Abstract
Over the last two decades, it has become evident that decreased bioavailability of endothelial nitric oxide (NO) produced from endothelial NO synthase (eNOS), referred to as endothelial dysfunction, plays a crucial role in the development and progression of atherosclerosis. Much progress has been made in understanding the mechanisms of decreased endothelial NO bioavailability at the levels of regulation of eNOS gene expression, eNOS enzymatic activity and NO inactivation. Initial studies suggest that increasing eNOS gene expression would improve endothelial NO release in the hope of inhibiting the progression of atherosclerosis. Recent experimental studies, however, do not always support this therapeutic concept and show some evidence that overexpression of eNOS in atherosclerosis may be even harmful for the disease progression.Thus, recent research to improve endothelial function in atherosclerosis has focused on regulation of eNOS enzymatic activity and prevention of NO inactivation by oxidative stress. Since the role of oxidative stress in endothelial NO bioavailability has been reviewed in a large number of comprehensive articles, this article focuses on the relevant regulatory mechanisms of eNOS enzymatic activity that are emerging to play a role in endothelial dysfunction in atherosclerosis.
Keywords: Arginase, Atherosclerosis, BH4, eNOS, L-arginine, Oxidative stress
The endothelium-derived nitric oxide (NO) is synthesized from the substrate L-arginine via endothelial NO synthase (eNOS) and plays a crucial role in regulating a wide spectrum of functions in the cardiovascular system, including vasorelaxation, inhibition of leukocyte-endothelial adhesion, vascular smooth muscle cell (SMC) migration and proliferation, as well as platelet aggregation.1 Physical or biochemical injury to the endothelium impairs production and/or function of endothelium-derived vasoprotective mediators of vascular health, such as NO, resulting in increased vascular contractions to vasoconstrictors such as endothelin-1, thromboxanes and serotonin,2,3 enhanced thrombus formation and exacerbated SMC proliferation and migration.1 It is, therefore, not surprising that loss of endothelial NO function is associated with several cardiovascular disorders, including atherosclerosis, which is due either to decreased production or to increased degradation of NO.4 Experimental and clinical studies provide evidence that defects of endothelial NO function, referred to as endothelial dysfunction, is not only associated with all major cardiovascular risk factors, such as hyperlipidemia, diabetes, hypertension, smoking and severity of atherosclerosis, but also has a profound predictive value for the future atherosclerotic disease progression.5–9 Therefore, the dysfunctional eNOS/NO pathway is considered as an early marker or a common mechanism for various cardiovascular disorders.
Although the underlying mechanisms of endothelial eNOS/NO dysfunction in atherosclerosis have been intensively studied and various mechanisms responsible for decreased endothelial NO bioactivity under the disease condition have been suggested, no single mechanism can fully explain the endothelial dysfunction. This may simply be due to the fact that atherosclerosis is a complex disease procedure and that multiple regulatory mechanisms are involved in endothelial NO bioactivity, particularly at the eNOS enzymatic level. Pathophysiologically, endothelial NO bioactivity is simply determined by the balance between synthesis and degradation of the molecule. Biochemically, the endothelial NO production is regulated at three different levels: 1) eNOS gene expression, 2) eNOS enzymatic activity and 3) degradation of NO (figure 1 ▶). Dysfunction of any of these mechanisms can cause endothelial dysfunction. This review article discusses the current knowledge of endothelial dysfunction in atherosclerosis, focusing on the mechanisms of deregulation of eNOS gene expression and enzymatic activity and also discusses future therapeutic perspectives to improve endothelial function in atherosclerosis. The detailed mechanisms of endothelial NO degradation by oxidative stress are comprehensively reviewed elsewhere.10,11
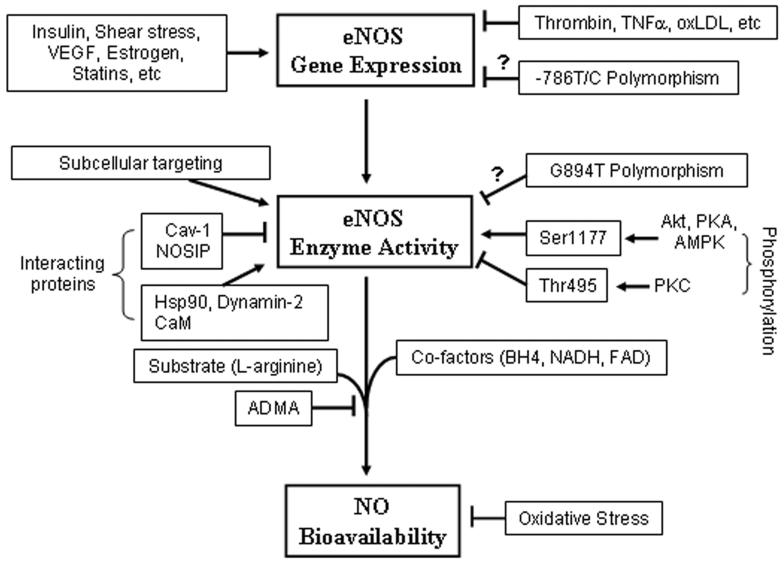
Figure 1.
Regulatory mechanisms of endothelial NO production at three different levels. While shear stress, insulin, VEGF, estrogen, and statins increase eNOS gene expression, several factors that are involved in atherosclerotic disease, such as thrombin, TNF-α and Ox-LDL, suppress eNOS gene expression. Whether the −786T/C substitution in the promoter region reduces eNOS gene expression is not clear. Subcellular targeting of eNOS regulated by co- or post-translational modifications, such as N-myristoylation and cysteine, palmitoylation is critical for optimal NO production. The mechanism by which localization influences eNOS activation is not fully understood. Interaction of eNOS with caveolin-1 (Cav-1) and NOSIP proteins reduces eNOS enzymatic activity, and interaction with heat shock protein 90 (Hsp90) and dynamin-2 increases eNOS enzymatic activity. Increase in intracellular Ca2+ concentration forms the Ca2+-calmodulin (CaM) complex, which also interacts with eNOS and stimulates eNOS enzymatic activity. Whether the eNOS Glu298Asp variant (G894T polymorphism) affects eNOS enzymatic activity remains obscure. Phosphorylation of human eNOS at serine 1177 (Ser1177) by protein kinases Akt, PKA or AMPK enhances, whereas phosphorylation of the enzyme at threonine 495 (Thr495) by PKC inhibits eNOS enzymatic activity. The eNOS enzymatic activity is also dependent on the availability of co-factors, such as BH4, NADH, FAD, and substrate L-arginine. Increase in production of endogenous ADMA reduces NO production. Increase in oxidative stress inactivates NO resulting in decreased NO bioavailability.
eNOS GENE EXPRESSION IN ATHEROSCLEROSIS
eNOS Gene Polymorphisms and Atherosclerosis
Human eNOS is encoded by a 26-exon gene on chromosome 7.12 Several clinical genetic studies identified different common eNOS gene polymorphisms that might be associated with atherosclerotic coronary artery disease. Among the identified eNOS polymorphisms, the G894T substitution within exon 7 resulting in the conversion of glutamate to aspartate at position 298 (Glu298Asp variant) is the one most frequently studied and has been reported to be associated with atherosclerotic coronary artery disease in two independent cohorts recruited from the Cambridge Heart AntiOxidant Studies (CHAOS-I and CHAOS-II).13 The Glu298Asp variant was also reported to be associated with an enhanced systemic pressor response to phenylephrine in a French cohort,14 a lower basal blood flow but a preserved response to adenosine in coronary arteries in Caucasians of German descent15 and vasospastic angina pectoris in a Japanese population,16 as well as with essential hypertension resistant to conventional antihypertensive therapy.17 While these studies showed association of the Glu298Asp variant with atherosclerotic coronary artery disease, other studies do not support these findings. No association of the eNOS variant with presence or severity of coronary artery disease or with myocardial infarction has been reported in several studies.18–21 No adverse effect of the Glu298Asp variant on survival was found in patients with ischemic heart failure.22 The reason for the controversy among the studies remains unclear. It could be due to selection of patient populations from different ethnic groups23,24 or different interactions of the genetic background with the environmental factors (i.e., cardiovascular risk factors present in the specific patient populations).25 One of the considerable limitations of these population studies is the lack of information on whether this polymorphism is functional, that is, whether the Glu298Asp variant is associated with reduced NO synthesis. It has been postulated that the Glu298Asp variant is associated with an increased susceptibility to proteolytic cleavage,26 but other studies suggest that this could have resulted from an artifact of experimental sample preparation.27,28 It is important to note that in 104 Caucasians undergoing coronary bypass graft surgery, the endothelial function as assessed by endothelium-dependent relaxations to various agonists in saphenous vein segments is strongly negatively associated with increased atherosclerotic risk factor profiles, and the presence of Glu298Asp variant exerts no additional effect.29 Similar results on the function of the Glu298Asp variant was also reported in Caucasian hypertensive patients by Rossi et al.30 Their study demonstrated that the Glu298Asp polymorphism does not affect the forearm blood flow responses to acetylcholine.30 These results of functional analysis suggest that the Glu298Asp polymorphism does not have a major direct functional effect on eNOS activity in atherosclerosis and may simply be an indirect genetic marker associated with the disease.
Other eNOS polymorphisms that are present in the eNOS promoter region and speculated to influence mRNA transcription and reduce eNOS gene expression are also described. The T to C substitution in the promoter region (−786T/C) has been reported to be able to influence transcriptional activity in vitro and found to associate with coronary artery vasospasm in a Japanese population.31 Most recently, Cattaruzza and colleagues32 reported that the −786T/C genotype, which results in the exchange of a cytosine for a thymidine at position −786, is associated with loss of eNOS mRNA and protein expression in cultured endothelial cells in response to shear stress, with reduced endothelium-dependent relaxations in saphenous veins obtained at surgery from carriers of the gene and with a higher frequency of coronary artery disease as assessed by quantitative angiography. Unfortunately, eNOS protein level in blood vessels from patients carrying the −786T/C polymorphism was not analyzed in this study. The question whether the −786T/C polymorphism affects eNOS expression in vivo remains unanswered. Similar to the Glu298Asp variant, inconsistent associations of the −786T/C polymorphism with endothelial functional measures and with clinical endpoints, such as myocardial infarction and cardiac death, were also shown.30,32 A meta-analysis of 26 studies involving 23,028 subjects showed only marginal increased risk of ischemic heart disease in individuals homozygous to Glu298Asp variant, and no significant association was found with the −786C allele.33 It seems that eNOS polymorphisms might represent only an indirect genetic marker for atherosclerosis. It remains possible that an assessment of the eNOS polymorphisms integrated into cardiovascular risk factors might increase the predictive value for clinical outcomes of coronary artery disease. It has also been suggested that very large scale genetic association studies of endothelial function or clinical outcomes are required, as are molecular studies, to obtain significant power to detect a statistical significance in such population studies.
eNOS Protein Expression in Atherosclerosis
In contrast to the association studies of eNOS gene polymorphisms, much more is known about eNOS mRNA and/or protein expression in atherosclerosis, particularly in animal models. Past studies provide substantial in vitro evidence that the protein expression level of eNOS can be altered by a number of hormonal substances or therapeutic drugs (figure 1 ▶).34–39 Although in vitro experiments demonstrate that a variety of atherogenic stimuli or mediators, such as oxidized low density lipoprotein (LDL), tumor necrosis factor (TNF)-α, thrombin and serum from patients with severe heart failure are able to suppress eNOS gene expression in cultured endothelial cells,34–39 there is not much information available for eNOS gene expression in vivo in human atherosclerosis. In 1998, Oemar and colleagues40 reported that arteries with advanced atherosclerotic plaques have intact endothelial coverage and that eNOS protein is not detectable in endothelial cells over the advanced atherosclerotic lesions as demonstrated by immunohistochemistry. One limitation of the study is that diseased carotid arterial specimens were compared with the atherosclerosis-resistant internal thoracic arteries.41 Studies with human aortic and coronary arterial tissues obtained from autopsy or from transplant donors found a significant decrease in eNOS gene expression in endothelial cells overlying advanced atherosclerotic lesions, but not in those of early atherosclerotic samples.42,43 In line with this report, most studies in atherosclerotic animal models demonstrate unchanged or even augmented expression of eNOS in atherosclerotic arteries, despite the presence of endothelial dysfunction.44–47 A most recent study with human coronary atherectomy specimens showed a higher eNOS gene expression in patients with acute coronary syndromes than those with stable angina.48 These results suggest that endothelial dysfunction in atherosclerosis, at least at the early disease stage, is not attributable to a decrease in eNOS gene expression.
Although various studies have shown that inhibition of eNOS either by pharmacological inhibitor or by eNOS gene knockout on the apolipoprotein E (ApoE−/−) background promotes atherogenesis,49–52 and eNOS gene transfer showed improvement of endothelial function and inhibition or regression of atherosclerotic lesions in animal models,53,54 controversial results are, however, reported in ApoE−/− mice which overexpress the eNOS gene (eNOS transgenic mice). In this mouse model, acceleration of atherosclerotic lesion formation was observed.55 This study contrasts with the results by van Haperen et al,56 who showed a reduction of atherosclerotic lesions using the same experimental approach (i.e., in ApoE−/− mice overexpressing eNOS gene). The controversy between the two studies is not clear. Nevertheless, one can assert that endothelial dysfunction in atherosclerosis is not primarily caused by decreased eNOS gene expression. Substantial evidence implicates that dysfunctional eNOS enzymatic activity and/or increased oxidative stress play predominant roles in atherosclerotic endothelial dysfunction. It is emerging that under certain conditions, eNOS can become pro-atherogenic, likely through production of reactive oxygen species – a so called “uncoupling of eNOS” (discussed in detail below). Accordingly, in animal models of atherosclerosis, removal of endothelial cells or infusion of eNOS inhibitor, not only prevented NO formation, but also reduced superoxide anion production.57,58 This hypothesis is supported by a recent study by Shi et al59 who showed that eNOS-deficient mice fed a high fat and high cholesterol diet developed much smaller aortic lesions than did wild-type control mice. It remains, therefore, a challenge to understand the mechanisms of deregulation of eNOS enzymatic activity in atherosclerosis.
eNOS ENZYMATIC DYSFUNCTION IN ATHEROSCLEROSIS
The enzyme activity of eNOS is affected by multiple factors (figure 1 ▶), including 1) the Glu298Asp variant that is speculated to influence eNOS enzymatic activity (discussed above), 2) post-translational modification associated with subcellular localization, 3) interacting proteins, such as caveolin-1 (Cav-1) and heat shock protein 90 (Hsp90), 4) co-factors, such as flavins, NADPH and tetrahydrobiopterin (BH4), 5) activation through Ca2+, 6) phosphorylation/dephosphorylation by protein kinases stimulated by hormonal agonists and 7) availability of substrate L-arginine. In the following section, we summarize and discuss the mechanisms that are emerging to play roles in eNOS enzymatic dysfunction in atherosclerosis.
eNOS-Protein Interaction
Concepts related to the regulation of intracellular signaling by alterations in the localization and association of distinct protein mediators are continually changing. Whereas the activation of eNOS has long been known to be dependent on protein-protein interactions, especially between calmodulin and eNOS, numerous additional eNOS-associated proteins have been identified over the last 5 years. It is now evident that endothelial NO production is not simply dependent on the expression of the eNOS enzyme, but is determined by an eNOS signaling complex that consists of the enzyme and a conglomerate of adaptor proteins, structural proteins, kinases, phosphatases and potentially also motor proteins that affect complex associations and determine intracellular localization.60,61
Caveolin-eNOS Interaction
Biochemical studies provide evidence that eNOS activity can be regulated by its localization in caveolae, the plasmalemmal vesicles in the cells.62 eNOS in the caveolae interacts with the major caveolae coat protein, Cav-1. This interaction tonically inhibits eNOS enzymatic activity.63,64 The formation of the Ca2+-calmodulin complex in endothelial cells upon stimulation with agonists can disrupt the interaction between eNOS and Cav-1 leading to enhanced eNOS enzymatic activity.64 Bucci et al65 demonstrated that a chimeric peptide with a cellular internalization sequence fused to Cav-1 scaffolding domain was efficiently incorporated into blood vessels and endothelial cells resulting in selective inhibition of acetylcholine-induced vasodilation and NO production. Consistent with its role in inhibiting eNOS activity, Cav-1 deficient mice demonstrates a higher eNOS activity.66,67 Moreover, Cav-1−/− and ApoE−/− double knockout mice, which are defective in hepatic LDL cholesterol clearance and have significantly higher levels of total cholesterol and triglycerides with no change in high density lipoprotein levels as compared with those which are ApoE−/−alone, either fed normal chow or Western high fat diet, have 70% to 80% reduction in atherosclerotic lesion burden despite the pro-atherogenic lipid profile.68 This result suggests that an increase in NO production in ApoE−/−/Cav-1−/− double knockout mice may protect against atherogenesis. The possible role of Cav-1/eNOS interaction in atherogenesis is also supported by the observation that serum from hypercholesterolemic patients and LDL upregulate Cav-1 expression without affecting eNOS protein level, augment caveolin-eNOS heterocomplex formation, and thereby attenuate NO production from the endothelial cells.69 Recently, an increase in Cav-1 protein level without change in eNOS expression in aortas was found in a type 1 diabetes mouse model, which is coupled with impaired endothelium-dependent relaxations.70 This result may implicate a role of increased Cav-1 expression in linking diabetes mellitus and atherosclerosis. Moreover, treatment of endothelial cells or ApoE−/− mice with statins decreases Cav-1 level and promotes eNOS activity.71–73 It remains to be determined, however, whether Cav-1/eNOS interaction indeed plays a role in atherogenesis in humans.
Hsp90-eNOS Interaction
Another intermediate protein that plays crucial roles in eNOS regulation is the 90 kDa heat shock protein (Hsp90).74–76 The interaction between Hsp90 and eNOS occurs in basal conditions and can be further enhanced by a variety of stimuli which trigger NO production.74,77 Binding with Hsp90 significantly increases eNOS activity74 which is mediated, in part, by the enhancement of calmodulin binding affinity to eNOS78,79 and also by facilitation of Ca2+/calmodulin to dissociate the interaction between Cav-1 and eNOS, thereby reversing the inhibitory action of Cav-1 on eNOS.80 In addition, Hsp90 was found to be crucial in eNOS serine 1179/1177 (bovine/human) phosphorylation. Hsp90 was shown to recruit serine/threonine protein kinase, Akt, to phosphorylate eNOS at serine 117981,82 and thereby increase eNOS activity. There is no direct evidence demonstrating that a change in Hsp90-eNOS interaction is involved in atherogenesis. The only indirect evidence was provided by an in vitro study in cultured endothelial cells showing that atorvastatin activates or phosphorylates eNOS on Ser1177, which is dependent on the ability of Hsp90 to recruit Akt in the eNOS complex.73 Whether this effect also contributes to the statin’s beneficial effects on vascular functions remains unknown.
Dynamin-2
Dynamin-2 is another protein which interacts with eNOS and positively regulates eNOS enzymatic activity.83,84 The mechanisms and the physiological significance of the regulatory mechanism of eNOS have not been studied, yet.
NOSIP-eNOS Interaction
NOSIP, eNOS-interacting protein, is a recently discovered novel protein with a molecular weight of 34 kDa. It is widely distributed in the cardiovascular system,85 the gastrointestinal tract86 and the nervous system of the rat,87 where it co-localizes with eNOS and/or neuronal NO synthase (nNOS). It interacts with eNOS and also nNOS.85 NOSIP displaces eNOS from the cell plasma membrane and relocates the enzyme to intracellular compartments resulting in the reduction of eNOS activity.85 The functional role of NOSIP under (physiol)-pathological conditions remains unknown.
eNOS PHOSPHORYLATION
In addition to modulation by protein-protein interaction, multiple signal transduction pathways converge to regulate eNOS by a phosphorylation process. The activation of the enzyme in response to multiple hormonal agonists such as estradiol, bradykinin and vascular endothelial growth factor (VEGF) occurs in association with elevations in cytosolic calcium concentrations.88–90 In contrast, eNOS activation by shear stress, isometric vessel contraction and insulin occurs independently of changes in intracellular calcium levels.91–93 Shear stress-induced enzyme activation is regulated by potassium channels, and it is prevented by tyrosine kinase inhibition indicating that the process also entails tyrosine phosphorylation.94–96 In addition to regulation by calcium and via tyrosine phosphorylation, multiple protein kinases modify eNOS activity through effects on phosphorylation of serine 1177 or 1179 in human or bovine endothelial cells, respectively. The responsible kinases include AMP-activated protein kinase (AMPK), protein kinase C (PKC), cAMP-dependent protein kinase (PKA) and Akt, which is also known as protein kinase B (PKB). Among the kinases, Akt/PKB seems the most well studied enzyme in regulation of eNOS activity.60 Factors that activate eNOS through Akt/PKB-mediated phosphorylation of Ser1177 include estradiol,97 shear stress,92 VEGF,98 insulin93 and high density lipoprotein.99 In contrast to the activation upon phosphorylation of Ser1177, phosphorylation of the threonine at position 495 yields attenuated eNOS activity.100 There seems to be evidence of coordinated regulation of eNOS activity by agonists, such as VEGF, which cause both phosphorylation of Ser1177 and dephosphorylation of Thr495.101 Recent work has further demonstrated that PKA signaling leads to eNOS phosphorylation at Ser1179 in bovine endothelial cells and dephosphorylation of Thr495, thereby enhancing enzymatic activity, whereas PKC promotes both the dephosphorylation of Ser1177 and the phosphorylation at Thr495, resulting in attenuated enzymatic activity.101 Furthermore, the dephosphorylation processes are mediated by phosphatases PP2A and PP1 acting selectively at Ser1177 and Thr495,102,103 respectively. Thus, eNOS activity is regulated by a complex combination of protein-protein interactions and signal transduction cascades involving regulation and phosphorylation events.
There is so far only indirect evidence for an involvement of functional changes in Akt/eNOS signaling in atherosclerotic endothelial dysfunction. The defect of Akt/eNOS signaling may play a primary role in endothelial dysfunction in type-2 diabetes mellitus. In aorta from diabetic animals, as well as in type 2 diabetic patients, Akt/eNOS phosphorylation has been shown to be decreased,104,105 at least partly via hyperglycemia-induced GlcNAc modification of the enzyme, which may explain the development and progression of diabetes-associated atherosclerosis.104 Hambrecht et al106 recently showed a significant increase in eNOS gene and protein level after only 4 weeks of regular physical exercise training, paralleled with a more pronounced eNOS phosphorylation on Ser1177 in patients with coronary artery disease,106 implicating a possible role of defective Akt/eNOS pathway in atherosclerosis.
eNOS UNCOUPLING
Recent research reveals a potential role of “eNOS uncoupling” in endothelial dysfunction in atherosclerosis in animal models. In “eNOS uncoupling,” electrons flowing from the reductase domain to the heme are diverted to molecular oxygen rather than to the substrate L-arginine, thereby resulting in production of superoxide instead of NO (figure 2 ▶).107,108 This mechanism also seems important for endothelial dysfunction associated with diabetes mellitus.109,110 Several biochemical mechanisms are proposed to be involved in eNOS uncoupling in atherosclerosis, such as BH4 deficiency, and increase in endogenous asymmetric dimethylarginine (ADMA), L-arginine deficiency and oxidative stress.
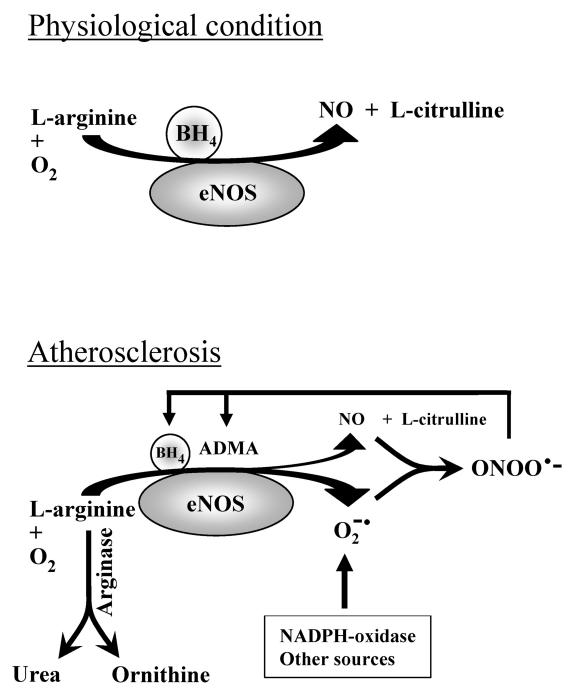
Figure 2.
Mechanisms of eNOS uncoupling in atherosclerotic endothelial dysfunction. (A) Under physiological conditions, endothelial cells produce NO from L-arginine in the presence of optimal concentration of the co-factor tetrahydrobiopterin (BH4). (B) Under pathological conditions, such as atherosclerosis, a decrease in endothelial BH4 production, an increase in formation of endogenous eNOS inhibitor ADMA and an increase in arginase activity which metabolizes L-arginine into urea and ornithine, causes eNOS uncoupling, a condition that leads to superoxide anion (O2−) production and less NO release. O2− produced from eNOS uncoupling and other enzymes reacts with NO to generate a more potent oxidant peroxynitrite (ONOO−) which further inactivates BH4 and increases ADMA accumulation in endothelial cells, leading to endothelial dysfunction and may promote atherogenesis.
BH4 Deficiency
The co-factor BH4 deficiency is proposed by most researchers as the mechanism involved in eNOS uncoupling which plays a role in endothelial dysfunction under various pathological conditions, including atherosclerosis.111,112 A normal endothelial NO generation by eNOS is dependent on the optimal concentration of the co-factor BH4, and a suboptimal concentration of BH4 leads to eNOS uncoupling.113 It has been demonstrated in clinical and animal studies that acute administration of BH4 or overexpression of GTPCH-1, the rate-limiting enzyme for BH4 synthesis, improves endothelial function associated with hypercholesterolemia, atherosclerosis and hyperglycemia.112,114–116 There are several studies demonstrating a decreased BH4 synthesis in the atherosclerotic vascular wall of atherosclerotic rabbits and ApoE−/− mice.55,117 Some controversial results have also been reported.118 The mechanism of decreased BH4 synthesis is unclear. It is suggested by several groups that oxidative stress causes BH4 deficiency by oxidation of BH4 in the cells.119–121 BH4 deficiency induces eNOS uncoupling, resulting in the generation of superoxide anions from uncoupled eNOS, which decreases BH4 levels further – a vicious cycle causing endothelial dysfunction (figure 2 ▶). This hypothesis was supported by the study of Alp et al. Either supplementation of BH4 to or overexpression of the BH4 synthesis rate-limiting enzyme GTPCH-1 in the ApoE−/−/eNOS transgenic mice reduced atherosclerotic lesion formation paralleled with an increase in NO formation and decrease in superoxide anion production.112 In line with this hypothesis, the antioxidant vitamin C has been shown to prevent oxidation and stabilization of BH4.122 Long term vitamin C administration improves endothelial function in a BH4-dependent manner in vivo in mice.118
An Endogenous Inhibitor of eNOS: Asymmetric Dimethylarginine (ADMA)
ADMA is a naturally occurring amino acid resulting from proteolysis of methylated arginine residues in proteins.123 The methylation of arginine is catalyzed by the enzyme protein arginine methyltransferase (PRMT) type I.124 No direct route of synthesizing ADMA from free arginine has been identified. ADMA is an endogenous inhibitor of eNOS. It competes with L-arginine to inhibit eNOS for NO production.125 Since the first discovery as an endogenous inhibitor by Vallance et al in 1992,126 the significant role of ADMA in endothelial dysfunction has been described in various pathological conditions, including atherosclerosis.127,128 A high concentration of plasma ADMA has been associated with several risk factors for atherosclerosis and elevated risk for acute coronary events.128–130 A recent study showed that plasma ADMA concentrations in patients who had newly diagnosed acute coronary syndromes are higher than the age-matched healthy control subjects.131 The amount of ADMA generated within a cell is dependent on the extent of arginine methylation in proteins and the rates of protein turnover. The mechanism that leads to an increase in ADMA plasma concentration in atherosclerosis is not clear. In vascular endothelial cells, type 1 PRMT is expressed and upregulated by LDL.132 Ninety percent of ADMA is metabolized by dimethylarginine dimethylaminohydrolase (DDAH).133 The enzyme can be inhibited by nitrosation caused by a potent oxidant peroxynitrate under the high-output NO production from eNOS expression, for example, under the condition of atherosclerosis.134 As a consequence, ADMA will be accumulated. A similar hypothesis to BH4 deficiency that causes eNOS uncoupling has been also proposed for ADMA accumulation: in the presence of high concentrations of ADMA, eNOS produces superoxide instead of NO, which leads to further oxidation of DDAH and further accumulation of ADMA.135 This hypothesis warrants further investigation.
Increased arginase activity
L-arginine is the exclusive substrate of eNOS for NO production.4 In the early 1990s, several groups demonstrated that acute and chronic supplementation of L-arginine improves endothelial vasodilator responses in cholesterol fed animals and in patients with hypercholesterolemia and atherosclerosis.136–139 L-arginine supplementation therapy as anti-atherosclerotic approach was supported by numerous studies,50,140–145 but is recently challenged by increasing the number of other studies showing no such effect or no sustained effect on endothelial function.50,145–149 Some studies even showed a harmful effect on atherosclerotic lesion formation150 in animal models, such as in ApoE−/−/iNOS−/−double knockout mice which are treated chronically with L-arginine supplementation. An increase in production of superoxide anion in atherosclerotic rabbit aortas treated with L-arginine has been recently described.151
The inconsistent results obtained with L-arginine in experimental and clinical studies may be due to the complex biochemical metabolisms of L-arginine.152,153 There is recently increasing evidence suggesting a potential role of arginase in regulation of endothelial NO production by competing with eNOS for the substrate L-arginine, whereby L-arginine is metabolized to urea and L-ornithine by arginase.152 There are two types of mammalian arginase, arginase I and II, encoded by different genes.154 Arginase I is located in the cytoplasm, expressed most abundantly in the liver, whereas arginase II is a mitochondrial enzyme, expressed primarily in extrahepatic tissues.155 The primary function of arginase I is thought to be involved in ammonia detoxification, whereas that of arginase II, in biosynthesis of polyamines and the amino acids, ornithine, proline and glutamate,156 plays a primary role of regulation of endothelial NO production.47,157 Interestingly, expression and activity of arginase II were found to be increased in human diabetic corpus cavernosum and inhibition of the enzyme enhances NO-dependent relaxation of corpus cavernosum smooth muscle158,159 suggesting a potential role of arginase II in negative regulation of NO production in diabetic erectile dysfunction. Most recently, an increase in arginase II expression or activity has been implicated in endothelial dysfunction associated with pulmonary hypertension,160 aging,161,162 ischemia-reperfusion-induced endothelial dysfunction,163 aortic coarctation hypertension,164 salt-induced hypertension165 and atherosclerosis.47
In contrast to the observation in rats and humans in which L-arginine evokes vascular relaxation by producing NO, it causes vasoconstriction in the mouse aorta.47 Of particular interest and importance is that the contraction induced by L-arginine is much more pronounced in atherosclerotic ApoE−/−mice compared with control animals, which is converted to a greater relaxation by arginase inhibitors in atherosclerotic ApoE−/−mice than wild-type animals, demonstrating a dominant role of increased arginase activity in atherosclerotic endothelial dysfunction.47 The greater relaxation induced by L-arginine under the inhibition of arginase in atherosclerotic aortas is paralleled with the higher eNOS expression in the atherosclerotic aortas. The results further support the concept that endothelial dysfunction in atherosclerosis is mainly due to decreased NO bioavailability rather than eNOS gene expression as discussed above.46 The simple administration of L-arginine alone as anti-atherosclerotic therapy may therefore not be suitable for some individuals and could even be harmful under certain conditions. Future research designed to target arginase specifically in the vasculature may provide a novel therapeutic approach to treat atherosclerosis and perhaps other cardiovascular disorders.
There is little information on regulatory mechanisms of arginase gene expression or activity in endothelial cells under disease conditions, including atherosclerosis. Arginase II enzymatic activity can be upregulated by thrombin and inflammatory cytokines that are important for atherothrombosis.47,157 Our study47 provided the first evidence, suggesting a role of RhoA/ROCK pathway in upregulation of arginase activity in human endothelial cells. In line with the results obtained from cultured cells, the increase in arginase II activity, but not protein expression, in atherosclerotic blood vessels of ApoE−/− mice is associated with increased RhoA protein level,47 suggesting that increased enzymatic activity of arginase II plays a predominant role in atherosclerotic endothelial dysfunction. This regulatory model of arginase II in human endothelial cells is further supported by the study of Bachetti and colleagues157 who showed that arginase activity, but not gene expression, in the cells was increased after 24-hour stimulation with an inflammatory cytokine mixture.157 The exact regulatory mechanisms of arginase activity in the cells and in atherosclerotic blood vessels remain elusive. Whether increased arginase activity contributes to eNOS uncoupling also remains an interesting topic for future research.
OXIDATIVE STRESS AND NO BIOACTIVITY IN ATHEROSCLEROSIS
There is considerable evidence showing the importance of oxidative stress in the pathogenesis of atherosclerosis. This aspect has been comprehensively reviewed in several articles.10,11 In this article we would like to point out that in atherosclerotic blood vessels there is an increase in enzyme expression that produces superoxide, such as the subcomponents of NADPH oxidase.166–168 It is emerging that besides several sources that generate superoxide (e.g., mitochondria, NADPH oxidase, xanthine oxidase, cytochrome p450-type enzymes, cyclooxygenase and lipoxygenase), eNOS seems to be an important source of superoxide generation via eNOS uncoupling mechanisms in the absence of substrate L-arginine or co-factors BH4 or in the presence of endogenous inhibitor ADMA as discussed above. It is also noteworthy to point out that an increase in superoxide generation not only quenches NO, but also enforces eNOS uncoupling, a vicious cycle which further enhances oxidative stress, resulting in further endothelial dysfunction (figure 2 ▶). Superoxide generation via eNOS uncoupling is emerging to play an important role in endothelial dysfunction in cardiovascular disorders, including atherosclerosis.111 Future research focusing on eNOS uncoupling would provide an interesting aspect for understanding the pathophysiology of endothelial dysfunction and also therapeutic strategy for atherosclerosis.
CONCLUSIONS
Endothelial dysfunction plays an important role in the pathogenesis of atherosclerotic coronary artery disease. Many mechanisms have been proposed to be responsible for endothelial dysfunction in atherosclerosis. Clinical genomic studies showed inconsistent results on the association of eNOS gene polymorphisms with endothelial dysfunction or clinical outcomes in patients with coronary artery disease. Meta-analysis of clinical studies suggests that eNOS polymorphisms are not a major risk but rather an indirect genetic marker for atherosclerosis. It is still possible that the eNOS polymorphisms could have more predictive value in certain ethnic populations with specified cardiovascular risk factor profiles. Substantial insight into mechanisms of endothelial dysfunction in atherosclerosis is provided by experimental studies in animal models and by clinical pathophysiological and pharmacological studies ex vivo and in vivo. However, one should be aware of species differences in response to pharmacological interventions, for example, L-arginine supplementation, when conclusions are based on the experiments conducted in different animal models. Results from different animal models may explain some controversial findings among different studies. Extrapolation of the results obtained from animal models to humans should be cautious. Experiments with ex vivo specimens from animal models, particularly from humans could provide the best information for translational medicine research. It is now becoming apparent that atherosclerotic endothelial dysfunction, particularly at the early disease stages is primarily caused by deregulation of eNOS enzymatic activity and inactivation of NO through oxidative stress rather than eNOS gene down-regulation. Besides various enzymes that produce oxidative stress, eNOS uncoupling appears to be an important mechanism contributing to increased oxidative stress in atherosclerosis. eNOS uncoupling can be induced by decreased production of co-factor BH4 or relative deficiency of the substrate L-arginine due either to increased arginase activity or accumulation of the endogenous eNOS inhibitor ADMA. It also seems to play an important role in endothelial dysfunction in atherosclerosis. Studies on regulation of eNOS enzymatic activity and maintenance of eNOS in the “coupled” state or on therapeutic approach for “re-coupling” of eNOS in atherosclerosis would be interesting areas for future research.
References
- 1.Yang Z, Luscher TF. Vascular endothelium. In: Lanzer P, Topol EJ, eds. Pan vascular medicine. Berlin - Heidelberg - New York: Springer;2002. 190–204.
- 2.Lamping K, Faraci F. Enhanced vasoconstrictor responses in eNOS deficient mice. Nitric Oxide 2003;8:207–213. [DOI] [PubMed] [Google Scholar]
- 3.Yang ZH, Diederich D, Schneider K, Siebenmann R, Stulz P, von Segesser L, Turina M, Buhler FR, Luscher TF. Endothelium-derived relaxing factor and protection against contractions induced by histamine and serotonin in the human internal mammary artery and in the saphenous vein. Circulation 1989;80:1041–1048. [DOI] [PubMed] [Google Scholar]
- 4.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109:III27–III32. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 6.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 7.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 8.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation 2004;109:2518–2523. [DOI] [PubMed] [Google Scholar]
- 9.Verbeuren TJ, Coene MC, Jordaens FH, Van Hove CE, Zonnekeyn LL, Herman AG. Effect of hypercholesterolemia on vascular reactivity in the rabbit. II. Influence of treatment with dipyridamole on endothelium-dependent and endothelium-independent responses in isolated aortas of control and hypercholesterolemic rabbits. Circ Res 1986;59:496–504. [DOI] [PubMed] [Google Scholar]
- 10.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 2005;25:29–38. [DOI] [PubMed] [Google Scholar]
- 11.Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004;84:1381–1478. [DOI] [PubMed] [Google Scholar]
- 12.Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem 1993;268:17478–17488. [PubMed] [Google Scholar]
- 13.Hingorani AD, Liang CF, Fatibene J, Lyon A, Monteith S, Parsons A, Haydock S, Hopper RV, Stephens NG, O’Shaughnessy KM, Brown MJ. A common variant of the endothelial nitric oxide synthase (Glu298—>Asp) is a major risk factor for coronary artery disease in the UK. Circulation 1999;100:1515–1520. [DOI] [PubMed] [Google Scholar]
- 14.Philip I, Plantefeve G, Vuillaumier-Barrot S, Vicaut E, LeMarie C, Henrion D, Poirier O, Levy BI, Desmonts JM, Durand G, Benessiano J. G894T polymorphism in the endothelial nitric oxide synthase gene is associated with an enhanced vascular responsiveness to phenylephrine. Circulation 1999;99:3096–3098. [DOI] [PubMed] [Google Scholar]
- 15.Naber CK, Baumgart D, Altmann C, Siffert W, Erbel R, Heusch G. eNOS 894T allele and coronary blood flow at rest and during adenosine-induced hyperemia. Am J Physiol Heart Circ Physiol 2001;281:H1908–H1912. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura M, Yasue H, Nakayama M, Shimasaki Y, Sumida H, Sugiyama S, Kugiyama K, Ogawa H, Ogawa Y, Saito Y, Miyamoto Y, Nakao K. A missense Glu298Asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet 1998;103:65–69. [DOI] [PubMed] [Google Scholar]
- 17.Jachymova M, Horky K, Bultas J, Kozich V, Jindra A, Peleska J, Martasek P. Association of the Glu298Asp polymorphism in the endothelial nitric oxide synthase gene with essential hypertension resistant to conventional therapy. Biochem Biophys Res Commun 2001;284:426–430. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Wilcken DE, Wang XL. The Glu-298—>Asp (894G—>T) mutation at exon 7 of the endothelial nitric oxide synthase gene and coronary artery disease. J Mol Med 1999;77:511–514. [DOI] [PubMed] [Google Scholar]
- 19.Hibi K, Ishigami T, Tamura K, Mizushima S, Nyui N, Fujita T, Ochiai H, Kosuge M, Watanabe Y, Yoshii Y, Kihara M, Kimura K, Ishii M, Umemura S. Endothelial nitric oxide synthase gene polymorphism and acute myocardial infarction. Hypertension 1998;32:521–526. [DOI] [PubMed] [Google Scholar]
- 20.Markus HS, Ruigrok Y, Ali N, Powell JF. Endothelial nitric oxide synthase exon 7 polymorphism, ischemic cerebrovascular disease, and carotid atheroma. Stroke 1998;29:1908–1911. [DOI] [PubMed] [Google Scholar]
- 21.Poirier O, Mao C, Mallet C, Nicaud V, Herrmann SM, Evans A, Ruidavets JB, Arveiler D, Luc G, Tiret L, Soubrier F, Cambien F. Polymorphisms of the endothelial nitric oxide synthase gene - no consistent association with myocardial infarction in the ECTIM study. Eur J Clin Invest 1999;29:284–290. [DOI] [PubMed] [Google Scholar]
- 22.McNamara DM, Holubkov R, Postava L, Ramani R, Janosko K, Mathier M, MacGowan GA, Murali S, Feldman AM, London B. Effect of the Asp298 variant of endothelial nitric oxide synthase on survival for patients with congestive heart failure. Circulation 2003;107:1598–1602. [DOI] [PubMed] [Google Scholar]
- 23.Marroni AS, Metzger IF, Souza-Costa DC, Nagassaki S, Sandrim VC, Correa RX, Rios-Santos F, Tanus-Santos JE. Consistent interethnic differences in the distribution of clinically relevant endothelial nitric oxide synthase genetic polymorphisms. Nitric Oxide 2005;12:177–182. [DOI] [PubMed] [Google Scholar]
- 24.Tanus-Santos JE, Desai M, Flockhart DA. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics 2001;11:719–725. [DOI] [PubMed] [Google Scholar]
- 25.Testa A, Spoto B, Tripepi G, Mallamaci F, Malatino L, Fatuzzo P, Maas R, Boeger R, Zoccali C. The GLU298ASP variant of nitric oxide synthase interacts with asymmetric dimethyl arginine in determining cardiovascular mortality in patients with end-stage renal disease. J Hypertens 2005;23:1825–1830. [DOI] [PubMed] [Google Scholar]
- 26.Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A 2000;97:2832–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald DM, Alp NJ, Channon KM. Functional comparison of the endothelial nitric oxide synthase Glu298Asp polymorphic variants in human endothelial cells. Pharmacogenetics 2004;14:831–839. [DOI] [PubMed] [Google Scholar]
- 28.Fairchild TA, Fulton D, Fontana JT, Gratton JP, McCabe TJ, Sessa WC. Acidic hydrolysis as a mechanism for the cleavage of the Glu(298)—>Asp variant of human endothelial nitric-oxide synthase. J Biol Chem 2001;276:26674–26679. [DOI] [PubMed] [Google Scholar]
- 29.Guzik TJ, Black E, West NE, McDonald D, Ratnatunga C, Pillai R, Channon KM. Relationship between the G894T polymorphism (Glu298Asp variant) in endothelial nitric oxide synthase and nitric oxide-mediated endothelial function in human atherosclerosis. Am J Med Genet 2001;100:130–137. [DOI] [PubMed] [Google Scholar]
- 30.Rossi GP, Taddei S, Virdis A, Cavallin M, Ghiadoni L, Favilla S, Versari D, Sudano I, Pessina AC, Salvetti A. The T-786C and Glu298Asp polymorphisms of the endothelial nitric oxide gene affect the forearm blood flow responses of Caucasian hypertensive patients. J Am Coll Cardiol 2003;41:938–945. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H, Motoyama T, Saito Y, Ogawa Y, Miyamoto Y, Nakao K. -786—>C mutation in the 5′-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation 1999;99:2864–2870. [DOI] [PubMed] [Google Scholar]
- 32.Cattaruzza M, Guzik TJ, Slodowski W, Pelvan A, Becker J, Halle M, Buchwald AB, Channon KM, Hecker M. Shear stress insensitivity of endothelial nitric oxide synthase expression as a genetic risk factor for coronary heart disease. Circ Res 2004;95:841–847. [DOI] [PubMed] [Google Scholar]
- 33.Casas JP, Bautista LE, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23028 subjects. Circulation 2004;109:1359–1365. [DOI] [PubMed] [Google Scholar]
- 34.Liao JK, Shin WS, Lee WY, Clark SL. Oxidized low-density lipoprotein decreases the expression of endothelial nitric oxide synthase. J Biol Chem 1995;270:319–324. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Kozai T, van der Loo B, Viswambharan H, Lachat M, Turina MI, Malinski T, Luscher TF. HMG-CoA reductase inhibition improves endothelial cell function and inhibits smooth muscle cell proliferation in human saphenous veins. J Am Coll Cardiol 2000;36:1691–1697. [DOI] [PubMed] [Google Scholar]
- 36.Alonso J, Sanchez de Miguel L, Monton M, Casado S, Lopez-Farre A. Endothelial cytosolic proteins bind to the 3′ untranslated region of endothelial nitric oxide synthase mRNA: regulation by tumor necrosis factor alpha. Mol Cell Biol 1997;17:5719–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, Yang Z, Luscher TF. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res 2001;89:583–590. [DOI] [PubMed] [Google Scholar]
- 38.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol 2002;22:8467–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agnoletti L, Curello S, Bachetti T, Malacarne F, Gaia G, Comini L, Volterrani M, Bonetti P, Parrinello G, Cadei M, Grigolato PG, Ferrari R. Serum from patients with severe heart failure downregulates eNOS and is proapoptotic: role of tumor necrosis factor-alpha. Circulation 1999;100:1983–1991. [DOI] [PubMed] [Google Scholar]
- 40.Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Luscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 1998;97:2494–2498. [DOI] [PubMed] [Google Scholar]
- 41.Lytle BW, Loop FD. Superiority of bilateral internal thoracic artery grafting: it’s been a long time comin’. Circulation 2001;104:2152–2154. [PubMed] [Google Scholar]
- 42.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol 1997;17:2479–2488. [DOI] [PubMed] [Google Scholar]
- 43.Fukuchi M, Giaid A. Endothelial expression of endothelial nitric oxide synthase and endothelin-1 in human coronary artery disease. Specific reference to underlying lesion. Lab Invest 1999;79:659–670. [PubMed] [Google Scholar]
- 44.Matsumoto T, D’uscio LV, Eguchi D, Akiyama M, Smith LA, Katusic ZS. Protective effect of chronic vitamin C treatment on endothelial function of apolipoprotein E-deficient mouse carotid artery. J Pharmacol Exp Ther 2003;306:103–108. [DOI] [PubMed] [Google Scholar]
- 45.Godecke A, Ziegler M, Ding Z, Schrader J. Endothelial dysfunction of coronary resistance vessels in apoE−/− mice involves NO but not prostacyclin-dependent mechanisms. Cardiovasc Res 2002;53:253–262. [DOI] [PubMed] [Google Scholar]
- 46.d’Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, Katusic ZS. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2001;21:1017–1022. [DOI] [PubMed] [Google Scholar]
- 47.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation 2004;110:3708–3714. [DOI] [PubMed] [Google Scholar]
- 48.Rossi ML, Marziliano N, Merlini PA, Bramucci E, Canosi U, Presbitero P, Arbustini E, Mannucci PM, Ardissino D. Phenotype commitment in vascular smooth muscle cells derived from coronary atherosclerotic plaques: differential gene expression of endothelial nitric oxide synthase. Eur J Histochem 2005;49:39–46. [DOI] [PubMed] [Google Scholar]
- 49.Cayatte AJ, Palacino JJ, Horten K, Cohen RA. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb 1994;14:753–759. [DOI] [PubMed] [Google Scholar]
- 50.Kauser K, da Cunha V, Fitch R, Mallari C, Rubanyi GM. Role of endogenous nitric oxide in progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol 2000;278:H1679–H1685. [DOI] [PubMed] [Google Scholar]
- 51.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS(−/−)Apoe(−/−) mice are ameliorated by enalapril treatment. J Clin Invest 2000;105:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001;104:448–454. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi T, Sumi D, Juliet PA, Matsui-Hirai H, Asai-Tanaka Y, Kano H, Fukatsu A, Tsunekawa T, Miyazaki A, Iguchi A, Ignarro LJ. Gene transfer of endothelial NO synthase, but not eNOS plus inducible NOS, regressed atherosclerosis in rabbits. Cardiovasc Res 2004;61:339–351. [DOI] [PubMed] [Google Scholar]
- 54.Mujynya-Ludunge K, Viswambharan H, Driscoll R, Ming XF, von Segesser LK, Kappenberger L, Yang Z, Vassalli G. Endothelial nitric oxide synthase gene transfer restores endothelium-dependent relaxations and attenuates lesion formation in carotid arteries in apolipoprotein E-deficient mice. Basic Res Cardiol 2005;100:102–111. [DOI] [PubMed] [Google Scholar]
- 55.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest 2002;110:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Haperen R, de Waard M, van Deel E, Mees B, Kutryk M, van Aken T, Hamming J, Grosveld F, Duncker DJ, de Crom R. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem 2002;277:48803–48807. [DOI] [PubMed] [Google Scholar]
- 57.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 1993;91:2546–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pritchard KA Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res 1995;77:510–518. [DOI] [PubMed] [Google Scholar]
- 59.Shi W, Wang X, Shih DM, Laubach VE, Navab M, Lusis AJ. Paradoxical reduction of fatty streak formation in mice lacking endothelial nitric oxide synthase. Circulation 2002;105:2078–2082. [DOI] [PubMed] [Google Scholar]
- 60.Sessa WC. eNOS at a glance. J Cell Sci 2004;117:2427–2429. [DOI] [PubMed] [Google Scholar]
- 61.Pelat M, Massion PB, Balligand JL. Nitric oxide “at heart”: emerging paradigms after a decade. Arch Mal Coeur Vaiss 2005;98:242–248. [PubMed] [Google Scholar]
- 62.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res 2004;94:1408–1417. [DOI] [PubMed] [Google Scholar]
- 63.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 1997;272:18522–18525. [DOI] [PubMed] [Google Scholar]
- 64.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem 1997;272:25907–25912. [DOI] [PubMed] [Google Scholar]
- 65.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 2000;6:1362–1367. [DOI] [PubMed] [Google Scholar]
- 66.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001;293:2449–2452. [DOI] [PubMed] [Google Scholar]
- 67.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 2001;276:38121–38138. [DOI] [PubMed] [Google Scholar]
- 68.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol 2004;24:98–105. [DOI] [PubMed] [Google Scholar]
- 69.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest 1999;103:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bucci M, Roviezzo F, Brancaleone V, Lin MI, Di Lorenzo A, Cicala C, Pinto A, Sessa WC, Farneti S, Fiorucci S, Cirino G. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler Thromb Vasc Biol 2004;24:721–726. [DOI] [PubMed] [Google Scholar]
- 71.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy- methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation 2001;103:113–118. [DOI] [PubMed] [Google Scholar]
- 72.Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation 2003; 107:2480–2486. [DOI] [PubMed] [Google Scholar]
- 73.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res 2001;89:866–873. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 1998;392:821–824. [DOI] [PubMed] [Google Scholar]
- 75.Balligand JL. Heat shock protein 90 in endothelial nitric oxide synthase signaling: following the lead(er)? Circ Res 2002;90:838–841. [DOI] [PubMed] [Google Scholar]
- 76.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 2002;90:866–873. [DOI] [PubMed] [Google Scholar]
- 77.Russell KS, Haynes MP, Caulin-Glaser T, Rosneck J, Sessa WC, Bender JR. Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. Effects on calcium sensitivity and NO release. J Biol Chem 2000;275:5026–5030. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem 2003;278:30821–30827. [DOI] [PubMed] [Google Scholar]
- 79.Song Y, Zweier JL, Xia Y. Determination of the enhancing action of HSP90 on neuronal nitric oxide synthase by EPR spectroscopy. Am J Physiol Cell Physiol 2001;281:C1819–C1824.11698240 [Google Scholar]
- 80.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem 2001;276:30359–30365. [DOI] [PubMed] [Google Scholar]
- 81.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A 2000;97:10832–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Q, Xia Y. Roles of 3-phosphoinositide-dependent kinase 1 in the regulation of endothelial nitric-oxide synthase phosphorylation and function by heat shock protein 90. J Biol Chem 2005;280:18081–18086. [DOI] [PubMed] [Google Scholar]
- 83.Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem 2001;276:14249–14256. [DOI] [PubMed] [Google Scholar]
- 84.Cao S, Yao J, Shah V. The proline-rich domain of dynamin-2 is responsible for dynamin-dependent in vitro potentiation of endothelial nitric-oxide synthase activity via selective effects on reductase domain function. J Biol Chem 2003;278:5894–5901. [DOI] [PubMed] [Google Scholar]
- 85.Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Muller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J 2001;15:79–89. [DOI] [PubMed] [Google Scholar]
- 86.Konig P, Dedio J, Muller-Esterl W, Kummer W. Distribution of the novel eNOS-interacting protein NOSIP in the liver, pancreas, and gastrointestinal tract of the rat. Gastroenterology 2002;123:314–324. [DOI] [PubMed] [Google Scholar]
- 87.Dreyer J, Hirlinger D, Muller-Esterl W, Oess S, Kuner R. Spinal upregulation of the nitric oxide synthase-interacting protein NOSIP in a rat model of inflammatory pain. Neurosci Lett 2003;350:13–16. [DOI] [PubMed] [Google Scholar]
- 88.Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 1999;96:2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T. Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem 1998;273:27383–27388. [DOI] [PubMed] [Google Scholar]
- 90.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997;100:3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand 2000;168:81–88. [DOI] [PubMed] [Google Scholar]
- 92.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–605. [DOI] [PubMed] [Google Scholar]
- 93.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 2001;276:30392–30398. [DOI] [PubMed] [Google Scholar]
- 94.Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res 1998;82:686–695. [DOI] [PubMed] [Google Scholar]
- 95.Fleming I, Bauersachs J, Busse R. Calcium-dependent and calcium-independent activation of the endothelial NO synthase. J Vasc Res 1997;34:165–174. [DOI] [PubMed] [Google Scholar]
- 96.Thorsgaard M, Lopez V, Buus NH, Simonsen U. Different modulation by Ca2+-activated K+ channel blockers and herbimycin of acetylcholine- and flow-evoked vasodilatation in rat mesenteric small arteries. Br J Pharmacol 2003;138:1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 2000;87:677–682. [DOI] [PubMed] [Google Scholar]
- 98.Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett 2000;477:258–262. [DOI] [PubMed] [Google Scholar]
- 99.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 2004;113:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 2001;276:16587–16591. [DOI] [PubMed] [Google Scholar]
- 101.Michell BJ, Chen Zp, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 2001;276:17625–17628. [DOI] [PubMed] [Google Scholar]
- 102.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry 2002;41:15845–15853. [DOI] [PubMed] [Google Scholar]
- 103.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 2001;88:E68–E75. [DOI] [PubMed] [Google Scholar]
- 104.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 2001;108:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, Luo H, van Breemen C. Compromised arterial function in human type 2 diabetic patients. Diabetes 2005;54:2415–2423. [DOI] [PubMed] [Google Scholar]
- 106.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003;107:3152–3158. [DOI] [PubMed] [Google Scholar]
- 107.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 2005;96:818–822. [DOI] [PubMed] [Google Scholar]
- 108.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 2005;102:9056–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol 2005;511:53–64. [DOI] [PubMed] [Google Scholar]
- 110.Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 2005;288:F1144–F1152. [DOI] [PubMed] [Google Scholar]
- 111.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol 2005;25:1551–1557. [DOI] [PubMed] [Google Scholar]
- 112.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 2004;24:445–450. [DOI] [PubMed] [Google Scholar]
- 113.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res 2003;37:121–127. [DOI] [PubMed] [Google Scholar]
- 114.Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation 2000;102:2172–2179. [DOI] [PubMed] [Google Scholar]
- 115.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest 2003;112:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res 2005;65:823–831. [DOI] [PubMed] [Google Scholar]
- 117.Vasquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol 2002;22:1655–1661. [DOI] [PubMed] [Google Scholar]
- 118.d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res 2003;92:88–95. [DOI] [PubMed] [Google Scholar]
- 119.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001;103:1282–1288. [DOI] [PubMed] [Google Scholar]
- 120.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 1999;263:681–684. [DOI] [PubMed] [Google Scholar]
- 121.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 2003;278:22546–22554. [DOI] [PubMed] [Google Scholar]
- 122.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 2001;276:40–47. [DOI] [PubMed] [Google Scholar]
- 123.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine: dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 2004;24:1023–1030. [DOI] [PubMed] [Google Scholar]
- 124.McBride AE, Silver PA. State of the arg: protein methylation at arginine comes of age. Cell 2001;106:5–8. [DOI] [PubMed] [Google Scholar]
- 125.Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 2004;134:2842S–2847S. [DOI] [PubMed] [Google Scholar]
- 126.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992;339:572–575. [DOI] [PubMed] [Google Scholar]
- 127.Boger RH, Ron ES. L-Arginine improves vascular function by overcoming deleterious effects of ADMA, a novel cardiovascular risk factor. Altern Med Rev 2005;10:14–23. [PubMed] [Google Scholar]
- 128.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation 1999;99:1141–1146. [DOI] [PubMed] [Google Scholar]
- 129.Valkonen VP, Paiva H, Salonen JT, Lakka TA, Lehtimaki T, Laakso J, Laaksonen R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 2001;358:2127–2128. [DOI] [PubMed] [Google Scholar]
- 130.Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J 2003;24:1912–1919. [DOI] [PubMed] [Google Scholar]
- 131.Bae SW, Stuhlinger MC, Yoo HS, Yu KH, Park HK, Choi BY, Lee YS, Pachinger O, Choi YH, Lee SH, Park JE. Plasma asymmetric dimethylarginine concentrations in newly diagnosed patients with acute myocardial infarction or unstable angina pectoris during two weeks of medical treatment. Am J Cardiol 2005;95:729–733. [DOI] [PubMed] [Google Scholar]
- 132.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 2000;87:99–105. [DOI] [PubMed] [Google Scholar]
- 133.Ogawa T, Kimoto M, Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem 1989;264:10205–10209. [PubMed] [Google Scholar]
- 134.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci U S A 2002;99:13527–13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boger RH. When the endothelium cannot say ‘NO’ anymore. ADMA, an endogenous inhibitor of NO synthase, promotes cardiovascular disease. Eur Heart J 2003;24:1901–1902. [DOI] [PubMed] [Google Scholar]
- 136.Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet 1991;338:1546–1550. [DOI] [PubMed] [Google Scholar]
- 137.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest 1992;90:1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest 1992;90:1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dubois-Rande JL, Zelinsky R, Roudot F, Chabrier PE, Castaigne A, Geschwind H, Adnot S. Effects of infusion of L-arginine into the left anterior descending coronary artery on acetylcholine-induced vasoconstriction of human atheromatous coronary arteries. Am J Cardiol 1992;70:1269–1275. [DOI] [PubMed] [Google Scholar]
- 140.Boger RH, Bode-Boger SM, Mugge A, Kienke S, Brandes R, Dwenger A, Frolich JC. Supplementation of hypercholesterolaemic rabbits with L-arginine reduces the vascular release of superoxide anions and restores NO production. Atherosclerosis 1995;117:273–284. [DOI] [PubMed] [Google Scholar]
- 141.Aji W, Ravalli S, Szabolcs M, Jiang XC, Sciacca RR, Michler RE, Cannon PJ. L-arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation 1997;95:430–437. [DOI] [PubMed] [Google Scholar]
- 142.Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest 1996;97:1989–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ceremuzynski L, Chamiec T, Herbaczynska-Cedro K. Effect of supplemental oral L-arginine on exercise capacity in patients with stable angina pectoris. Am J Cardiol 1997;80:331–333. [DOI] [PubMed] [Google Scholar]
- 144.Tousoulis D, Davies G, Tentolouris C, Crake T, Toutouzas P. Coronary stenosis dilatation induced by L-arginine. Lancet 1997;349:1812–1813. [DOI] [PubMed] [Google Scholar]
- 145.Oomen CM, van Erk MJ, Feskens EJ, Kok FJ, Kromhout D. Arginine intake and risk of coronary heart disease mortality in elderly men. Arterioscler Thromb Vasc Biol 2000;20:2134–2139. [DOI] [PubMed] [Google Scholar]
- 146.Blum A, Hathaway L, Mincemoyer R, Schenke WH, Kirby M, Csako G, Waclawiw MA, Panza JA, Cannon RO 3rd. Oral L-arginine in patients with coronary artery disease on medical management. Circulation 2000;101:2160–2164. [DOI] [PubMed] [Google Scholar]
- 147.Walker HA, McGing E, Fisher I, Boger RH, Bode-Boger SM, Jackson G, Ritter JM, Chowienczyk PJ. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol 2001;38:499–505. [DOI] [PubMed] [Google Scholar]
- 148.Wennmalm A, Edlund A, Granstrom EF, Wiklund O. Acute supplementation with the nitric oxide precursor L-arginine does not improve cardiovascular performance in patients with hypercholesterolemia. Atherosclerosis 1995;118:223–231. [DOI] [PubMed] [Google Scholar]
- 149.Miller HI, Dascalu A, Rassin TA, Wollman Y, Chernichowsky T, Iaina A. Effects of an acute dose of L-arginine during coronary angiography in patients with chronic renal failure: a randomized, parallel, double-blind clinical trial. Am J Nephrol 2003;23:91–95. [DOI] [PubMed] [Google Scholar]
- 150.Chen J, Kuhlencordt P, Urano F, Ichinose H, Astern J, Huang PL. Effects of chronic treatment with L-arginine on atherosclerosis in apoE knockout and apoE/inducible NO synthase double-knockout mice. Arterioscler Thromb Vasc Biol 2003;23:97–103. [DOI] [PubMed] [Google Scholar]
- 151.Simonet S, Rupin A, Badier-Commander C, Coumailleau S, Behr-Roussel D, Verbeuren TJ. Evidence for superoxide anion generation in aortas of cholesterol-fed rabbits treated with L-arginine. Eur J Pharmacol 2004;492:211–216. [DOI] [PubMed] [Google Scholar]
- 152.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 1998;336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Loscalzo J. Adverse effects of supplemental L-arginine in atherosclerosis: consequences of methylation stress in a complex catabolism? Arterioscler Thromb Vasc Biol 2003;23:3–5. [DOI] [PubMed] [Google Scholar]
- 154.Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD. Cloning and characterization of the human type II arginase gene. Genomics 1996;38:118–123. [DOI] [PubMed] [Google Scholar]
- 155.Morris SM Jr, Bhamidipati D, Kepka-Lenhart D. Human type II arginase: sequence analysis and tissue-specific expression. Gene 1997;193:157–161. [DOI] [PubMed] [Google Scholar]
- 156.Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol Genet Metab 2004;81:S38–S44. [DOI] [PubMed] [Google Scholar]
- 157.Bachetti T, Comini L, Francolini G, Bastianon D, Valetti B, Cadei M, Grigolato P, Suzuki H, Finazzi D, Albertini A, Curello S, Ferrari R. Arginase pathway in human endothelial cells in pathophysiological conditions. J Mol Cell Cardiol 2004;37:515–523. [DOI] [PubMed] [Google Scholar]
- 158.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun 2001;283:923–927. [DOI] [PubMed] [Google Scholar]
- 159.Cama E, Colleluori DM, Emig FA, Shin H, Kim SW, Kim NN, Traish AM, Ash DE, Christianson DW. Human arginase II: crystal structure and physiological role in male and female sexual arousal. Biochemistry 2003;42:8445–8451. [DOI] [PubMed] [Google Scholar]
- 160.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 2004;18:1746–1748. [DOI] [PubMed] [Google Scholar]
- 161.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003;108:2000–2006. [DOI] [PubMed] [Google Scholar]
- 162.Sakai Y, Masuda H, Kihara K, Kurosaki E, Yamauchi Y, Azuma H. Involvement of increased arginase activity in impaired cavernous relaxation with aging in the rabbit. J Urol 2004;172:369–373. [DOI] [PubMed] [Google Scholar]
- 163.Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J 2003;17:2328–2330. [DOI] [PubMed] [Google Scholar]
- 164.Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 2004;44:935–943. [DOI] [PubMed] [Google Scholar]
- 165.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 2005;288:R1057–R1062. [DOI] [PubMed] [Google Scholar]
- 166.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of NADH/NADPH oxidase p22phox in human coronary arteries. Circulation 1999;100:1494–1498. [DOI] [PubMed] [Google Scholar]
- 167.Kalinina N, Agrotis A, Tararak E, Antropova Y, Kanellakis P, Ilyinskaya O, Quinn MT, Smirnov V, Bobik A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2002;22:2037–2043. [DOI] [PubMed] [Google Scholar]
- 168.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002;105:1429–1435. [DOI] [PubMed] [Google Scholar]