Introduction
The link between Crohn's disease (CD) and infections is complex and can have implications both clinically (problems in diagnosis, differential diagnosis, approach to management and complications) and scientifically (issues in etiology and pathogenesis).
This article discusses common enteric pathogens that can mimic CD; addresses diagnostic problems associated with diarrhea, relapse in CD, and infectious complications of CD, and explores theories regarding the etiology and pathogenesis of idiopathic inflammatory bowel disease (IBD).
Common Enteric Pathogens Can Mimic CD
Intestinal infections can mimic the classic endoscopic and clinical (abdominal pain, diarrhea, and weight loss) features of IBD, and can present with identical extraintestinal manifestations (eg, reactive arthritis, erythema nodosum).
Yersinia Enterocolitica and Yersinia Pseudotuberculosis
The most common gastrointestinal symptoms associated with these infections are acute abdominal pain and diarrhea. At endoscopy, aphthoid and elongated ulcers may be seen in the terminal ileum, cecum, and ascending colon. Some patients present with an appendicitis-like syndrome. Laparotomy typically discloses a thickened ileal bowel wall and mesenteric lymphadenitis. Pathologic examination of the resection specimen may show transmural inflammation. A minority of patients present with long-standing disease (several months). The differential diagnosis is made by culture (from stools or from a surgical specimen) or serology. The relationship between these infections and CD is not entirely clear; however, Yersinia infection may precede or superinfect CD, and Yersinia DNA has been found in tissues of patients with long-standing CD.[1-3]
Campylobacter Species
Patients with Campylobacter enteritis usually present with abdominal pain, diarrhea (possibly bloody), fever, nausea, and vomiting. Endoscopy shows focal colitis, which may be difficult to distinguish from CD. Stool culture allows the differentiation from CD. Rectal biopsies usually show acute, self-limited colitis. Campylobacter may superinfect CD, but generally does not persist in patients with CD.[4,5]
Salmonella Species
Salmonella is the most common cause of foodborne outbreaks of gastroenteritis. Infection with this organism often causes diffuse lesions in the colon (which may be difficult to differentiate from ulcerative colitis) but has been associated with ileitis as well. Differential diagnosis against CD may be problematic on computed tomography scan or endoscopy; stool culture and biopsy can help in these cases. Mucosal biopsies show a clinical picture of acute colitis unless there is superinfection of preexisting IBD. The organism usually does not persist in tissues from patients with CD.[6,7]
Shigella Species
Shigella species cause bacillary dysentery (mainly in subtropical and tropical climates). Symptoms are watery diarrhea, abdominal pain, and fever, followed by rectal tenesmus and the passage of blood and mucus. Endoscopy shows focal colitis, which must be differentiated from CD. Differential diagnosis is done by stool culture and biopsy (Figure 1). Shigella species may superinfect CD but do not persist in CD tissues.[8]
Figure 1.
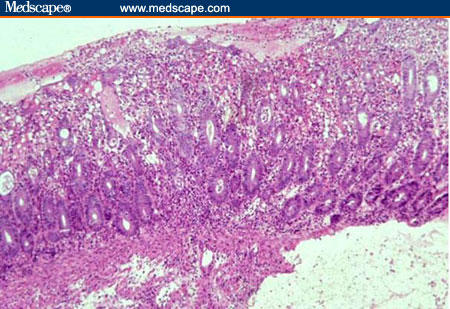
Mucosal biopsy from a patient with culture-proven Shigella colitis showing loss of surface epithelial cells, presence of a mixed inflammatory cell infiltrate, and numerous crypt abscesses (H&E x 125).
Escherichia coli
The association between E coli and CD is complex. E coli O157:H7 infection may mimic CD clinically, radiologically, and endoscopically. Differential diagnosis is by stool culture. E coli is also a common isolate from surgical specimens with CD, and E coli antibodies are found in higher numbers and higher titers in patients with CD vs controls.[9] Immunohistochemical examination of CD bowel sections has shown the presence of E coli in the mucus, on the surface epithelium, in ulcer beds, along fissures, in abscesses, and in inflammatory cells in the lamina propria (macrophages and giant cells). E coli has also been associated with bacterial translocation and postoperative infectious complications in CD.[10] Recently, a French group identified a specific pathogenic E coli strain (strain LF82, or adherent-invasive E coli) that can colonize ileal mucosa, invade intestinal epithelial cells, and survive and replicate within macrophages.[11]
Less Common Pathogens
Acute amebic colitis can mimic a first attack of colonic CD, and patients with CD can be carriers of Entamoeba histolytica. Because steroids can provoke amebic activity and may lead to fulminant colitis, it is important to determine whether amebae are present. Stool and biopsy examination, and even serology can be negative. Therefore, it is not unreasonable to treat patients who are suspected of infection with metronidazole concomitant with steroid therapy.[12] The association between Chlamydia trachomatis infection and CD is unclear. This pathogen has been isolated from biopsies of patients with CD. Chlamydia infection has also been diagnosed serologically in patients with CD.[13]Aeromonas hydrophila can cause a chronic, segmental colitis mimicking CD. Differential diagnosis is by stool culture.[14] CD must also be differentiated from abdominal tuberculosis,[15]Mycobacterium avium-intracellulare infection, chronic giardiasis, histoplasmosis, cryptococcosis, Blastocystis hominis infection, actinomycosis, Actinomyces israelii infection, and basidiobolomycosis.
Abdominal tuberculosis can be part of a multiorgan disease process or, less commonly, presents as a primary disease. It has been rare in western countries since the introduction of tuberculostatic drugs. Recently, the incidence of this entity has increased due to the AIDS epidemic and because of immigration patterns. The disease can be seen in otherwise healthy, immunocompetent patients. Differentiation from CD can be very difficult on clinical and histologic grounds if culture is negative. Erroneous treatment with steroids or a tumor necrosis factor-alpha inhibitor can lead to exacerbation of tuberculosis. Mycobacterium avium-intracellulare can cause chronic ileal infection, with formation of strictures and granulomas in AIDS patients. Giardiasis is a common infection and shares many symptoms with CD; it can complicate CD and cause exacerbation of the disease. Symptoms usually diminish upon treatment with metronidazole. Cryptococcosis and histoplasmosis can cause multifocal granulomatous enterocolitis, mostly in immunodeficient patients (AIDS, Job's syndrome); they are rarely seen in apparently immunocompetent patients. Treatment with steroids can be fatal. Blastocystis hominis can cause terminal ileitis in patients, without any apparent predisposing factors. It can be present in CD, but its pathogenic potential under these circumstances is unclear. This entity can be treated with metronidazole. Actinomycosis is a rare chronic infection that can present in otherwise healthy individuals, with abscesses and fistulae. Clinically, the latter may be difficult to differentiate from CD; the differential diagnosis can be made by anaerobic culture and histology. Actinomycosis can also complicate preexisting CD. Basidiobolomycosis is usually encountered in the tropics and presents typically with induration of subcutaneous tissues. Visceral involvement mimicking CD is extremely rare; it can have a fatal outcome.
Diagnostic Problems Associated With Diarrhea
Diarrhea and Colitis in Immunocompetent Patients
Acute Diarrhea (< 1 Week)
Acute watery diarrhea is often of viral etiology. Stool cultures will be negative. Acute viral gastroenteritis caused by Rotavirus, Norwalk agent, or Adenovirus may provoke exacerbation of preexisting CD.[16] The latter has also been observed with Rubella virus and Epstein-Barr virus infection.[17] Bloody diarrhea indicates a break in the mucosal lining and is often of bacterial origin. Stool cultures can confirm the bacterial nature of the diarrhea, but are frequently negative. In this setting, one must also consider diverticular disease, ischemia, malignancy, and drug-induced colitis.[18] A diagnosis of CD must be made cautiously in a patient with a first attack of colitis. Most patients with infectious colitis experience an acute onset of the disease and present within 1 week, often with fever. Onset of diarrhea in IBD is more often insidious.[19] A positive stool culture does not necessarily exclude a diagnosis of IBD.[20] Histology may assist in the differential diagnosis because focal basal plasmacytosis is a good predictive factor for a diagnosis of IBD. If acute colitis remains unclassified, patients should be followed up because up to 50% may develop IBD within 2.5-3 years.[21]
Fulminant Colitis
Fulminant colitis is a life-threatening condition that may be complicated by toxic megacolon. Ulcerative colitis and infectious bowel disease are major potential causes in this setting; CD is a less common cause. Early and precise diagnosis are essential for adequate treatment. Stool cultures, a search for cytomegalovirus (CMV) and Clostridium difficile enterotoxin, and testing for ova and parasites must be performed in all patients with fulminant colitis. Colectomy is indicated for toxic dilatation due to IBD, whereas patients with infectious colitis are generally treated without operation.[22]
Chronic Diarrhea (> 2 Weeks in Children; > 4 Weeks in Adults)
An infectious origin remains possible in cases of chronic diarrhea. Giardia lamblia infection of the small intestine can cause chronic abdominal pain, watery, foul-smelling stools associated with abdominal distension, anorexia, nausea, vomiting, weight loss, and frank malabsorption. Malabsorption can include steatorrhea, lactase deficiency, and malabsorption of fat-soluble vitamins and vitamin B12.[23,24]
The symptoms of chronic giardiasis can mimic Crohn's disease. Infectious bacterial enteritis usually resolves within 1-2 weeks with or without antibiotic treatment. Occasionally, cases of Clostridium difficile colitis can cause crampy abdominal pain and diarrhea for a period of several weeks before either worsening to require medical treatment or spontaneously resolving. Chlamydiae are frequently found in colonic biopsies of patients with chronic colitis. Facultatively enteropathogenic organisms such as Klebsiella pneumoniae and Pseudomonas aeruginosa have also been isolated from mucosal biopsies of patients with chronic colitis. These pathogens are also found in the mucosal biopsy specimens of patients with Crohn's disease, but the significance of this observation is uncertain in terms of the etiology and pathogenesis of Crohn's disease.[25] Histopathology aids in the differential diagnosis. Ideally, segmental colonic biopsies and an ileal biopsy are assessed along with information regarding endoscopic features and clinical data.[26,27] In infectious colitis, the microscopic features vary with the duration of the disease. Early changes include mucosal edema, cryptitis, crypt ulcers, and abscesses. Later on in the disease course, regeneration along with residual focal cryptitis is seen. This pattern may be confused with CD because of the focal nature of the changes.[28]
The distinction between IBD and infectious colitis relies primarily on the absence of features that orient towards a diagnosis of IBD (ie, architectural distortion and basal plasmacytosis; Figure 2).[29,30]
Figure 2.
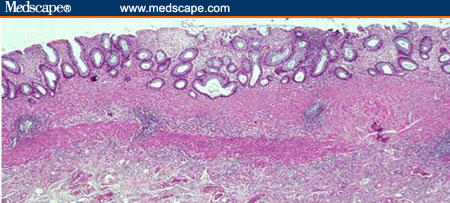
Colonic biopsy from patient with established, quiescent ulcerative colitis showing diffuse architectural abnormalities: shortened crypts, branching crypts, and thickening of the muscularis mucosae (H&E x 125).
However, the characteristic features that help establish a precise diagnosis of IBD are often not present in biopsies obtained during the initial phase of disease. Crypt distortion and diffuse basal plasmocytosis first appear after 2 weeks of disease and subsequently increase in frequency.[31] Therefore, there may be utility in obtaining a second set of biopsies 4 weeks (or later) after initial onset. Granulomas in the lamina propria not associated with damaged crypts are a corroborating feature of CD. The typical CD granuloma is an often ill-circumscribed collection of epithelioid histiocytes. Multinucleated giant cells are not characteristic and necrosis is usually not apparent. However, noncaseating granulomas, small collections of epithelioid histiocytes, and isolated giant cells may be present in infectious colitis.
Diarrhea and Colitis in Immunocompromised Patients
The differential diagnosis of diarrhea in patients with HIV infection includes a large number of bacterial, viral, and parasitic pathogens as well as malignancies such as lymphoma and Kaposi's sarcoma.[32] IBD rarely occurs in association with HIV infection. CD has been found to develop in a patient with established HIV infection, but remission of long-standing CD upon infection with HIV has also been noted.[33,34] With CD4 T-cell depletion, IBD disease activity may improve, but extreme reduction in CD4+ cell count may be associated with active disease (due to AIDS-related infection?).[35]
The precise etiologic diagnosis of diarrhea may also present a clinical problem in transplant patients receiving immunosuppressive therapy. An increased incidence of active IBD after liver transplantation for sclerosing cholangitis, despite immunosuppressive therapy, has been documented. Because patients who have undergone liver transplantation may also be prone to superimposed infection, the differential diagnosis between active IBD and infection must be made when the patient presents with severe colitis.[36,37] Persistent afebrile diarrhea with erosive enterocolitis has been observed in mycophenolate mofetil-treated renal transplant patients. In approximately 60% of cases, an infectious origin can be demonstrated. In the remaining 40%, biopsies may show a peculiar pattern that has been compared with CD and graft-vs-host-disease. Lesions may be caused by the local presence of mycophenolic acid or its metabolites.[38]
Relapse in CD
Relapse in CD has been associated with several factors. Infection, either enteric or systemic, may trigger a relapse by activating the gastrointestinal mucosal immune system. Enteropathogenic bacteria such as C difficile, Campylobacter jejuni, and enteropathogenic E coli have been isolated in a small number of patients with CD with symptomatic relapse. Superinfection of CD with enteropathogenic microorganisms may induce relapse, in general, but seems not to be very common.[39] The association between C difficile and IBD exacerbations has been studied extensively because of the possible link to prior antibiotic use in patients with IBD; findings have been controversial. In some studies, all patients with IBD with C difficile infection had taken antibiotics within the previous 6 months, whereas in other studies it was reported that fewer than 30% of patients had been treated with antibiotics.[40,41] Isolation of C difficile alone is of uncertain pathologic significance because infection may spontaneously clear without treatment.[42] Viral infections, especially with CMV, are another potential cause of IBD exacerbation, particularly in fulminant cases.[43]
Infectious Complications of CD
Extraluminal Spread of Enteric Bacteria
Endotoxemia occurs in most patients with active CD. Wound sepsis and intra-abdominal abscess can occur after elective operation for CD, even in the absence of macroscopic contamination. Both findings may be related to the high rate of bacterial translocation from the bowel lumen in patients with CD. Bacterial translocation may be favored by distension of the intestine proximal to CD-induced strictures.[44-46]
Opportunistic Infections and CD
Specific attention must be given to opportunistic infections because of the increased use of potent immunosuppressive drugs to treat IBD and because of the potential complications. Opportunistic infections can occur in the digestive tract or as an extraintestinal complication. The association between CMV infection and CD is complex. CMV enterocolitis may mimic CD in AIDS patients, but can also occur in patients without evidence of an immunocompromised state.[47-49] CMV infection may play a role in the natural history of refractory IBD (more frequently in ulcerative colitis than in CD) and in some of the related complications.[50,51] Histology is the gold standard for diagnosis, but should be assessed in the context of serology and results of PCR (polymerase chain reaction) studies (Figure 3).
Figure 3.
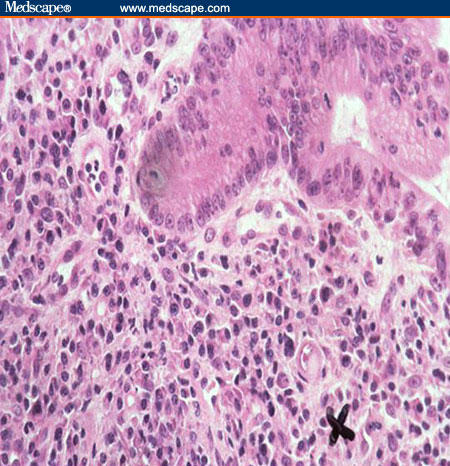
CMV inclusion (marked by "X") in a biopsy from a patient with severely active ulcerative colitis (H&E x 400).
Infection and the Etiology and Pathogenesis of CD
The precise etiology of CD remains unknown. Three major theories are currently under consideration: (1) disease develops due to a reaction to a persistent intestinal infection; (2) disease is due to the existence of a defective mucosal barrier to luminal antigens; and (3) disease results from a dysregulated host immune response to ubiquitous pathogens. In each of these theories, either pathogenic or resident luminal bacteria constantly stimulate the mucosal and systemic immune systems to perpetuate the inflammatory cascade.[52] The dysregulated host immune response in CD is demonstrated by the fact that there is considerable evidence for an ongoing T-helper cell type 1 response, with excess interleukin-12, interferon-gamma, and tumor necrosis factor-alpha.[53] The hypothesis that bacteria are implicated in the pathogenesis of CD is supported by several clinical and experimental observations.
First, there are some indications that childhood infections are a risk factor for development of CD and may in fact precede the early onset of disease.[54] Alternatively, the relative lack of infectious episodes due to improved domestic hygiene has also been associated with CD.[55] Second, it has been convincingly demonstrated that fecal stream diversion prevents recurrence of CD in the neoterminal ileum after curative ileal resection.[56]
The third line of evidence comes from the large number of rodent models of spontaneous chronic intestinal inflammation that have recently been developed. In the majority of these models, disease developed as a consequence of immune manipulations, suggesting a central role for the immune system in the regulation of intestinal inflammation. It is noteworthy, however, that inflammation usually did not develop in mice maintained in germ-free conditions. This finding suggests that disease may develop as a result of a dysregulated inflammatory response to components of the normal bowel flora.[57] Development of colitis in interleukin-10 gene-deficient mice can be prevented by administration of probiotics (Lactobacillus reuteri, L plantarum 299V, or genetically modified L lactis secreting interleukin-10) or by antibiotic therapy (treatment with ciprofloxacin or neomycin and metronidazole).[58-61] In humans, the use of probiotics in the treatment of CD is still controversial. Although preliminary results with probiotic therapy in humans with ulcerative colitis and pouchitis have been encouraging, their efficacy in treatment or maintenance of remission of CD remains to be clarified.[62] The role of antibiotic therapy for CD has been investigated for a number of years. It was found that metronidazole may have a role in the management of acute relapse in CD.[63] This agent may also delay and attenuate symptomatic recurrence of CD in the neoterminal ileum.[64] Ciprofloxacin in combination with metronidazole is well tolerated and appears to play a beneficial role in achieving clinical remission in patients with active CD, particularly when there is involvement of the colon.[65]
Finally, the recent discovery that the CARD15/NOD2 gene is involved in the genetic predisposition to CD has for the first time provided evidence of a molecular link between bacteria and inflammation of the digestive tract. The CARD15 protein product can be activated by components of the bacterial wall and induces the activation of NF kappa B, a proinflammatory molecule. Sensing of bacteria is impaired in patients carrying risk variant alleles for CD (ie, the 3 main CARD15/NOD2 mutations -- R702W, G908R, and 1007fs).[66]
Although there are numerous data implicating microorganisms in the pathogenesis of CD, it is still unclear whether the disease can be caused by the presence of specific bacteria or whether a global disturbance of the intestinal flora is involved. Several specific pathogens have been well studied. The literature on Mycobacterium paratuberculosis and measles virus is profuse but, at present, is still inconclusive.[67-70] Other microorganisms that have been implicated are Listeria monocytogenes and Pseudomonas fluorescens.[71,72] Study of the fecal or mucosa-associated bowel flora in patients with CD has recently gained new impetus by the development of genetic methods for identification of bacteria. These methods are primarily based on the detection or amplification and sequencing of 16S ribosomal RNA or DNA, which is specific for bacteria and ideally allows identification at a single-species level. In fecal samples from patients with CD, the biodiversity is usually high. However, Enterobacteriaceae are observed significantly more frequently in patients with CD than in healthy controls, and a large proportion of the dominant flora may belong to yet undefined phylogenetic groups.[73] The intestinal mucus layer of patients with CD harbors higher numbers of bacteria compared with controls and may be less protective against the endogenous microflora.[74] Findings from investigation of the mucosal flora have suggested that changes in the flora in patients with IBD are not secondary to inflammation, but may be the result of a specific host response.[75]
Concluding Remarks
This review illustrates many aspects of the complex relationship between CD and infectious disease. Infectious microorganisms have been implicated in the etiology and pathogenesis of CD for a long period. Much research has been performed in this field, but thus far, no definitive answers have emerged. The recent discovery that the CARD15/NOD2 gene is involved in the genetic predisposition to CD will undoubtedly provide an extra impetus to this research. More important to clinicians is the finding that infectious microorganisms can also be present in early or established CD, either as apparently "innocent bystanders" or as the cause of disease exacerbations -- this raises problems in diagnosis, differential diagnosis, and approach to management and complications.
Contributor Information
Gert De Hertogh, Resident, Department of Morphology & Molecular Pathology, University Hospital KU Leuven, Leuven, Belgium.
Karel Geboes, Professor, Department of Morphology & Molecular Pathology, University Hospital KU Leuven, Leuven, Belgium.
References
- 1. Vantrappen G, Ponette E, Geboes K, Bertrand P. Yersinia enteritis and enterocolitis: gastroenterological aspects. Gastroenterology. 1977;72:220-227. [PubMed] [Google Scholar]
- 2. Saebo A, Lassen J. Acute and chronic gastrointestinal manifestations associated with Yersinia enterocolitica infection. A Norwegian 10-year follow-up study on 458 hospitalized patients. Ann Surg. 1992;215:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamps LW, Madhusudhan KT, Havens JM, et al. Pathogenic Yersinia DNA is detected in bowel and mesenteric lymph nodes from patients with Crohn's disease. Am J Surg Pathol. 2003;27:220-227. [DOI] [PubMed] [Google Scholar]
- 4. Drake AA, Gilchrist MJ, Washington JA II, Huizenga KA, Van Scoy RE. Diarrhea due to Campylobacter fetus subspecies jejuni. A clinical review of 63 cases. Mayo Clin Proc. 1981;56:414-423. [PubMed] [Google Scholar]
- 5. Blaser MJ, Hoverson D, Ely IG, Duncan DJ, Wang WL, Brown WR. Studies of Campylobacter jejuni in patients with inflammatory bowel disease. Gastroenterology. 1984;86:33-38. [PubMed] [Google Scholar]
- 6. Day DW, Mandal BK, Morson BC. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978;2:117-131. [DOI] [PubMed] [Google Scholar]
- 7. Szilagyi A, Gerson M, Mendelson J, Yusuf NA. Salmonella infections complicating inflammatory bowel disease. J Clin Gastroenterol. 1985;7:251-255. [DOI] [PubMed] [Google Scholar]
- 8. Rutgeerts P, Geboes K, Ponette E, Coremans G, Vantrappen G. Acute infective colitis caused by endemic pathogens in western Europe: endoscopic features. Endoscopy. 1982;14:212-219. [DOI] [PubMed] [Google Scholar]
- 9. Tabaqchali S, O'Donoghue DP, Bettelheim KA. Escherichia coli antibodies in patients with inflammatory bowel disease. Gut. 1978;19:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilnyckyj A, Greenberg H, Bernstein CN. Escherichia coli O157:H7 infection mimicking Crohn's disease. Gastroenterology. 1997;112:995-999. [DOI] [PubMed] [Google Scholar]
- 11. Darfeuille-Michaud A. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn's disease. Int J Med Microbiol. 2002;292:185-193. [DOI] [PubMed] [Google Scholar]
- 12. Korelitz BI. When should we look for amebae in patients with inflammatory bowel disease? J Clin Gastroenterol. 1989;11:373-375. [PubMed] [Google Scholar]
- 13. Orda R, Samra Z, Levy Y, Shperber Y, Scapa E. Chlamydia trachomatis and inflammatory bowel disease--a coincidence? J R Soc Med. 1990;83:15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farraye FA, Peppercorn MA, Ciano PS, Kavesh WN. Segmental colitis associated with Aeromonas hydrophila. Am J Gastroenterol. 1989;84: 436-438. [PubMed] [Google Scholar]
- 15. Shah S, Thomas V, Mathan M, et al. Colonoscopic study of 50 patients with colonic tuberculosis. Gut. 1992;33:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gebhard RL, Greenberg HB, Singh N, et al. Acute viral enteritis and exacerbations of inflammatory bowel disease. Gastroenterology. 1982;83:1207-1209. [PubMed] [Google Scholar]
- 17. Kangro HO, Chong SK, Hardiman A, Heath RB, Walker-Smith JA. A prospective study of viral and mycoplasma infections in chronic inflammatory bowel disease. Gastroenterology. 1990;98:549-553. [DOI] [PubMed] [Google Scholar]
- 18. Siproudhis L, Mahmoud H, Briand N, et al. [Causal assessment of drug-induced acute colitis. A prospective study of 58 consecutive cases] [article in French]. Gastroenterol Clin Biol. 1998;22:778-784. [PubMed] [Google Scholar]
- 19. Schumacher G, Sandstedt B, Kollberg B. A prospective study of first attacks of inflammatory bowel disease and infectious colitis. Clinical findings and early diagnosis. Scand J Gastroenterol. 1994;29:265-274. [DOI] [PubMed] [Google Scholar]
- 20. Schumacher G, Kollberg B, Sandstedt B, et al. A prospective study of first attacks of inflammatory bowel disease and non-relapsing colitis. Microbiologic findings. Scand J Gastroenterol. 1993;28:1077-1085. [DOI] [PubMed] [Google Scholar]
- 21. Notteghem B, Salomez JL, Gower-Rousseau C, et al. [What is the prognosis in unclassified colitis? Results of a cohort study of 104 patients in the Northern-Pas-de-Calais region] [article in French]. Gastroenterol Clin Biol. 1993;17:811-815. [PubMed] [Google Scholar]
- 22. Schofield PF. Toxic dilatation and perforation in inflammatory bowel disease. Ann R Coll Surg Engl. 1982;64:318-320. [PMC free article] [PubMed] [Google Scholar]
- 23. Moore GT, Cross WM, McGuire D, et al. Epidemic giardiasis at a ski resort. N Engl J Med. 1969;281:402-407. [DOI] [PubMed] [Google Scholar]
- 24. Barbour AG, Nichols CR, Fukushima T. An outbreak of giardiasis in a group of campers. Am J Trop Med Hyg. 1976;25:384-389. [DOI] [PubMed] [Google Scholar]
- 25. Horing E, Gopfert D, Schroter G, von Gaisberg U. Frequency and spectrum of microorganisms isolated from biopsy specimens in chronic colitis. Endoscopy. 1991;23:325-327. [DOI] [PubMed] [Google Scholar]
- 26. Borsch G, Schmidt G. Endoscopy of the terminal ileum. Diagnostic yield in 400 consecutive examinations. Dis Colon Rectum. 1985;28:499-501. [DOI] [PubMed] [Google Scholar]
- 27. Dejaco C, Oesterreicher C, Angelberger S, et al. Diagnosing colitis: a prospective study on essential parameters for reaching a diagnosis. Endoscopy. 2003;35:1004-1008. [DOI] [PubMed] [Google Scholar]
- 28. Kumar NB, Nostrant TT, Appelman HD. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis). Am J Surg Pathol. 1982;6:523-529. [DOI] [PubMed] [Google Scholar]
- 29. Surawicz CM, Belic L. Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology. 1984;86:104-113. [PubMed] [Google Scholar]
- 30. Schumacher G, Sandstedt B, Mollby R, Kollberg B. Clinical and histologic features differentiating non-relapsing colitis from first attacks of inflammatory bowel disease. Scand J Gastroenterol. 1991;26:151-161. [DOI] [PubMed] [Google Scholar]
- 31. Schumacher G, Kollberg B, Sandstedt B. A prospective study of first attacks of inflammatory bowel disease and infectious colitis. Histologic course during the 1st year after presentation. Scand J Gastroenterol. 1994;29:318-332. [DOI] [PubMed] [Google Scholar]
- 32. Smith PD, Lane HC, Gill VJ, et al. Intestinal infections in patients with the acquired immunodeficiency syndrome (AIDS). Etiology and response to therapy. Ann Intern Med. 1988;108:328-333. [DOI] [PubMed] [Google Scholar]
- 33. Bernstein BB, Gelb A, Tabanda-Lichauco R. Crohn's ileitis in a patient with longstanding HIV infection. Am J Gastroenterol. 1994;89:937-939. [PubMed] [Google Scholar]
- 34. James SP. Remission of Crohn's disease after human immunodeficiency virus infection. Gastroenterology. 1988;95:1667-1669. [DOI] [PubMed] [Google Scholar]
- 35. Yoshida EM, Chan NH, Herrick RA, et al. Human immunodeficiency virus infection, the acquired immunodeficiency syndrome, and inflammatory bowel disease. J Clin Gastroenterol. 1996;23:24-28. [DOI] [PubMed] [Google Scholar]
- 36. Befeler AS, Lissoos TW, Schiano TD, et al. Clinical course and management of inflammatory bowel disease after liver transplantation. Transplantation. 1998;65:393-396. [DOI] [PubMed] [Google Scholar]
- 37. Dvorchik I, Subotin M, Demetris AJ, et al. Effect of liver transplantation on inflammatory bowel disease in patients with primary sclerosing cholangitis. Hepatology. 2002;35:380-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maes BD, Dalle I, Geboes K, et al. Erosive enterocolitis in mycophenolate mofetil-treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation. 2003;75:665-672. [DOI] [PubMed] [Google Scholar]
- 39. Weber P, Koch M, Heizmann WR, Scheurlen M, Jenss H, Hartmann F. Microbic superinfection in relapse of inflammatory bowel disease. J Clin Gastroenterol. 1992;14:302-308. [DOI] [PubMed] [Google Scholar]
- 40. Meyers S, Mayer L, Bottone E, Desmond E, Janowitz HD. Occurrence of Clostridium difficile toxin during the course of inflammatory bowel disease. Gastroenterology. 1981;80:697-670. [PubMed] [Google Scholar]
- 41. McLaren L, Bartlett JG, Gitnick G. Infectious agents in inflammatory bowel disease : collaborative studies. Gastroenterology. 1981;80:1228A. [Google Scholar]
- 42. Keighley MR, Youngs D, Johnson M, Allan RN, Burdon DW. Clostridium difficile toxin in acute diarrhoea complicating inflammatory bowel disease. Gut. 1982;23:410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berk T, Gordon SJ, Choi HY, Cooper HS. Cytomegalovirus infection of the colon: a possible role in exacerbations of inflammatory bowel disease. Am J Gastroenterol. 1985;80:355-360. [PubMed] [Google Scholar]
- 44. Higgens C, Allan RN, Keighley MR, Arabi Y, Alexander-Williams J. Sepsis following operation for inflammatory intestinal disease. Dis Colon Rectum. 1980;23:102-105. [DOI] [PubMed] [Google Scholar]
- 45. Laffineur G, Lescut D, Vincent P, Quandalle P, Wurtz A, Colombel JF. [Bacterial translocation in Crohn disease] [article in French]. Gastroenterol Clin Biol. 1992;16:777-781. [PubMed] [Google Scholar]
- 46. Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caroline DF, Hilpert PL, Russin VL. CMV colitis mimicking Crohn's disease in a patient with acquired immune deficiency syndrome (AIDS). Can Assoc Radiol J. 1987;38:227-228. [PubMed] [Google Scholar]
- 48. Wajsman R, Cappell MS, Biempica L, Cho KC. Terminal ileitis associated with cytomegalovirus and the acquired immune deficiency syndrome. Am J Gastroenterol. 1989;84:790-793. [PubMed] [Google Scholar]
- 49. Taniwaki S, Kataoka M, Tanaka H, Mizuno Y, Hirose M. Multiple ulcers of the ileum due to Cytomegalovirus infection in a patient who showed no evidence of an immunocompromised state. J Gastroenterol. 1997;32:548-552. [DOI] [PubMed] [Google Scholar]
- 50. Orvar K, Murray J, Carmen G, Conklin J. Cytomegalovirus infection associated with onset of inflammatory bowel disease. Dig Dis Sci. 1993;38:2307-2310. [DOI] [PubMed] [Google Scholar]
- 51. Vega R, Bertran X, Menacho M, et al. Cytomegalovirus infection in patients with inflammatory bowel disease. Am J Gastroenterol. 1999;94:1053-1056. [DOI] [PubMed] [Google Scholar]
- 52. Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S-11S. [PubMed] [Google Scholar]
- 53. MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. [DOI] [PubMed] [Google Scholar]
- 54. Wurzelmann JI, Lyles CM, Sandler RS. Childhood infections and the risk of inflammatory bowel disease. Dig Dis Sci. 1994;39:555-560. [DOI] [PubMed] [Google Scholar]
- 55. Gent AE, Hellier MD, Grace RH, Swarbrick ET, Coggon D. Inflammatory bowel disease and domestic hygiene in infancy. Lancet. 1994;343:766-767. [DOI] [PubMed] [Google Scholar]
- 56. Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771-774. [DOI] [PubMed] [Google Scholar]
- 57. Powrie F, Leach MW. Genetic and spontaneous models of inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Ther Immunol. 1995;2:115-123. [PubMed] [Google Scholar]
- 58. Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107-1114. [DOI] [PubMed] [Google Scholar]
- 59. Madsen KL, Doyle JS, Tavernini MM, Jewell LD, Rennie RP, Fedorak RN. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology. 2000;118:1094-1105. [DOI] [PubMed] [Google Scholar]
- 60. Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 61. Schultz M, Veltkamp C, Dieleman LA, et al. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71-80. [DOI] [PubMed] [Google Scholar]
- 62. Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm Bowel Dis. 2000;6:107-115. [DOI] [PubMed] [Google Scholar]
- 63. Keighley MR. Infection and the use of antibiotics in Crohn's disease. Can J Surg. 1984;27:438-441. [PubMed] [Google Scholar]
- 64. Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology. 1995;108:1617-1621. [DOI] [PubMed] [Google Scholar]
- 65. Greenbloom SL, Steinhart AH, Greenberg GR. Combination ciprofloxacin and metronidazole for active Crohn's disease. Can J Gastroenterol. 1998;12:53-56. [DOI] [PubMed] [Google Scholar]
- 66. Hugot JP, Zouali H, Lesage S. Lessons to be learned from the NOD2 gene in Crohn's disease. Eur J Gastroenterol Hepatol. 2003;15:593-597. [DOI] [PubMed] [Google Scholar]
- 67. Hermon-Taylor J, Bull TJ, Sheridan JM, Cheng J, Stellakis ML, Sumar N. Causation of Crohn's disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000;14:521-539. [DOI] [PubMed] [Google Scholar]
- 68. Chamberlin W, Graham DY, Hulten K, et al. Review article: Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Aliment Pharmacol Ther. 2001;15:337-346. [DOI] [PubMed] [Google Scholar]
- 69. Borody TJ, Leis S, Warren EF, Surace R. Treatment of severe Crohn's disease using antimycobacterial triple therapy--approaching a cure? Dig Liver Dis. 2002;34:29-38. [DOI] [PubMed] [Google Scholar]
- 70. Robertson DJ, Sandler RS. Measles virus and Crohn's disease: a critical appraisal of the current literature. Inflamm Bowel Dis. 2001;7:51-57. [DOI] [PubMed] [Google Scholar]
- 71. Chen W, Li D, Paulus B, Wilson I, Chadwick VS. Detection of Listeria monocytogenes by polymerase chain reaction in intestinal mucosal biopsies from patients with inflammatory bowel disease and controls. J Gastroenterol Hepatol. 2000;15:1145-1150. [DOI] [PubMed] [Google Scholar]
- 72. Wei B, Huang T, Dalwadi H, Sutton CL, Bruckner D, Braun J. Pseudomonas fluorescens encodes the Crohn's disease-associated I2 sequence and T-cell superantigen. Infect Immun. 2002;70:6567-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seksik P, Rigottier-Gois L, Gramet G, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117:1089-1097. [DOI] [PubMed] [Google Scholar]
- 75. Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44-54. [DOI] [PubMed] [Google Scholar]


