Abstract
Background
Persistent infection with carcinogenic human papillomavirus (HPV) is linked to high-grade lesions and cervical cancer. To better understand the natural history of HPV, we sought to determine the rates of incident and cleared carcinogenic HPV infection, by age, among women aged 15–49 years and to explore risk factors for incident infection.
Methods
Women enrolled in an earlier HPV prevalence survey (500 of 800 who were HPV-negative and all 121 who were HPV-positive) were invited to participate in follow-up HPV testing at their periodic health examination one year later. A cervical soft-brush specimen for HPV testing and a smear for cytologic examination were obtained, and participants completed a questionnaire on their demographic characteristics and sexual history.
Results
Two hundred and fifty-three (50.6%) previously HPV-negative women and 54 (44.6%) previously HPV-positive women were retested. The mean interval between visits was 14.0 (standard deviation 2.0, median 13.5, range 9.0–21.3) months. Incident HPV infection occurred in 11.1% (28/253) of the women overall, with the highest rate, 25.0% (6/24), in the 15–19-year age group. In the univariate analyses, risk factors for incident HPV were the median number of sexual partners in the past year (≤ 1 v. ≥ 2: odds ratio [OR] 8.2, 95% confidence interval [CI] 3.0–22.2; p < 0.001) and the median number of sexual partners over a lifetime (> 3 v. ≤ 3: OR 3.0, 95% CI 1.2–7.2; p = 0.014). In multivariate logistic regression modelling adjusted for age, median number of sexual partners in the past year, median number of sexual partners over a lifetime, marital status, current smoking and current use of oral contraceptives, only the median number of sexual partners in the past year remained significantly associated with incidence (OR 6.2, 95% CI 1.6–24.5; p = 0.009). Of the previously HPV-positive women, 51.9% (28/54) had cleared the infection.
Interpretation
Incident infection with carcinogenic HPV was highest in women aged 15–19 years, and risk factors were consistent with a sexually transmitted infection. A large proportion of the women who were HPV-positive appeared to have cleared the infection after one year.
Persistent infection with one or more carcinogenic types of human papillomavirus (HPV) is an important etiologic factor in the development of cervical intraepithelial neoplasia and the progression to cervical cancer.1,2,3 HPV DNA has been detected in up to 99.7% of invasive cervical cancers worldwide.4
Carcinogenic types of HPV are highly prevalent in Ontario women, infecting about one in 4 women aged 20–24 years.5 Despite this high prevalence of HPV infection, the incidence of cervical intraepithelial neoplasia and invasive cervical carcinoma in women infected with HPV is relatively low.1,2,3,6,7,8 HPV is often transiently detectable in young women, and lower prevalence rates are expected in older age groups as infection clears or becomes latent.1,2,5,6,7,8,9 Risk-factor profiles also vary with age.5 Determining age-specific rates of infection and clearance and potential risk factors in low-risk women selected from family practice settings will further our understanding of the natural history of HPV. This knowledge will also help to clarify the role of HPV testing in primary screening to identify women at risk of developing cervical cancer in the Canadian general population.
Although HPV incidence and clearance have been studied in women attending maternal and child health programs,3 family planning clinics,7 a multistate health maintenance organization,8 HIV clinics10,11 and student health centres in various countries,2,9 findings in these groups may not reflect the range of ages and corresponding risk profiles of women in a family practice setting. To our knowledge, no studies have investigated age-specific HPV incidence and clearance in a group of low-risk women drawn from Canadian family practices. As a follow-up to our survey of HPV prevalence in randomly selected women accessing the primary health care system in Ontario,5 we examined annual incidence and clearance rates in the same group of Ontario women and extended our investigation of risk factors.
Methods
Thirty-one of the 33 physician practices that enrolled women in the earlier survey5 agreed to participate in the follow-up study; however, 4 practices were not successful in recalling any patients for retesting. Each practice was sent a list that included all previously HPV-positive women and a randomly selected sample of previously HPV-negative women. Physicians and their staff were blinded to the women's HPV status. The office staff of each family physician contacted the women by telephone or mail to ask them to return for their annual cervical cytologic screening appointment, with the option of continued participation in the research study. Women were seen for follow-up HPV testing from July 1999 to May 2000. If repeat cytology had been performed already, but less than 3 months had elapsed, the woman was still eligible for follow-up. In addition to written informed consent obtained at initial testing, women who continued to take part in the study gave verbal consent. The St. Joseph's Healthcare Research Ethics Board approved both the initial and follow-up protocols.
To determine incidence rates, we used an electronic random selection function to identify 500 of the 955 women, stratified by age and region, who participated in the prevalence study and who had tested negative for 13 carcinogenic types of HPV by the hybrid capture II (HCII) assay and had tested negative for all HPV types by polymerase chain reaction (PCR) testing with genotyping, as previously described.5 To determine clearance rates, we selected all women who had tested positive for carcinogenic HPV infection (n = 121) by the HCII assay. Fig. 1 shows the patient flow during recruitment and follow-up.
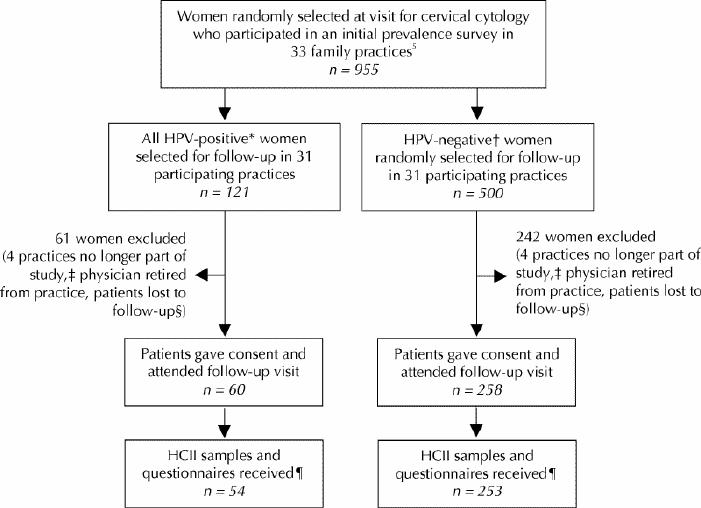
Fig. 1: Flow diagram showing how 253 HPV-negative and 54 HPV-positive women were recruited for 1-year follow-up from among those who had been enrolled in our previous population-based survey of HPV prevalence in Ontario.5 *HPV-positivity for follow-up purposes was determined by the hybrid capture II (HCII) assay (Digene Corporation, Gaithersburg, Md.). †HPV-negative women were found to be negative by both HCII and polymerase chain reaction (PCR) testing; PCR testing indicates the presence of any type of HPV, and genotyping may be used to identify carcinogenic types. ‡Practices that withdrew from the study or discontinued recruitment. §Women were considered lost to follow-up if they refused to participate in the follow-up survey, did not have the physicians' office procedures (no study samples taken at visit for cervical cytology and/or or no appointment within 3 months of Pap test), changed physicians, did not attend their appointment, could not have Pap test (third trimester of pregnancy or recent childbirth), could not be contacted or had no reason/contact recorded (practices did not complete follow-up for lists provided). ¶Several samples were lost or improperly stored and/or questionnaires were not completed by patients or forwarded by the practices.
A cervical soft-brush specimen was obtained by the family physician for HPV DNA testing by the HCII assay, in addition to the annual cytologic smear. The detailed clinical procedures and specimen transport have been described previously.5 The HCII assay is a second-generation signal amplification–based DNA probe test with chemoluminescent readout that is used to detect the presence of one or more of 13 carcinogenic HPV types as a group (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). The detailed assay procedure has been described previously.12 Briefly, the mean relative light units of triplicate positive controls containing HPV 16 DNA at 1.0 pg/mL constituted the cutoff value, and specimens with relative light units equal to or above this level were regarded as positive.
Changes in the risk factors investigated in the earlier study were determined for returning women by using a self-administered follow-up questionnaire. These risk factors included age category, geographic region, marital status, highest level of education completed, lifetime number of sexual partners, number of sexual partners in the past year, age at first intercourse, parity, current smoking status, condom use, current use of oral contraceptives and history of sexually transmitted disease (STD). We performed univariate regression analyses for the same subset of factors previously identified as significant correlates of positivity.5 The multivariate model was adjusted for age, marital status, lifetime number of sexual partners, number of sexual partners in the past year, current cigarette smoking and current use of oral contraceptives.
Results
Of the 800 women who were previously HPV-negative, 500 (62.5%) were randomly selected for follow-up HPV testing, and 253 (50.6%) of these women were retested by the HCII assay (Fig. 1). Fifty-four (44.6%) of the 121 previously HPV-positive women were retested by the HCII assay. Incidence and clearance analyses were thus based on 307 (49.4%) women who had given informed consent and for whom HCII testing data were available. The mean interval between annual periodic health examinations was 14.0 (standard deviation [SD] 2.0, median 13.5 months, range 9.0–21.3) months. The 307 women who had follow-up HPV testing had a mean age of 32.7 years (SD 9.4). Questionnaires were missing for 2 (0.7%) women at the time of analysis. The incidence and clearance rates of carcinogenic HPV infection, based on the 5-year age cohorts, are shown in Fig. 2.
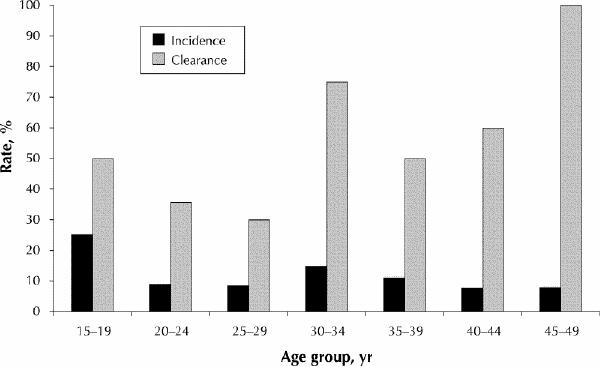
Figure 2: Age-specific incidence and clearance rates for carcinogenic types of HPV determined by the hybrid capture II (HCII) assay performed at a follow-up visit after an average interval of 14 months on 253 women who had been negative for HPV by both the HCII assay and PCR testing (incidence) and 54 who had been positive by the HCII assay (clearance).
Overall, 11.1% (28/253) of the women showed incident HPV infection at their return visit for cytologic screening. The results of the univariate and multivariate regression analyses appear in Table 1. In univariate analyses, factors associated with incident carcinogenic HPV infection were the median number of sexual partners in the past year (≤ 1 v. ≥ 2: odds ratio [OR] 8.2, 95% confidence interval [CI] 3.0–22.2; p < 0.001) and the median number of sexual partners over a lifetime (> 3 v. ≤ 3: OR 3.0, 95% CI 1.2–7.2; p = 0.014). A nearly significant statistical association between single or separated/divorced marital status and incident HPV infection (OR 2.3, 95% CI 1.0–5.1; p = 0.053) prompted inclusion of marital status in the multivariate analyses. In multivariate logistic regression modelling adjusted for age, median number of sexual partners in the past year, median number of sexual partners over a lifetime, marital status, current smoking and current use of oral contraceptives, only the median number of partners in the past year remained significantly associated with incidence (OR 6.2, 95% CI 1.6–24.5; p = 0.009).
Table 1
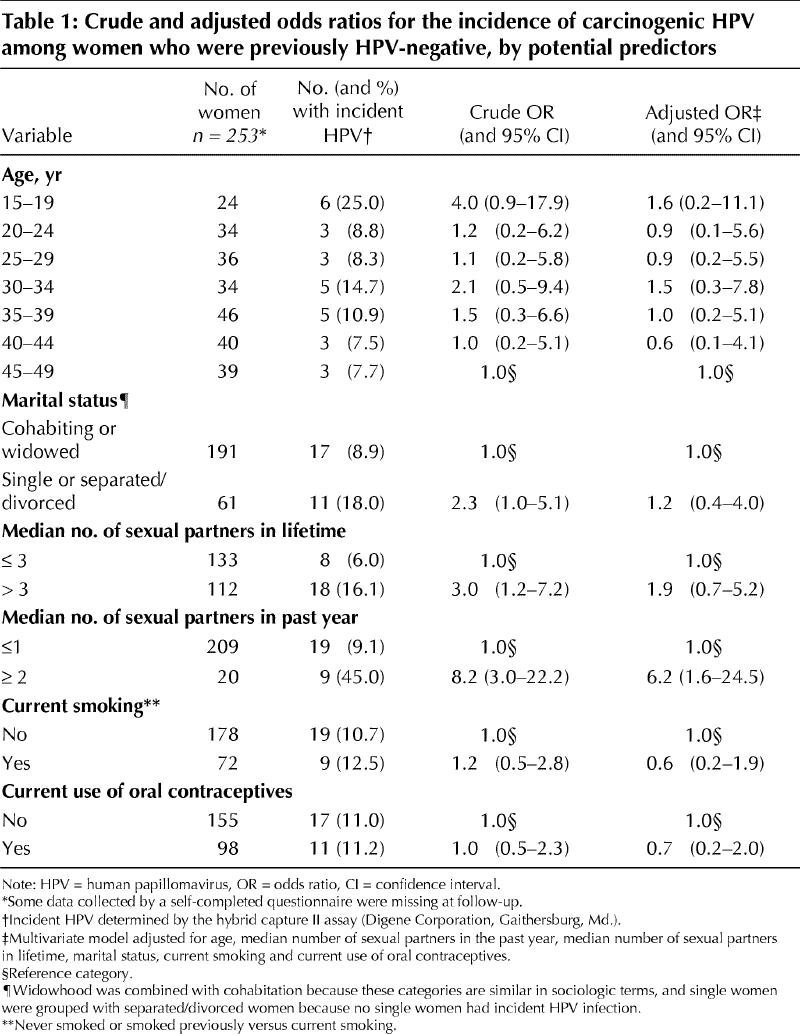
No statistically significant association was found between incidence of HPV and history of any of 6 STDs as a group (chlamydia, genital herpes, genital warts, gonorrhea, syphilis, trichomoniasis), and the number of cases was not sufficient to examine STDs individually (data not shown).
Among women who were previously HPV-positive according to the HCII assay, 51.9% (28/54) appeared to have cleared the infection.
Interpretation
These results suggest that the acquisition and clearance of carcinogenic types of HPV, as a group, were common among these women recruited from primary care settings across Ontario. The highest incidence of HPV in the 15–19-year age group (25%) is consistent with the highest prevalence of 24% that we reported previously in women aged 20–24 years in the inception cohort.5
The risks associated with changing sexual partners, both in the previous year and over a lifetime, were consistent with what has been reported for incident infection.1,2,3,6,7 Moscicki and colleagues followed HPV-negative women for a median period of 50 months, finding that a history of herpes simplex virus and a history of vulvar warts were also risk factors and that current use of oral contraceptives was a protective factor.7 If HPV infection was persistent, current cigarette smoking was a risk factor for squamous intraepithelial neoplasia. A history of STD has been identified as a risk factor for HPV infection.7,13 In this group of low-risk women, we did not detect any association between self-reported STD history and incidence of HPV. The women in our survey were not tested for HIV, but longitudinal studies that compared HIV-negative and HIV-positive cohorts of women have shown that HPV is more prevalent in HIV-positive women and that the probability of HPV persistence is associated with the degree of immune suppression.10,11
Although we were hampered by small numbers in the analysis of clearance, our results confirmed what others have found, namely, that a sizeable proportion of women are no longer HPV-positive after one year.2,6,8,9 Variability in the expression of HPV and the ability of laboratory testing to detect it are also possible reasons why some women would have been HPV-negative.9 Because the HCII assay tests for the presence of one or more carcinogenic HPV types as a group, it is plausible that some of the women who were HPV-positive both at baseline and at follow-up had in fact cleared the initial infection and were subsequently re-infected with a different or even the same HPV type. A study that followed Brazilian women over several years for specific types of HPV showed that women with persistent HPV type 16 or 18 infection had a relative risk of squamous intraepithelial lesions of 10.2 (95% CI 5.9–17.6) as compared with women who were HPV-negative at baseline.3
In cross-sectional studies, the sensitivity and specificity of a single HCII test for the detection of high-grade lesions have been found to be 88% and 89% respectively.14 It appears that the potential role of HPV testing in primary screening to detect women at increased risk of developing cervical cancer is limited by its low specificity, especially in younger women. Our previous survey showed that the age-specific prevalence of infection with carcinogenic types of HPV is much lower after the age of 29 years.5 Consistent with the notion that HPV screening may be more useful in older women, testing by the HCII assay has been shown to have higher specificity in women aged 29 years and older with ASCUS (atypical squamous cells of undetermined significance) cytology.15 Because of the sexual mode of transmission of HPV and the general decrease in incidence of infection after the age of 24 years, it appears that HPV positivity after the age of 29 years is likely to be a result of persistent infection.
The small sample size and our limited knowledge concerning which factors are responsible for clearing HPV infection precluded a more thorough investigation of clearance in this group. The small sample size and single follow-up visit also prevented meaningful analysis of any association between cervical pathology and persistence or clearance of HPV.
Further studies of this common sexually transmitted viral infection among cohorts of women and men are needed to extend our understanding of what factors are important for the development of viral persistence, squamous intraepithelial lesions and, subsequently, cervical cancer. The role that HPV testing might play in the primary screening of women in developing countries, where there is limited access to cytologic screening services, requires further investigation.
Members of the Survey of HPV in Ontario Women (SHOW) Group: Principal Investigator: John Sellors. Coinvestigators: Janusz Kaczorowski, Alice Lytwyn, Jim Mahony. Participating family physicians: Central East Region: Barbara Alexander, Catherine Andrew, Sadrudin Bardai, Lucie Blouin, Alice Bluemke, Janice Boxall, Heather Currie, Peter Deimling, Val Hester, Andrea Hirscheimer, Peter Jacyk, Eshrat Sayani, Peggy Wilkens; Central West Region: Cameron Duthie, Karin Euler, Martha Taylor, Sophie Xenoyannis, Preston Zuliani; Eastern Region: Marie-Josée Deslauriers, Janet Dollin, Kathryn Lockington; Northeastern Region: David Crookston, Daniel Krawczuk, William McMullen, John Mulloy; Northwestern Region: Corinna Chung, Rhonda Diamond, Janet Noy, Sven Pederson, John Vaudry; Southwestern Region: Kate Bailey, Sharon Doyle, Emer Dudley, Andrea Steen.
Acknowledgments
We extend our sincere thanks to Dr. Margaret Fearon, Ontario Public Health Laboratories, Toronto, Ont., for the assistance provided to the project.
Footnotes
This article has been peer reviewed.
Contributors: All authors contributed ideas to this paper and reviewed the manuscript for important intellectual content. Dr. Sellors contributed to the study design and provided expertise in the discussion of results. Ms. Karwalajtys coordinated the data collection, analyzed data and drafted the manuscript. Dr. Kaczorowski contributed to the study design and supervised the analysis and reporting of results. Dr. Mahony contributed to the study design and coordinated the laboratory analysis. Dr. Lytwyn contributed to the study design. Ms. Chong performed the laboratory analyses and contributed to the reporting of results. Ms. Sparrow coordinated the data collection and performed initial analyses. Dr. Lorincz contributed to the study design.
Funding was provided as a research grant by the Division of STD Prevention and Control, Bureau of HIV/AIDS and STD, Health Canada, Ottawa, Ont., and as an unrestricted research grant by the Pharmaceuticals Division, 3M Corporation, Minneapolis, Minn. The HCII kits were provided at no charge by Digene Corporation, Gaithersburg, Md.
Competing interests: None declared for Ms. Karwalajtys, Dr. Kaczorowski, Dr. Mahony, Dr. Lytwyn, Ms. Chong and Ms. Sparrow. Dr. Sellors received partial support from Digene Corporation, Gaithersburg, Md., the manufacturer of the HCII kits, to attend a conference on HPV in Geneva, Switzerland, in 2001. Dr. Lorincz is an employee of Digene Corporation.
Correspondence to: Dr. John W. Sellors, Program for Appropriate Technology in Health, 1455 NW Leary Way, Seattle WA 98107-5136, USA; fax 206 285-6619; jsellors@path.org
References
- 1.Koutsky LA, Holmes KK, Critchlow CW, Stevens CE, Paavonen J, Beckmann AM, et al. A cohort study of the risk of intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992;327:1272-8. [DOI] [PubMed]
- 2.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338: 423-8. [DOI] [PubMed]
- 3.Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001;286:3106-14. [DOI] [PubMed]
- 4.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:1-3. [DOI] [PubMed]
- 5.Sellors JW, Mahony JB, Kaczorowski J, Lytwyn A, Bangura H, Chong S, et al. Human papillomavirus infection in Ontario women: prevalence and predictors. CMAJ 2000;163(5):503-8. [PMC free article] [PubMed]
- 6.Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, et al. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis 1995;171:1026-30. [DOI] [PubMed]
- 7.Moscicki AB, Nills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 2001;285(23):2995-3002. [DOI] [PubMed]
- 8.Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis 1994;169:235-40. [DOI] [PubMed]
- 9.Wheeler CM, Greer CE, Becker TM, Hunt WC, Anderson SM, Manos MM. Short-term fluctuations in the detection of cervical human papillomavirus DNA. Obstet Gynecol 1996;88(2):261-8. [DOI] [PubMed]
- 10.Ahdieh L, Klein RS, Burk R, Cu-Uvin S, Schuman P, Duerr A, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis 2001;184:682-90. [DOI] [PubMed]
- 11.Hankins C, Coutlee F, Lapointe N, Simard P, Tran T, Samson J, et al. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. Canadian Women's HIV Study Group. CMAJ 1999; 160 (2): 185-91. [PMC free article] [PubMed]
- 12.Lorincz A. Molecular methods for the detection of human papillomavirus infection. Obstet Gynecol Clin North Am 1996;23:707-30. [PubMed]
- 13.Munoz N, Kato I, Bosch FX, Eluf-Neto J, de Sanjose S, Ascunce N, et al. Risk factors for HPV DNA detection in middle-aged women. Sex Transm Dis 1996; 23(6):504-10. [DOI] [PubMed]
- 14.Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, et al. HPV DNA testing in cervical cancer screening; results from women in a high-risk province of Costa Rica. JAMA 2000;238:87-93. [DOI] [PubMed]
- 15.Sherman ME, Schiffman M, Cox JT, ALTS group. Effects of age and human papilloma viral load on colposcopy triage: data from the randomized Atypical Squamous Cells of Undetermined Significance/Low Grade Squamous Intraepithelial Lesion Triage Study (ALTS). J Natl Cancer Inst 2002;94(2):102-7. [DOI] [PubMed]


