require future consideration (Verdonk et al., 1992). This Appendix explores these potential errors and suggests a method for minimizing the effects of attenuation on measurements of velocity in soft tissues (in this case myocardium).
Fig. A1 depicts three representative through-transmitted radiofrequency traces. The water reference trace (top panel) occurs later in time than either of the myocardial specimen traces, indicating the slower speed of sound in water compared to that in myocardial tissue. The earliest myocardial specimen trace in time corresponds to parallel insonification (bottom panel) and the later one corresponds to perpendicular insonification (middle panel). The effects of attenuation (weaker pulse amplitude and changed pulse shape compared to the water reference trace) are most apparent for parallel insonification. While clearly still experiencing attenuation, the trace corresponding to perpendicular insonification appears more “water-like” in shape. Previous work by Wear has examined the effects of frequency-dependent attenuation and dispersion on measurements of velocity in which a specific timing marker (e.g., a zero-crossing, maximum, or minimum) is designated for the specimen and the reference waveforms (Wear, 2000). In the case of sufficiently weakly dispersive media, like myocardium, the effect of dispersion on the received signal is not significant. (Previous studies in this laboratory have predicted a 2.5 m/s difference for phase velocities at 1 and 9 MHz for perpendicular insonification in freshly excised canine myocardium (O'Donnell et al., 1981; Waters et al., 2003; Waters et al., 2000).) The study by Wear showed that for media that exhibit a frequency-dependent attenuation, the pulse spreads over time and the magnitude of the estimate of the difference in arrival times between the specimen and the reference depends on the point in the waveform that is analyzed (Wear, 2000). Similarly, the calculated time difference (and resulting calculated velocity) depends on the choice of percentage of magnitude of the analytic signal when using that technique for timing mark detection. Furthermore, when comparing results in which the attenuation varies (here as a consequence of changing angle of insonification relative to the predominant direction of the myofibers), the measured anisotropy of velocity also depends on that choice. Table II shows results of calculation of the anisotropy of velocity for specific percentages of the magnitude of analytic signal for a representative specimen. The measured anisotropy of velocity is seen to increase with decreasing choice of percentage. This effect on measured velocity indicates that this method of velocity determination is biased because of changing pulse shape resulting from the frequency-dependent effects of attenuation. Similarly to what was shown by Wear for timing mark detection using a particular marker on the radiofrequency waveform (Wear, 2000), a timing mark detection algorithm based on percentage of analytic signal appears to be more strongly affected by certain frequency components of the specimen trace because it only takes into account a certain point on the corresponding analytic signal which has been shown to depend on the attenuation of the specimen.
Fig. A1.
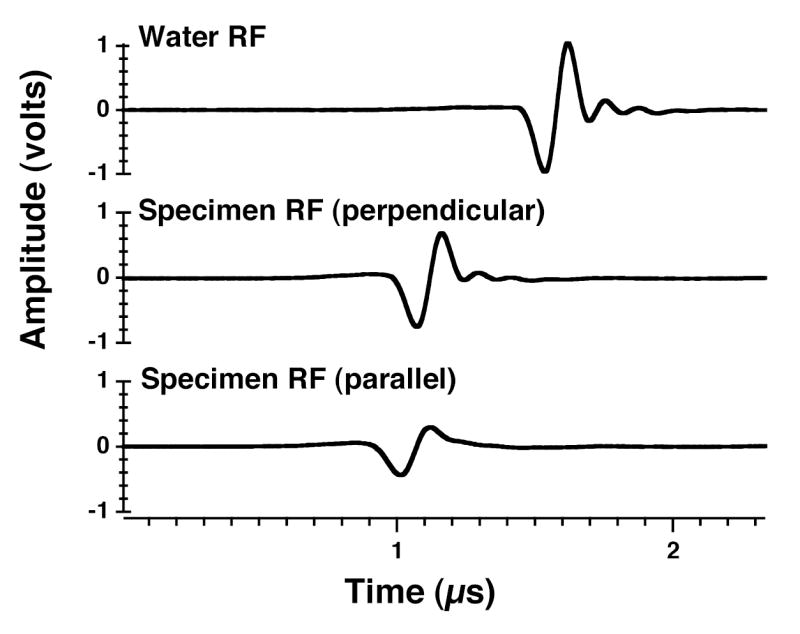
Radiofrequency traces for water reference (top panel) and a representative myocardial specimen at perpendicular insonification (minimum attenuation) (middle panel) and at parallel insonification (maximum attenuation) (bottom panel).
Table II.
Comparison of calculated velocities using different methods of timing mark detection in a representative specimen.
| Method of Timing Mark Determination | Measured Apparent Anisotropy of Velocity (m/s) |
|---|---|
| RF Correlation | 8.9 |
| Zero Crossing | 8.8 |
| Percentage Magnitude Analytic Signal | |
| 15% | 21.5 |
| 30% | 15.7 |
| 45% | 14.5 |
| 60% | 13.6 |
| 75% | 13.1 |
| 90% | 12.1 |
| 100% (peak) | 11.7 |
In order to facilitate an objective measure of timing differences that would reflect all present frequency components and, as a consequence, be less dependent on the effects of changing pulse shape resulting from attenuation in the specimen, we determined differences in signal arrival times by cross-correlation of the specimen and water reference through-transmitted radiofrequency signals. Use of the radiofrequency trace (rather than the analytic signal) includes all measured information for use in determining timing marks. The work by Wear observed that when using a zero crossing method of timing mark detection, errors in group velocity estimates are minimized when the zero crossing marker is chosen near the center of the waveform (Wear, 2000). Furthermore, that study demonstrated that in media such as myocardium that have a faster speed of sound than in water, the effects of attenuation caused calculated velocities to be increasingly too high as timing marks were chosen nearer the leading edge of the waveforms and they were, correspondingly, increasingly too low as timing marks were chosen nearer the trailing edge of the waveforms (Wear, 2000). Because the effects of pulse spreading due to attenuation appear to have an approximately symmetric effect on velocity estimates for points nearer the leading edge of the waveform compared to points nearer the trailing edge of the waveform (Wear, 2000), this suggests that determining timing differences from radiofrequency trace correlation, which uses all points of the waveform in determining timing differences, may yield results closer to those obtained from timing mark choices nearer the center of the waveform and thus with minimized errors as a result of the effects of attenuation. Table II shows results of calculated anisotropy of velocity using radiofrequency trace correlation for comparison to results of specific choices of percentage of magnitude analytic signal for the representative specimen. In addition, results from radiofrequency trace correlation are seen to agree with results using a zero crossing method of timing mark detection as employed in the Mol and Breddels work (Mol and Breddels, 1982).
In order to compare current measurements of velocity made with the radiofrequency cross-correlation method for timing mark detection with previous measurements from this laboratory (Verdonk et al., 1992) that employed 15% of the magnitude of analytic signal for timing mark detection, we analyzed our current data on formalin-fixed lamb myocardial specimens and also reanalyzed the historical data on formalin-fixed human myocardial specimens using both methods. Measured mean values of the anisotropy were in good agreement between the two data sets (22 m/s for the current lamb myocardial data compared to 19 m/s for the previous human myocardial data using 15% analytic signal for timing mark detection, and 12 m/s for current lamb myocardial data compared to 12 m/s for previous human myocardial data using cross-correlation of the radiofrequency traces for timing mark detection). The larger measured anisotropy of velocity observed when using a percentage analytic signal method for timing mark detection would be expected because of the larger attenuation for parallel compared to perpendicular insonification and corresponding dependence of this method on pulse shape which is affected by changing attenuation in the rotating specimens. For these reasons, timing mark detection based on cross-correlation of the radiofrequency traces may be a preferable choice for measurements of velocity in weakly dispersive specimens that exhibit attenuation different from the reference medium and for specimens that exhibit an anisotropy of velocity. In addition, the cross-correlation of radiofrequency traces method of timing mark detection is more straightforward and less arbitrary than that based on a percentage of the magnitude of analytic signal.
References
- Akashi N, Kushibiki J, Chubachi N, Dunn F. "Acoustic properties of selected bovine tissues in the frequency range 20-200 MHz,". J Acoust Soc Am. 1995;98:3035–3039. doi: 10.1121/1.413827. [DOI] [PubMed] [Google Scholar]
- Bamber JC, Hill CR. "Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature,". Ultrasound Med Biol. 1979;5:149–157. doi: 10.1016/0301-5629(79)90083-8. [DOI] [PubMed] [Google Scholar]
- Bamber JC, Hill CR, King JA, Dunn F. "Ultrasonic propagation through fixed and unfixed tissues,". Ultrasound Med Biol. 1979;5:159–165. doi: 10.1016/0301-5629(79)90084-x. [DOI] [PubMed] [Google Scholar]
- Del Grosso VA, Mader CW. "Speed of sound in pure water,". J Acoust Soc Am. 1972;52:1442–1446. [Google Scholar]
- Fei DY, Shung KK. "Ultrasonic backscatter from mammalian tissues,". J Acoust Soc Am. 1985;78:871–876. doi: 10.1121/1.393115. [DOI] [PubMed] [Google Scholar]
- Fei DY, Shung KK, Wilson TM. "Ultrasonic backscatter from bovine tissues: Variation with pathology,". J Acoust Soc Am. 1987;81:166–172. doi: 10.1121/1.395026. [DOI] [PubMed] [Google Scholar]
- Goss SA, Frizzell LA, Dunn F, Dines KA. "Dependence of the ultrasonic properties of biological tissue on constituent proteins,". J Acoust Soc Am. 1980;67:1041–1044. [Google Scholar]
- Mol CR, Breddels PA. "Ultrasound velocity in muscle,". J Acoust Soc Am. 1982;71:455–461. doi: 10.1121/1.387467. [DOI] [PubMed] [Google Scholar]
- O'Donnell M, Jaynes ET, Miller JG. "Kramers-Kronig relationship between ultrasonic attenuation and phase velocity,". J Acoust Soc Am. 1981;69:6960–701. [Google Scholar]
- Saijo Y, Tanaka M, Okawai H, Sasaki H, Nitta SI, Dunn F. "Ultrasonic tissue characterization of infarcted myocardium by scanning acoustic microscopy,". Ultrasound Med Biol. 1997;23:77–85. doi: 10.1016/s0301-5629(96)00174-3. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Saijo Y, Tanaka M, Nitta S. "Influence of tissue preparation on the acoustic properties of tissue sections at high frequencies,". Ultrasound Med Biol. 2003;29:1367–1372. doi: 10.1016/s0301-5629(03)00991-8. [DOI] [PubMed] [Google Scholar]
- Shung KK, Reid JM. "Ultrasonic scattering from tissues,". Proc IEEE Ultrasonics Symposium 77 CH. 1977;1264-1SU:230–233. [Google Scholar]
- Shung KK, Reid JM. "Ultrasound velocity in major bovine blood vessel walls,". J Acoust Soc Am. 1978;64:692–694. doi: 10.1121/1.381996. [DOI] [PubMed] [Google Scholar]
- Verdonk ED, Wickline SA, Miller JG. "Anistropy of ultrasonic velocity and elastic properties in normal human myocardium,". J Acoust Soc Am. 1992;92:3039–3050. doi: 10.1121/1.404200. [DOI] [PubMed] [Google Scholar]
- Waters KR, Hughes M, Mobley J, Miller JG. "Differential forms of the Kramers-Kronig dispersion relations,". IEEE Trans Ultrason Ferroelec Freq Contr. 2003;50:68–76. doi: 10.1109/tuffc.2003.1176526. [DOI] [PubMed] [Google Scholar]
- Waters KR, Hughes MS, Mobley J, Brandenburger GH, Miller JG. "On the applicability of Kramers-Kronig relations for ultrasonic attenuation obeying a frequency power law,". J Acoust Soc Am. 2000;108:556–563. doi: 10.1121/1.429586. [DOI] [PubMed] [Google Scholar]
- Wear KA. "The effects of frequency-dependent attenuation and dispersion on sound speed measurements: Applications in human trabecular bone,". IEEE Trans on Ultrason Ferroelec Freq Contr. 2000;47:265–273. doi: 10.1109/58.818770. [DOI] [PMC free article] [PubMed] [Google Scholar]


