Abstract
Background: The IgG1 and IgG3 antibodies are considered cytophilic and protective against Plasmodium falciparum, whereas IgG2 and IgG4 are thought to block protective mechanisms.
Objectives: The main objective was to measure antibodies directed against erythrocyte binding antigen-175 (EBA-175) peptide 4 and analyze the relationship between such antibodies and clinical malaria attack.
Methods: Using an enzyme-linked immunosorbent assay, a retrospective analysis of naturally acquired antibodies to synthetic peptide from EBA-175 peptide 4 has been carried out in 158 school children from the village of Dienga in Gabon.
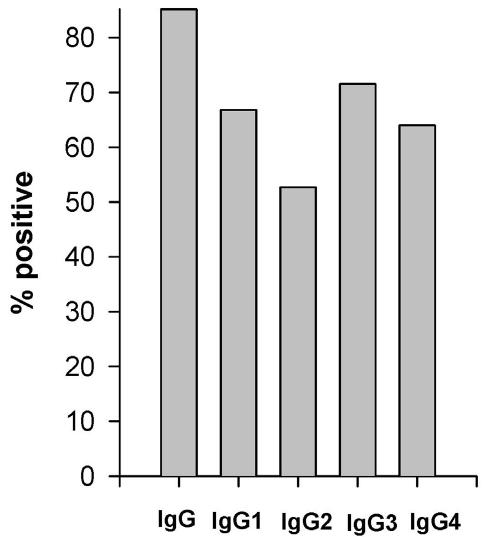
Results: The overall prevalence rates of antibodies to EBA-175 peptide 4 were 85.2%, 66.8%, 52.6%, 71.6% and 64.0% for total IgG, IgG1, IgG2, IgG3 and IgG4, respectively. Protection from clinical malaria, determined after a prospective 1-year study, was associated with the levels of IgG and IgG1 antibodies that increased with age.
Conclusion: Together, these data suggest that age/exposure-related acquisition of anti-EBA-175 antibodies may contribute to the development of clinically protective immunity and could be taken into account in malaria control strategies when they are confirmed.
Keywords: Clinical malaria, EBA-175 peptide 4, Immune response, Plasmodium falciparum
Currently efforts are focused on the development of a vaccine against the human malaria parasite, Plasmodium falciparum, as an alternative to drug therapy or vector control. P. falciparum uses a 175 kDa sialic acid binding protein ligand known as erythrocyte binding antigen-175 (EBA-175) for erythrocyte invasion.1–4 The gene encoding EBA-175 has been sequenced from both the FCR3 and CAMP strains of P. falciparum.5–7 While it is now well established that EBA-175 is a dimorphic antigen, the role that dimorphism plays in host-parasite interactions (including putative differences in red blood cell invasion efficiency) remains unclear. However, the initial molecular interaction between the parasite and the erythrocyte involves binding of a conserved domain (region II) of EBA-175 to sialic acid residues from glycophorin A followed by proteolytic cleavage of EBA-175 and binding of the dimorphic F and C segments to the glycophorin A back bone.7 A conserved region of 42 amino acids (aa) of EBA-175, termed EBA-peptide 4 (1062–1103) within region V, has been implicated in the binding to erythrocytes,8 although it is not essential for the initial sialic acid-dependent binding.4
EBA-175 is considered a potential vaccine candidate because it induces antibodies that inhibit malaria merozoite invasion.9 The recombinant fragments of EBA-175 are recognized by human sera from malaria endemic areas.10 Antibodies raised in mice against EBA-peptide 4 blocked binding of native EBA-175 to human erythrocytes and inhibited merozoite invasion in vitro.8,9 Importantly, an alternative invasion pathway by P. falciparum merozoites commonly occurs in the field parasites11 and can also be induced in vitro by targeted disruption of the EBA-175 gene.12 EBA-175 has a universal role in merozoite invasion since the antibodies against region II block invasion pathways that do not involve sialic acid.13 It has been demonstrated that EBA-175 is also expressed on pre-erythrocytic parasites14 and that immunization with EBA-region II protects Aotus monkeys from P. falciparum challenge.15 Although EBA-175 antigen is likely to be involved in the development of protective immunity against malaria, the antibody response to EBA-175 in humans living in malaria endemic regions remains poorly characterized,16 including the response to EBA-peptide 4 which is predicted to include a B-cell epitope.6
The aim of the present study was to measure antibodies directed against EBA peptide 4, as well as isotype distribution in school children living in Dienga, Gabon. The influence of age on the levels of these antibodies has been investigated, as well as their relationship with parasite density and occurrence of clinical malaria attack during a 12-month follow-up.
Materials and Methods
Subjects and field methods
The village under study was Dienga in southeast Gabon where a clinical, biological and parasitological follow-up was carried out among the primary school-going population during the whole malaria transmission season of 1995.17 Clinical and parasitological data allowed us to distinguish between protected and unprotected children. Briefly, protected children were defined as those who never presented during the whole survey with a febrile episode (defined as axillary temperature >37°C) associated with neither P. falciparum parasitemia >400/μl nor the presence of 4-aminoquinoline metabolite in urine. Unprotected children were defined as those who presented with at least one malaria attack defined as the association of fever and parasitemia ≥5000/μl.17,18 The unique criterion of selection was the availability of sufficient quantity of plasma, which was obtained for 158 children.
Moreover, from February 1995 to March 1996, all children were routinely screened for P. falciparum infection by finger prick blood sampling every 2 weeks and also whenever fever occurred. The thick blood smears were prepared and stained with Giemsa. Parasite densities were recorded as the number of parasites/μl of blood, assuming an average leukocyte count of 8000/μl. Each value of parasite density was simultaneously adjusted to age and date of sampling by calculating the ratio of individual parasite density (parasite density + 1) to the geometric mean of all (parasite density + 1) values recorded at each date of sampling in the group of children of the same age. For each child who presented at least six recorded thick blood smears, the geometric mean of date and age adjusted (GMA) parasite densities was calculated. For the need of statistical analysis, log-transformed values of the GMA parasite density were considered.
Antigen and antibody measurements
A synthetic peptide used as antigen was EBA-peptide 4 (aa 1062-1103:SNNEYKVNEREDERTLTKEYEDIVLKSHMNRESDDGELYDEN). This peptide was synthesized by Interactiva Biotechnology (Ulm, Germany). The plasma was collected from 158 children at the end of the follow-up. Antibodies were measured by enzyme-linked immunosorbent assay (ELISA) using 1 μg/ml of EBA peptide 4, a 100-fold diluted plasma and a 2,000-fold diluted goat anti-human IgG (Fc specific) conjugated to alkaline phosphatase (Sigma, St. Louis, MO, USA). Bound enzyme was detected with p-nitrophenylphosphate and the absorbance was read at 405 nm.
IgG subclass analysis was carried out using 50-fold diluted plasma, mouse anti-human IgG1, IgG2, IgG3 and IgG4 antibodies (codes: LMH 1013, 1022, 1032 and 1042; Caltag Laboratories, Burlingame, CA, USA) at a final concentration of 1, 0.25, 0.5, 0.25 μg/ml, respectively, and a goat anti-mouse IgG at respective final concentrations of 2.5, 5, 1.3 and 2.5 μg/ml. Bound enzyme was detected as described above. Reference positive and negative control plasmas were included in each plate and results were expressed in arbitrary units (AU) calculated as previously described.19,20 The thresholds for positivity were set at 45.2 AU for anti-EBA-peptide 4 IgG and 35.6, 93.3, 31.7 and 23.3 AU for IgG1, IgG2, IgG3 and IgG4 isotypes, respectively, as determined from the mean reactivity plus 2 SD of 20 plasmas from non-immune subjects.
Statistical analysis
Differences in proportions were analyzed using the χ2 test. Differences in means were tested by Student’s unpaired t test on linear or log-transformed values. When variable distribution was not normalized by log transformation, the non-parametric Mann-Whitney U test was employed. Associations between quantitative variables were assessed by the Spearman’s rank test of correlation. A logistic regression using the maximum likelihood ratio method (LR procedure of BMDP) was used to adjust antibody responses simultaneously on age and protection status. For all tests, P values <0.05 were considered significant.
Results
Recognition of EBA-175 peptide 4 by human antibodies
Clinical, parasitological and hematological results have been previously reported.17,18 Sex, presence of the sickle cell trait and P. falciparum parasite density at time of blood draw were equally distributed among children who had clinical malaria (unprotected) and those who never experienced a malaria attack (protected) during the 12-month follow-up. The overall prevalence rates were 85.5%, 66.8%, 52.6%, 71.6% and 64.0%, respectively for total IgG, IgG1, IgG2, IgG3 and IgG4 (figure 1 ▶). Both IgG1 and IgG3 antibody prevalence rates and levels were higher in IgG positive samples (χ2 test and Mann-Whitney U test, P = 0.0001 in both tests for IgG1, and P = 0.009 and P = 0.0004, respectively for IgG3). The levels of IgG4 antibodies were higher in IgG1 and IgG2 responders compared to non-responders (Mann-Whitney U test, P = 0.006, P <0.0001, respectively) and were correlated to the levels of each of the other IgG subclasses (Spearman: rho = 0.326, P = <0.0001 for IgG1; rho = 0.322, P = <0.0001 for IgG2; and rho = 0.126, P = <0.07 for IgG3). The prevalence rate and the levels of IgG1 antibodies were both higher in IgG2 responders than in non-responders (χ2 test, P = 0.03; Spearman: rho = 0.183, P = 0.009).
Figure 1.
Proportions of individuals with plasma IgG and IgG subclass antibodies against EBA-175 peptide 4, as tested by ELISA.
Influence of age on EBA-175 antibody response
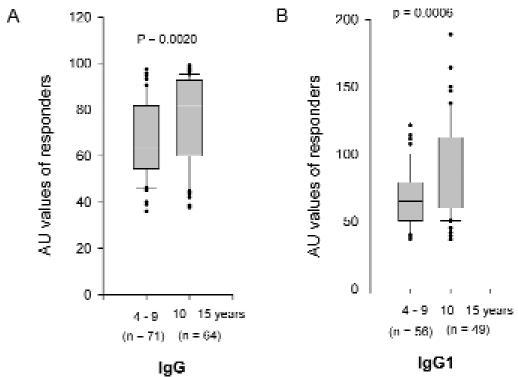
The prevalence rate (Mann-Whitney U test, P = 0.006) and the level (Spearman: rho = 0.238, P = 0.0006) of IgG1 antibodies to EBA-peptide 4 increased with age (figure 2A ▶). Total IgG level also increased with age (Spearman: rho = 0.212, P = 0.002) (figure 2B ▶). While anti-EBA-175 IgG2, IgG3 and IgG4 antibodies did not vary with age, the ratios of IgG1:IgG2, IgG1:IgG3, and IgG1:IgG4 antibodies increased with age (Spearman: rho = 0.252, P = 0.0006; rho = 0.153, P = 0.05; and rho = 0.276, P = 0.0003, respectively).
Figure 2.
Distribution of IgG (A) and IgG1 (B) levels in children <10 years old and those who are 10 years and older. IgG and IgG1 levels differed between both groups of children (Mann-Whitney U test, P = 0.0020 and 0.0006, respectively).
Relationships between parasite density and anti-EBA-175 antibody response
Individuals with IgG antibodies to EBA-175 exhibited higher GMA parasite density than those without IgG (Mann-Whitney U test, P = 0.04). GMA parasite density also correlated to IgG1 levels (Spearman: rho = 0.126, P = 0.08).
Association of EBA-175-specific antibodies and resistance to clinical malaria
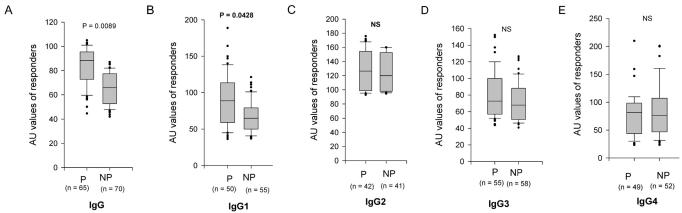
Children were monitored for malaria infection and clinical disease during a prospective 1-year study. EBA-175-specific antibodies titers were compared between unprotected (who presented with a clinical malaria attack) and protected individuals (who did not). The prevalence rate of total IgG was similar in both groups, but their level was higher in protected individuals (Mann-Whitney U test, P = 0.0089) (figure 3A ▶). Furthermore, the prevalence rate (χ2 test, P = 0.02) and the level (Mann-Whitney U test, P = 0.0428) of IgG1 antibody were higher in protected than in unprotected children (figure 3B ▶). The logistic regression demonstrated that both age (P = 0.049) and IgG1 antibodies (P = 0.045, OR = 2.4, 95% CI: 1.1–5.5) were independent predictors of protection status. Moreover, the ratio of cytophilic antibodies IgG1:IgG3 was also increased in protected as compared to unprotected children (Mann-Whitney U test, P = 0.02). The prevalence rates and levels of IgG2, IgG3 and IgG4 antibodies were similar in both groups of children (figure 3C, 3D and 3E ▶).
Figure 3.
Distribution of plasma antibodies levels (in arbitrary units, AU) to EBA-175 peptide 4 in children who had clinical malaria (unprotected = NP) and those who had an asymptomatic infection (protected = P). (A) total IgG, (B) IgG1, (C) IgG2, (D) IgG3, (E) IgG4. The 10th, 25th, 75th and 90th percentile boxes for the AU values of each IgG subclass are presented and the horizontal line within these percentile boxes indicate the median AU. There were significant differences in the distributions of total IgG and IgG1 levels between both groups of children (Mann-Whitney U test, P = 0.009 and 0.043, respectively).
Discussion
The immunological effector mechanisms responsible for protection against malaria are poorly understood and may vary according to transmission dynamics21,22 and age of exposure to malaria parasites.23 The development of anti-disease immunity in children repeatedly infected with P. falciparum is thought to be related to the rise of immune responses to certain parasite antigens. In areas of stable transmission, antibodies recognizing monomorphic epitopes,21.22 polymorphic epitopes24 and variant epitopes on proteins showing clonal antigenic variation25–27 have all been suggested to contribute to this protection. In this study, the anti-EBA-175 peptide 4 IgG isotypes were analyzed in children who either presented with or not with a malaria attack during a prospective 1-year follow-up study. To overcome the confounding effects of antigen polymorphism, we deliberately focused on a conserved sequence, commonly expressed by P. falciparum. Our study demonstrated that IgG1, IgG2, IgG3 and IgG4 antibodies from most of the children recognized EBA-175 peptide 4. This clearly indicates that IgG antibodies compete for binding to EBA-175 epitopes and is consistent with the role of an isotype imbalance in the resistance and/or susceptibility to malaria infection.28 A similar subclass distribution has been reported in studies using EBA-175 recombinant antigens,29 merozoite surface protein 1 (MSP1)30 and MSP2.31,32 Interestingly, our study showed that protection against clinical malaria was associated with the presence and levels of IgG1 antibodies to EBA-175 peptide 4 (figure 3B ▶), as well as with the levels of total IgG (figure 3A ▶). Furthermore, the levels of total IgG and IgG1 antibodies increased with age (figure 2 ▶). Overall, there were no significant differences in the distribution of IgG2, IgG3 and IgG4 levels between those who were protected or not against malaria (figure 3C, 3D and 3E ▶). Although a possible change in EBA-175 antibody levels through the period of follow-up is not taken into account, these results are in agreement with previous findings concerning the antibody response of naturally infected individuals to P. falciparum antigens. It has been shown, in a large population study in Gambia, that antibodies to EBA-175 were predominantly of the IgG1 and IgG3 subclasses and increased with age. Although the presence of such antibody was not associated with clinical protection against malaria, there was a trend indicating that individuals with high anti-EBA-175 region II IgG levels presented with some protection.29 Jakobsen et al.33 reported contrasting observations in antibody response to EBA peptide (aa 1076 to 1096) among individuals living in different endemic regions. Indeed, sera from donors living in Indonesia, Nigeria and Sudan with long exposure to malaria, had low or negligible IgG reactivity to EBA peptide (aa 1076 to 1096). By contrast, Tanzanian children with clinical malaria had higher IgG reactivity to this peptide compared to those with asymptomatic infections. The fact that EBA peptide (aa 1076 to 1096) is composed of 20 amino acids within the EBA-peptide 4 (42 aa) lacking therefore an epitope, may explain the difference in antibody response directed against this peptide and EBA-peptide 4.
In our study, anti-EBA-175 IgG and IgG1 were associated with protection against clinical malaria, but other subclasses were not. We suggest that this protection of Gabonese children with high IgG levels was mainly related to the IgG1 subclass, as demonstrated by its higher prevalence rate and levels among IgG responders. Nevertheless, the cytophilic antibodies IgG1 and IgG3 act in cooperation with cells in parasite-killing effector responses, such as opsonization and antibody-dependent cellular inhibition and, therefore, both are believed to be involved in protection against P. falciparum.34 Since IgG3 antibody seems to be more efficient than IgG1 in cooperating with accessory cells35,36 and in activating the complement pathway,37 it might be surprising that anti-EBA-175 specific IgG3 does not appear to be associated with protection. One likely explanation is that IgG4 and IgG3 recognize similar epitopes from EBA-175 peptide 4 and that IgG4 competes with IgG3 for binding.
Similar isotypic analysis has been performed in various endemic areas, but did not reveal a clear pattern of relationships between isotype distribution and parasite density or malaria attack incidence. This may be due to parasite and host genetic factors, to immunoassay used or to the design of the field study.38 The epidemiological studies in Papua New Guinea indicated that IgG antibodies to MSP2 were associated with a reduced risk of fever associated with malaria infection and with a reduced risk of malaria-related anemia,39,40 but did not characterize the protective subclass. Conversely, a study in Senegal indicated that an increase in anti-malarial IgG3 with age was associated with a decreased risk of clinical malaria, but the specificity of the antibodies was not investigated.41 Our study showed an association between parasite density and anti-EBA-175 IgG and IgG1 antibodies. Indeed, the GMA parasite density was higher in IgG positive than in negative individuals, as well as in individuals with high levels of IgG1 antibodies. Such association probably results from a higher parasite density more efficiently triggering the immune system to produce EBA-175 antibodies. We determined the subclass of EBA-175 specific IgG in plasma from individuals immunized by natural exposure to malaria. The most important question regarding the potential of EBA-175 as a vaccine antigen is whether EBA-175-specific immune responses are significantly involved in protective immunity to malaria. Our data showed that EBA-175 is naturally antigenic, that IgG1 antibodies to EBA-175 peptide 4 are associated with protection against clinical malaria and further investigation of relationships between EBA-175 antibodies and malaria attack incidence is justified.
Acknowledgments
We thank Adrian Luty and Pascal Millet for help in collecting field data and Michel Cot for help in statistical analysis. We also thank Daniel Camus for helpful discussions.
Grant Support: State of Gabon, by Total-Fina-Elf and by the French Ministry of Foreign Affairs
References
- 1.Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 1985;230:553–556. [DOI] [PubMed] [Google Scholar]
- 2.Klotz FW, Orlandi PA, Reuter G, Cohen SJ, Haynes JD, Schauer R, Howard RJ, Palese P, Miller LH. Binding of Plasmodium falciparum 175-kilodalton erythrocyte binding antigen and invasion of murine erythrocytes requires N-acetylneuraminic acid but not its O-acetylated form. Mol Biochem Parasitol 1992;51:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlandi PA, Klotz FW, Haynes JD. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2–3)Gal- sequences of glycophorin A. J Cell Biol 1992;116:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 1994;264:1941–1944. [DOI] [PubMed] [Google Scholar]
- 5.Kain KC, Lanar DE. Determination of genetic variation within Plasmodium falciparum by using enzymatically amplified DNA from filter paper disks impregnated with whole blood. J Clin Microbiol 199;29:1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim BK. EBA-175: an erythrocyte-binding ligand of Plasmodium falciparum. Parasitol Today 1995;11:213–217. [DOI] [PubMed] [Google Scholar]
- 7.Ware LA, Kain KC, Lee Sim BK, Haynes JD, Baird JK, Lanar DE. Two alleles of the 175-kilodalton Plasmodium falciparum erythrocyte binding antigen. Mol Biochem Parasitol 1993;60:105–109. [DOI] [PubMed] [Google Scholar]
- 8.Sim BK. Sequence conservation of a functional domain of erythrocyte binding antigen 175 in Plasmodium falciparum. Mol Biochem Parasitol 1990;41:293–295. [DOI] [PubMed] [Google Scholar]
- 9.Sim BK, Orlandi PA, Haynes JD, Klotz FW, Carter JM, Camus D, Zegans ME, Chulay JD. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol 1990;111:1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugherty JR, Murphy CI, Doros-Richert LA, Barbosa A, Kashala LO, Ballou WR, Snellings NJ, Ockenhouse CF, Lanar DE. Baculovirus-mediated expression of Plasmodium falciparum erythrocyte binding antigen 175 polypeptides and their recognition by human antibodies. Infect Immun 1997;65:3631–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okoyeh JN, Pillai CR, Chitnis CE. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect Immun 1999;67:5784–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed MB, Caruana SR, Batchelor AH, Thompson JK, Crabb BS, Cowman AF. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci U S A 2000;97:7509–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narum DL, Haynes JD, Fuhrmann S, Moch K, Liang H, Hoffman SL, Sim BK. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect Immun 2000;68:1964–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grüner AC, Brahimi K, Letourneur F, Rénia L, Eling W, Snounou G, Druihle P. EBA-175 expression on malaria sporozoites. 12th British Society of Parasitology Malaria meeting. Leeds, U.K, 2001.
- 15.Jones TR, Narum DL, Gozalo AS, Aguiar J, Fuhrmann SR, Liang H, Haynes JD, Moch JK, Lucas C, Luu T, Magill AJ, Hoffman SL, Sim BK. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. J Infect Dis 2001;183:303–312. [DOI] [PubMed] [Google Scholar]
- 16.Jakobsen PH, Heegaard PM, Koch C, Wasniowska K, Lemnge MM, Jensen JB, Sim BK. Identification of an erythrocyte binding peptide from the erythrocyte binding antigen, EBA-175, which blocks parasite multiplication and induces peptide-blocking antibodies. Infect Immun 1998;66:4203–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deloron P, Ringwald P, Luty AJ, Renaut A, Minh TN, Mbessy JR, Millet P. Relationships between malaria prevalence and malaria-related morbidity in school children from two villages in central Africa. Am J Trop Med Hyg 1999;61:99–102. [DOI] [PubMed] [Google Scholar]
- 18.Migot-Nabias F, Luty AJ, Ringwald P, Vaillant M, Dubois B, Renaut A, Mayombo RJ, Minh TN, Fievet N, Mbessi JR, Millet P, Deloron P. Immune responses against Plasmodium falciparum asexual blood-stage antigens and disease susceptibility in Gabonese and Cameroonian children. Am J Trop Med Hyg 1999;61:488–494. [DOI] [PubMed] [Google Scholar]
- 19.Rasheed FN, Bulmer JN, De Francisco A, Jawla MF, Jakobsen PH, Jepson A, Greenwood BM. Relationships between maternal malaria and malarial immune responses in mothers and neonates. Parasite Immunol 1995;17:1–10. [DOI] [PubMed] [Google Scholar]
- 20.Migot-Nabias F, Deloron P, Ringwald P, Dubois B, Mayombo J, Minh TN, Fievet N, Millet P, Luty A. Immune response to Plasmodium falciparum liver stage antigen-1: geographical variations within central Africa and their relationship with protection from clinical malaria. Trans R Soc Trop Med Hyg 2000;94:557–562. [DOI] [PubMed] [Google Scholar]
- 21.Druilhe P, Perignon JL. A hypothesis about the chronicity of malaria infection. Parasitol Today 1997;13:353–357. [DOI] [PubMed] [Google Scholar]
- 22.Jensen JB, Hoffman SL, Boland MT, Akood MA, Laughlin LW, Kurniawan L, Marwoto HA. Comparison of immunity to malaria in Sudan and Indonesia: crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci U S A 1984;81:922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today 1995;11:105–111. [DOI] [PubMed] [Google Scholar]
- 24.Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, Takacs B, Schonfeld HJ, Holder AA, Greenwood BM. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol 1992;14:321–337. [DOI] [PubMed] [Google Scholar]
- 25.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg 1989;83:293–303. [DOI] [PubMed] [Google Scholar]
- 26.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med 1998;4:358–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giha HA, Staalsoe T, Dodoo D, Roper C, Satti GM, Arnot DE, Hviid L, Theander TG. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol Lett 2000;71:117–126. [DOI] [PubMed] [Google Scholar]
- 28.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun 1992;60:1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okenu DM, Riley EM, Bickle QD, Agomo PU, Barbosa A, Daugherty JR, Lanar DE, Conway DJ. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect Immun 2000;68:5559–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silveira LA, Dorta ML, Kimura EA, Katzin AM, Kawamoto F, Tanabe K, Ferreira MU. Allelic diversity and antibody recognition of Plasmodium falciparum merozoite surface protein 1 during hypoendemic malaria transmission in the Brazilian amazon region. Infect Immun 1999;67:5906–5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor RR, Smith DB, Robinson VJ, McBride JS, Riley EM. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun 1995;63:4382–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg 1998;58:406–413. [DOI] [PubMed] [Google Scholar]
- 33.Jakobsen PH, Heegaard PM, Koch C, Wasniowska K, Lemnge MM, Jensen JB, Sim BK. Identification of an erythrocyte binding peptide from the erythrocyte binding antigen, EBA-175, which blocks parasite multiplication and induces peptide-blocking antibodies. Infect Immun 1998;66:4203–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 1990;172:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegelberg HL. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol 1974;19:259–294. [DOI] [PubMed] [Google Scholar]
- 36.van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today 1993;14:215–221. [DOI] [PubMed] [Google Scholar]
- 37.Burton DR, Gregory L, Jefferis R. Aspects of the molecular structure of IgG subclasses. Monogr Allergy 1986;19:7–35. [PubMed] [Google Scholar]
- 38.Aucan C, Traore Y, Tall F, Nacro B, Traore-Leroux T, Fumoux F, Rihet P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun 2000;68:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.al-Yaman F, Genton B, Anders RF, Falk M, Triglia T, Lewis D, Hii J, Beck HP, Alpers MP. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am J Trop Med Hyg 1994;51:593–602. [DOI] [PubMed] [Google Scholar]
- 40.al-Yaman F, Genton B, Anders R, Taraika J, Ginny M, Mellor S, Alpers MP. Assessment of the role of the humoral response to Plasmodium falciparum MSP2 compared to RESA and SPf66 in protecting Papua New Guinean children from clinical malaria. Parasite Immunol 1995;17:493–501. [DOI] [PubMed] [Google Scholar]
- 41.Aribot G, Rogier C, Sarthou JL, Trape JF, Balde AT, Druilhe P, Roussilhon C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa). Am J Trop Med Hyg 1996;54:449–457. [DOI] [PubMed] [Google Scholar]