Abstract
Vasopressin acts on renal collecting duct cells to stimulate translocation of aquaporin-2(AQP2)-containing membrane vesicles from throughout the cytoplasm to the apical region. The vesicles fuse with the plasma membrane to increase water permeability. To identify the intracellular membrane compartments that contain AQP2, we carried out LC-MS/MS-based proteomic analysis of immunoisolated AQP2-containing intracellular vesicles from rat IMCD. Immuno-gold EM and immunoblotting confirmed heavy AQP2 labeling of immunoisolated vesicles. Vesicle proteins were separated by SDS-PAGE, followed by in-gel trypsin digestion in consecutive gel slices and identification by LC-MS/MS. Identification of Rab GTPases 4, 5, 18, and 21 (associated with early endosomes), Rab7 (late endosomes), and Rab11 and Rab25 (recycling endosomes) indicate that a substantial fraction of intracellular AQP2 is present in endosomal compartments. In addition, several endosome-associated SNARE proteins were identified including syntaxin-7, syntaxin-12, syntaxin-13, Vti1a, VAMP2, and VAMP3. Rab3 was not found, however, either by mass spectrometry or immunoblotting, suggesting a relative lack of AQP2 in secretory vesicles. Additionally, we identified markers of the trans-Golgi network (TGN), components of the exocyst complex, and several motor proteins including myosin IC, non-muscle myosin IIA&B, myosin VI, and myosin IXB. Beyond this, identification of multiple ER-resident proteins and ribosomal proteins indicated that a substantial fraction of intracellular AQP2 is present in rough endoplasmic reticulum (RER). These results show that AQP2-containing vesicles are heterogeneous and that intracellular AQP2 resides chiefly in endosomes, TGN, and RER.
Index words: proteomics, endosomes, myosin, vasopressin, mass spectrometry
Introduction
Aquaporin 2 (AQP2) is the vasopressin-regulated molecular water channel of the renal collecting duct, where it constitutes the major route of water movement across the apical plasma membrane. Vasopressin rapidly increases the water permeability of the collecting duct epithelium by binding to V2 receptors in the basolateral plasma membrane and inducing the cAMP-dependent trafficking of AQP2-containing vesicles from throughout the cytoplasm to the apical region of collecting duct principal cells, followed by fusion of these vesicles with the apical membrane of collecting duct cells (1). Although this fundamental mechanism is well established, there is little information about the specific intracellular protein trafficking pathways involved and the nature of the intracellular compartments in which AQP2 resides.
Until now, studies to identify the intracellular localization of AQP2 in collecting duct cells have depended largely on two fundamental approaches, namely immuno-electron microscopy and immunofluorescence immunocytochemistry with confocal microscopy. Immuno-electron microscopy (1) (2) has demonstrated that aquaporin-2 resides in intracellular vesicles distributed throughout the cytoplasm of collecting duct cells. However, it has not been feasible to identify the specific intracellular compartments that contain aquaporin-2 by immuno-electron microscopy, in part, because fixatives needed for high quality structural preservation markedly decrease the ability of aquaporin-2 antibodies to recognize the target protein. The second approach, viz. immunofluorescence immunocytochemistry with confocal microscopy (3) (4) (5), lacks sufficient spatial resolution to identify aquaporin-2 localization in subcellular compartments with certainty, even with double labeling using antibodies to compartment-specific marker proteins.
Studies in other experimental systems have identified several alternative pathways for trafficking to the plasma membranes of cells (6) (7). First, transport vesicles from the trans-Golgi network (TGN) can travel directly to the plasma membrane in the secretory pathway as “secretory vesicles”. Second, membrane proteins can be delivered to the apical plasma membrane via so-called “recycling endosomes”. Recycling endosomes can receive membrane traffic directly from the TGN or from early endosomes formed as a result of endocytosis. Third, proteins initially targeted to the basolateral plasma membrane via the exocyst complex may travel to the apical plasma membrane by transcytosis (8). AQP2 trafficking to the apical plasma membrane of collecting duct cells may utilize one or more of these pathways.
The objective of the present studies is to identify the intracellular compartments in which AQP2 resides in unstimulated inner medullary collecting duct (IMCD) cells freshly isolated from rat kidneys. To do this, we have devised a proteomics-based strategy to identify the proteins associated with AQP2 in immunoisolated intracellular vesicles using LC-MS/MS for large-scale protein identification. The identified proteins can be expected to include markers of specific intracellular compartments (e.g. members of the Rab family of small GTPases and SNARE proteins), pinpointing the location of AQP2 in IMCD cells during the unstimulated steady state.
Methods
The general protocol for immunoisolation of AQP2 vesicles from rat IMCD is summarized in Figure 1. Details of the procedures involved are described in the following.
Figure 1.

Flow diagrams for (A) immunoisolation of intracellular AQP2 vesicles and (B) proteomic analysis of AQP2 vesicles.
Production of and biotinylation of chicken anti-AQP2 antibody
An anti-peptide antibody against the COOH-terminal 15 amino acids of rat AQP2 was raised in chickens (Aves Labs, Inc., Tigard, OR 97223). The sequence of the immunizing peptide was CVELHSPQSLPRGSKA, including an N-terminal cysteine to allow facile linkage to the carrier protein, keyhole limpet hemocyanin (KLH). This antibody was extracted and affinity purified from the egg yolks by the supplier. We biotinylated it using the EZ-Link Sulfo-NHS-LC Biotinylation Kit (Pierce, Rockford, IL), according to the manufacturer’s instructions. Briefly, a 12-fold molar excess of solubilized Sulfo-NHS-LC-biotin was added to the antibody in PBS and incubated at room temperature for 30 minutes. The solution was then run through a 10 ml desalting column to remove excess biotin. Ten 1 ml fractions were collected.
Preparation of an intracellular membrane fraction by differential centrifugation
Animal experiments were conducted under the auspices of approved NHLBI animal protocol 2-KE-3. Six Sprague-Dawley rats, weighing between 200–250g were injected intraperitoneally with furosemide (5 mg/rat) twenty minutes before decapitation and removal of both kidneys. Furosemide dissipates the medullary osmolality gradient and thereby prevents osmotic shock to the cells (9). Inner medullas were dissected from the kidneys, minced with a razor blade, and homogenized using a Dounce homogenizer in one ml of isolation fluid (10 mM triethanolamine, 250 mM sucrose, in 250 ml water, pH to 7.6, plus 8 mg/l PMSF and 0.08 mg/l leupeptin). The homogenate was centrifuged at 4,000 Xg for ten minutes at 4°C (Tomy, MTX-150). The pellet was resuspended in 1 ml of isolation fluid then rehomogenized and recentrifuged under the same conditions. The pellets were discarded and the supernatants were pooled and centrifuged at 17,000 Xg for twenty minutes at 4°C (Sorvall RC2-B centrifuge with SS34 rotor). The supernatant was collected and centrifuged at 200,000 Xg for one hour at 4°C (Beckman ultracentrifuge with Ti-80 rotor). The pellet was resuspended in 500 μl of PBS and was centrifuged for another hour under the same conditions. The resulting pellet was resuspended in 520 μl PBS. This low-density membrane suspension was used for immunoisolation of AQP2-containing vesicles.
Previous studies have concluded that the 200,000 Xg pellet from this procedure is virtually devoid of plasma membranes based on immunoblotting with antibodies to plasma membrane marker proteins (10) (11). To verify this conclusion, we carried out a control experiment in IMCD cell suspensions using surface biotinylation to label plasma membrane proteins (12). An inner medullary collecting duct (IMCD) suspension was prepared from 2 rats as described (13). Working at 4°C to prevent endocytosis, the IMCD suspension was first gently pelleted via centrifugation (50 Xg for 10 seconds) and then washed three times by resuspension in 1ml cold biotinylation solution (215 mM NaCl, 4 mM KCl, 2.5 mM Na2HPO4, 5.5 mM glucose, and 10 mM triethanolamine, pH 7.4). The 2 mM CaCl2, 1.2 mM MgSO4, resulting pellet containing the IMCD cells was resuspended in 1ml biotinylation solution plus 2 mg Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) and incubated for 1 hour. The IMCD cells then washed two times with 1ml biotin quenching solution (0.1 mM CaCl2, 1 mM MgCl2, and 260 mM glycine in PBS, pH 7.4) followed by incubation with the same solution for 20 minutes. The biotin quenching solution was subsequently replaced with sucrose-based isolation fluid (described above), and the IMCD cells were homogenized and subjected to differential centrifugation as described in the previous paragraph. Detection of biotinylated proteins in the 17,000 Xg and 200,000 Xg pellets was accomplished by SDS-PAGE, followed by electroblotting to nitrocellulose membranes and exposure of the resulting nitrocellulose blots to a streptavidin-horseradish peroxidase (HRP) conjugate (Pierce, Rockford, IL). Biotinylated proteins were then visualized by chemiluminescence (See Immunoblotting, below). If plasma membranes have been successfully excluded from the 200,000 Xg pellet (prior to AQP2 vesicle immunoisolation), biotinylated proteins should be absent from this pellet.
Immunoisolation of AQP2 containing vesicles
Dynal M-280 streptavidin-coated magnetic beads were mixed with biotinylated anti-AQP2 antibody on a Dynal Sample Mixer (15 mg beads with 38.8 μg of biotinylated anti-AQP2 antibody). An excess of free biotin (0.075 μmoles) was added to each mixture and mixed for 30 minutes at 22°C to bind any streptavidin sites not occupied by the antibody. Control beads were handled in the same way except that the chicken anti-AQP2 was replaced with 38.8 μg of chicken non-immune IgY. Beads were separated with a magnet, and the supernatant discarded. The beads were then resuspended with 1% BSA in wash solution (5 mM Tris-HCl [pH 7.5], 0.5 mM EDTA, 1 M NaCl) and mixed for 30 min to block non-specific binding. The beads were then washed in a 0.1% BSA solution in PBS four times for five minutes each. Low-density membranes (200,000 Xg pellet, as described in previous section, 750 μg protein), were added to each bead mixture and mixed for 17 hrs at 4°C. The supernatant was then discarded and the beads were then washed in 0.1% BSA in PBS three times for five minutes each. The supernatant was discarded and proteins were eluted and solubilized by addition of 100 μl Laemmli sample buffer (10 mM Tris, pH 6.8, 1.5% SDS, 6% glycerol), followed by heating to 100°C for 10 minutes. Protein concentration in the supernatant was assessed using the BCA Protein Assay (Pierce, Rockford, IL).
Separation by 1-D SDS-PAGE gel and trypsinization
Immunoisolated AQP2-vesicle proteins were separated by SDS-PAGE using 12% polyacrylamide minigels (Bio Rad Laboratories, Hercules, CA). Gels were then stained for 15 min with Coomassie blue to visualize the proteins. The entire sample lane was cut into 35 sequential slices of approximately 1–2 mm thickness. Each of the 35 slices was then destained with 25 mM NH4HCO3/50% acetonitrile (ACN) for ten-minute intervals until entirely destained. Gel samples were dried, then reduced with 10 mM DTT in 25 mM NH4HCO3 for 1 hr at 56°C. The supernatant was removed and an aqueous solution containing 55 mM iodoacetamide in 25 mM NH4HCO3 was added for 45 min in darkness at 22°C to alkylate the reduced cysteine residues. The supernatant was then removed and gels were washed with 25 mM NH4HCO3 for 10 min. Gel pieces were dehydrated with 25 mM NH4HCO3/50%ACN and dried. Proteins were trypsinized using 12.5 ng/μl sequencing-grade modified trypsin (Promega, Madison, WI) diluted in 25 mM NH4HCO3 and incubated at 37°C for 16 hrs. Peptides were extracted from the gel by sonication in a 50% ACN/0.5% formic acid solution, then dried and reconstituted with 0.1% formic acid.
LC-MS/MS
Tryptic peptides from each gel block were analyzed by 1-dimensional LC-MS/MS using a modified configuration of the ProteomeX 2D LC/MS workstation (Thermo Finnigan, San Jose, CA). Chromatographic separation of peptides was accomplished using two Zorbax 300SB-C18 peptide traps (Agilent Technologies, Wilminton, DE), working in alternating fashion (replacing the standard strong cation exchange and reverse phase columns), while the standard ESI source was replaced by a nanospray ionization source and a reversed-phase PicoFrit TM column (BioBasic C18, 75 mm x 10cm, tip=15 μm, New Objective, Woburn, MA). The peptides were loaded onto the traps in alternating fashion using an autosampler (Surveyor, Thermo Electron, San Jose, CA). After washing with 0.1% formic acid, the peptides were eluted by 0–60% solvent B in solvent A (A = 0.1% formic acid; B = acetonitrile) in 30 min at a flow rate of about 200 nl/min (75 μl/min prior to splitting).
Inclusion criteria for identified peptides
The mass/charge (m/z) ratios of peptides and their fragmented ions were recorded by a method that allows the acquisition of three MS2 scans (i.e., for the three highest intensity peaks in MS1 scans) following each full MS scan. The raw datafiles were searched against the rat protein database from NCBI and rat ab initio protein database from Ensembl using the BioWorks (Version 3.1) software (Thermo Finnigan, San Jose, CA) based on the Sequest algorithm. The search parameters included the following: precursor-ion mass accuracy = 3 amu; fragment-ion mass accuracy = 1 amu; modification allowed for carboxyamidomethylation; and 2 missed cleavages allowed. After the peptide sequence and protein identification from BioWorks software was carried out, the identified peptide sequences were initially qualified and filtered using the cross correlation score (Xcorr) at the following threshold: Xcorr > 1.5 for 1+ ion, 2.0 for 2+ ion, and 2.5 for 3+ ion. For each identified peptide sequence that passed the filter threshold, proteins identified from two or more different peptides were selected if they achieved the following criteria: 1) peptide sequence had the highest Xcorr score for a particular CID spectrum; 2) peptide sequence had a delta normalized correlation (deltaCn) score > 0.1; and 3) peptide sequence had good quality CID spectra by visual inspection. In addition, manual inspections were carried out for identifications based on a single peptide if such peptides corresponded to proteins involved in endosomal trafficking, cytoskeletal organization, or various functions at the plasma membrane (putative cargo proteins), according to gene ontology classifications obtained using Rat Genome Database (http://rgd.mcw.edu/) and Harvester software (EMBL, http://harvester.embl.de/). All identified peptide sequences were searched using BLAST to obtain the best possible unique protein ID, thus eliminating redundant annotations.
A full list of proteins whose identifications were validated by identification of 2 or more component peptides or 1 peptide and manual inspection of spectrum is given in Supplementary Table 2. Supplementary Tables 3 and 4 give a summary of the raw (unvalidated) data. Supplementary Tables 2–4 can be found at URL: http://dir.nhlbi.nih.gov/reviews/lkem/pisitkun1.asp (login: lkem; password: reviewdata). Supplementary Table 1 is contained in this manuscript.
Supplementary Table 1.
Proteins identified with 2 or more unique peptides in AQP2-immunoisolated vesicles.
| Protein name | Accession | Gene name | # peptides | # unique peptides |
|---|---|---|---|---|
| Endosomal Trafficking | ||||
| annexin A2 | NP_063970 | Anxa2 | 51 | 18 |
| annexin A4 | NP_077069 | Anxa4 | 7 | 5 |
| annexin A5 | NP_037264 | Anxa5 | 9 | 6 |
| adaptor protein complex AP-1, beta 1 subunit or adaptor-related protein complex 2, beta 1 subunit | NP_058973 NP_542150 | Ap1B1 Ap2B1 | 9 | 5 |
| adaptor protein complex AP-2, alpha 2 subunit | NP_112270 | Ap2A2 | 7 | 7 |
| adaptor-related protein complex 2, mu 1 subunit | NP_446289 | Ap2M1 | 2 | 2 |
| ADP-ribosylation factor 1 or 2 or 3 | NP_071963 NP_031503 NP_543180 | Arf1 Arf2 Arf3 | 5 | 2 |
| ADP-ribosylation factor 6 | NP_077066 | Arf6 | 3 | 2 |
| similar to Ac39/physophilin | XP_214672 | Atp6V0D1 | 3 | 3 |
| clathrin, heavy polypeptide | NP_062172 | Cltc | 16 | 12 |
| similar to coatomer protein complex subunit alpha | XP_222899 | Copa | 3 | 3 |
| coatomer protein complex, subunit beta 2 (beta prime) | NP_068533 | Copb2 | 3 | 2 |
| similar to Myoferlin | XP_220031 | Fer1L3 | 2 | 2 |
| guanine nucleotide binding protein, alpha inhibiting 3 | NP_037238 | Gnai3 | 2 | 2 |
| guanine nucleotide-binding protein, beta-1 or -2 or –3 | NP_112249 NP_112299 NP_068630 | Gnb1 Gnb2 | 8 | 4 |
| cation-dependent mannose-6-phosphate receptor | NP_001007701 | M6Pr | 5 | 4 |
| myxovirus (influenza virus) resistance 1 or 2 | NP_775119 NP_599177 | Mx1 Mx2 | 4 | 2 |
| N-ethylmaleimide sensitive fusion protein attachment protein alpha | NP_542152 | Napa | 8 | 5 |
| Rab1 | NP_112352 | Rab1 | 15 | 3 |
| Rab2 | NP_113906 | Rab2 | 10 | 5 |
| Rab5B | XP_213824 | Rab5B | 2 | 2 |
| Rab5C | XP_213463 | Rab5C | 3 | 2 |
| Rab7 | NP_076440 | Rab7 | 12 | 6 |
| Rab10 | NP_059055 | Rab10 | 2 | 2 |
| Rab11A or 11B | NP_112414 NP_116006 | Rab11A Rab11B | 2 | 2 |
| Rab14 | NP_446041 | Rab14 | 7 | 3 |
| Rab18 | XP_225453 | Rab18 | 2 | 2 |
| Rab25 | XP_227404 | Rab25 | 3 | 3 |
| reticulon 4 | NP_114019 | Rtn4 | 13 | 4 |
| secretory carrier membrane protein 1 | XP_342175 | Scamp1 | 3 | 2 |
| secretory carrier membrane protein 2 | NP_076445 | Scamp2 | 4 | 2 |
| similar to gp250 precursor | XP_217115 | Sorl1 | 3 | 2 |
| sortilin 1 | XP_342318 | Sort1 | 3 | 2 |
| syntaxin 7 | NP_068641 | Stx7 | 11 | 4 |
| syntaxin 12 or 13 | NP_075228 AAC18967 | Stx12 | 5 | 4 |
| vesicle-associated membrane protein 2 | NP_036795 | Vamp2 | 10 | 3 |
| vesicle-associated membrane protein, associated protein A or B | NP_113819 NP_068619 | Vapa Vapb | 2 | 2 |
| valosin-containing protein | NP_446316 | Vcp | 6 | 5 |
| 14-3-3, zeta polypeptide | NP_037143 | Ywhaz | 3 | 3 |
| Endoplasmic Reticulum | ||||
| aldehyde dehydrogenase family 3, subfamily A2 | NP_113919 | Aldh3A2 | 2 | 2 |
| similar to hypothetical protein FLJ14511 | XP_232987 | Alg2 | 2 | 2 |
| sarcoplasmic/endoplasmic reticulum calcium ATPase 2 | P11507 | Atp2A2 | 9 | 5 |
| calreticulin | NP_071794 | Calr | 3 | 2 |
| calnexin | NP_742005 | Canx | 22 | 6 |
| carboxylesterase 2 | NP_598270 | Ces2 | 3 | 3 |
| cytochrome b-5 | NP_071581 | Cyb5 | 7 | 4 |
| similar to oligosaccharyltransferase | XP_233581 | Ddost | 9 | 5 |
| diaphorase 1 | NP_620232 | Dia1 | 3 | 3 |
| similar to RIKEN cDNA 1200007D18 | XP_213272 | Ergic32 | 4 | 3 |
| endoplasmic retuclum protein 29 | NP_446413 | Erp29 | 4 | 4 |
| similar to alpha glucosidase II, alpha subunit | XP_215144 | Ganab | 2 | 2 |
| glucosidase 1 | NP_113937 | Gcs1 | 4 | 4 |
| glucose regulated protein, 58 kDa | NP_059015 | Grp58 | 19 | 15 |
| similar to hexose-6-phosphate dehydrogenase precursor | XP_233688 | H6Pd | 2 | 2 |
| hydroxysteroid 11-beta dehydrogenase 1 | NP_058776 | Hsd11B1 | 10 | 4 |
| hydroxysteroid 11-beta dehydrogenase 2 | NP_058777 | Hsd11B2 | 9 | 7 |
| heat shock 70kD protein 5 | NP_037215 | Hspa5 | 11 | 9 |
| hypoxia up-regulated 1 | NP_620222 | Hyou1 | 9 | 6 |
| inositol 1,4,5-triphosphate receptor 1 | NP_001007236 | Itpr1 | 2 | 2 |
| similar to Vesicular integral-membrane protein VIP36 precursor | XP_214428 | Lman2 | 12 | 6 |
| prolyl 4-hydroxylase, beta polypeptide | NP_037130 | P4Hb | 7 | 6 |
| proteolipid protein 2 | NP_997484 | Plp2 | 14 | 2 |
| cyclophilin B | NP_071981 | Ppib | 14 | 4 |
| similar to alpha glucosidase II, beta subunit | XP_238534 | Prkcsh | 3 | 2 |
| coated vesicle membrane protein | NP_113910 | Rnp24 | 4 | 2 |
| serine (or cysteine) proteinase inhibitor, clade H, member 1 | NP_058869 | Serpinh1 | 3 | 3 |
| signal peptidase complex 18kD | NP_113911 | Spc18 | 3 | 2 |
| similar to RIKEN cDNA 5730406I15 | XP_214994 | Spc25 | 4 | 3 |
| signal sequence receptor, alpha | NP_001008891 | Ssr1 | 4 | 2 |
| signal sequence receptor 4 | NP_058895 | Ssr4 | 11 | 4 |
| integral membrane protein Tmp21-I | NP_445919 | Tmp21 | 8 | 5 |
| similar to Endoplasmin precursor | XP_343193 | Tra1 | 9 | 9 |
| thioredoxin domain containing 7 | NP_001004442 | Txndc7 | 13 | 8 |
| UDP-glucose ceramide glucosyltransferase-like 1 | NP_598280 | Ugcgl1 | 2 | 2 |
| Putative Cargo | ||||
| agrin | NP_786930 | Agrn | 4 | 2 |
| kidney aminopeptidase M | NP_112274 | Anpep | 3 | 2 |
| apolipoprotein E | NP_620183 | Apoe | 2 | 2 |
| aquaporin 1 | NP_036910 | Aqp1 | 6 | 3 |
| aquaporin 2 | NP_037041 | Aqp2 | 11 | 2 |
| ATPase, Na+/K+ transporting, alpha 1 polypeptide | NP_036636 | Atp1A1 | 39 | 12 |
| ATPase, Na+/K+ transporting, alpha 2 polypeptide | NP_036637 | Atp1A2 | 41 | 6 |
| ATPase, Na+/K+ transporting, beta 1 polypeptide | NP_037245 | Atp1B1 | 12 | 5 |
| similar to Cadherin-16 precursor | XP_226203 | Cdh16 | 18 | 7 |
| chloride channel K1 | NP_445779 | Clcnk1 | 2 | 2 |
| ceruloplasmin | NP_036664 | Cp | 8 | 6 |
| FXYD domain-containing ion transport regulator 2 isoform A or B | NP_663769 NP_059045 | Fxyd2 | 4 | 2 |
| insulin-like growth factor 2 receptor | NP_036888 | Igf2R | 4 | 4 |
| integrin alpha 2 | XP_345157 | Itga2 | 4 | 3 |
| similar to VLA-3 alpha subunit | XP_340885 | Itga3 | 2 | 2 |
| similar to integrin alpha v subunit | XP_230950 | Itgav | 5 | 4 |
| integrin beta 1 | NP_058718 | Itgb1 | 3 | 3 |
| aminopeptidase Vp165 | NP_598258 | Lnpep | 18 | 10 |
| Lutheran blood group | NP_113940 | Lu | 8 | 3 |
| membrane bound C2 domain containing protein | NP_058945 | Mbc2 | 12 | 9 |
| chloride ion pump-associated 55 kDa protein | NP_659553 | Pcyox1 | 2 | 2 |
| Rap1A or 1B | NP_002875 NP_599173 | Rap1A Rap1B | 8 | 4 |
| similar to band 7 protein | XP_219858 | Spfh1 | 2 | 2 |
| similar to CDNA sequence BC036333 | XP_214372 | Spfh2 | 2 | 2 |
| tumor-associated calcium signal transducer 1 | NP_612550 | Tacstd1 | 8 | 4 |
| transmembrane emp24 protein transport domain containing 4 | NP_872353 | Tmed4 | 2 | 2 |
| transmembrane protein 4 | NP_064337 | Tmem4 | 3 | 2 |
| Ribosomal | ||||
| acidic ribosomal protein P0 | NP_071797 | Arbp | 13 | 7 |
| similar to ribosome-binding protein p34 | NP_001008281 | Mgc105677 | 5 | 2 |
| similar to 60S ribosomal protein L11 | XP_342950 | Rpl11 | 10 | 2 |
| similar to 60S ribosomal protein L12 | XP_216039 | Rpl12 | 4 | 3 |
| ribosomal protein L13 | NP_112363 | Rpl13 | 10 | 2 |
| ribosomal protein L27 | NP_071959 | Rpl27 | 2 | 2 |
| ribosomal protein L30 | NP_073190 | Rpl30 | 5 | 2 |
| ribosomal protein L31 | NP_071951 | Rpl31 | 3 | 2 |
| ribosomal protein L6 | NP_446423 | Rpl6 | 4 | 2 |
| 60S ribosomal protein L7a | P62425 | Rpl7A | 4 | 3 |
| ribosomal protein L8 | XP_343279 | Rpl8 | 4 | 2 |
| 60S acidic ribosomal protein P2 | P02401 | Rplp2 | 6 | 3 |
| ribosomal protein S11 | NP_112372 | Rps11 | 3 | 2 |
| ribosomal protein S13 | NP_569116 | Rps13 | 4 | 3 |
| ribosomal protein S15a | NP_446434 | Rps15A | 3 | 2 |
| similar to 40S ribosomal protein S16 | XP_341816 | Rps16 | 4 | 3 |
| 40S ribosomal protein S18 | P62271 | Rps18 | 5 | 2 |
| similar to 40S ribosomal protein S2 | XP_214903 | Rps2 | 4 | 2 |
| ribosomal protein S26 | NP_037356 | Rps26 | 8 | 2 |
| 40S ribosomal protein S3 | P62909 | Rps3 | 3 | 3 |
| ribosomal protein S4, X-linked | NP_001007601 | Rps4X | 2 | 2 |
| ribosomal protein S6 | NP_058856 | Rps6 | 3 | 2 |
| similar to 40S ribosomal protein S7 | XP_345691 | Rps7 | 3 | 2 |
| ribosomal protein S8 | NP_113894 | Rps8 | 9 | 4 |
| ribosomal protein S9 | NP_112370 | Rps9 | 3 | 2 |
| Nuclear | ||||
| similar to KIAA0788 protein | XP_215831 | Ascc3L1 | 3 | 2 |
| similar to Probable RNA-dependent helicase p72 | XP_235480 | Ddx17 | 12 | 7 |
| similar to Probable RNA-dependent helicase p68 | NP_001007614 | Ddx5 | 5 | 4 |
| H1 histone family, member 4 | NP_579819 | H1F4 | 2 | 2 |
| histone 2a | NP_068612 | H2A | 29 | 2 |
| H2A histone family, member Z | NP_073165 | H2Afz | 11 | 2 |
| H3 histone, family 3B | NP_446437 | H3F3B | 2 | 2 |
| histone 1, H2bl | NP_072173 | Hist1H2Bl | 5 | 3 |
| germinal histone H4 gene | NP_073177 | Hist1H4B | 18 | 4 |
| heterogeneous nuclear ribonucleoprotein A3 | NP_937765 | Hnrnpa3 | 4 | 2 |
| similar to heterogeneous nuclear ribonucleoprotein H3 isoform a | XP_342132 | Hnrph3 | 7 | 3 |
| poly(rC) binding protein 1 | NP_035995 | Pcbp1 | 3 | 2 |
| neuronal differentiation-related gene | NP_647549 | Prp19 | 3 | 3 |
| similar to splicing factor Prp8 | XP_213385 | Prpf8 | 2 | 2 |
| similar to heterogeneous nuclear ribonucleoprotein G | XP_229192 | Rbmx | 9 | 3 |
| similar to RIKEN cDNA 1810061H24 | XP_214697 | Sf3B3 | 10 | 8 |
| similar to PTB-associated splicing factor | XP_342921 | Sfpq | 3 | 3 |
| similar to 116 kDa U5 small nuclear ribonucleoprotein component | XP_213492 | Snrp116 | 2 | 2 |
| similar to Small nuclear ribonucleoprotein Sm D2 | XP_214847 | Snrpd2 | 4 | 3 |
| small nuclear ribonucleoparticle-associated protein | NP_112379 | Snrpn | 3 | 2 |
| Cytoskeletal-Motor | ||||
| cytoplasmic beta-actin or gamma-actin | NP_112406 P63259 | Actb Actg1 | 75 | 17 |
| actin, gamma 2 | NP_037025 | Actg2 | 3 | 3 |
| similar to RIKEN cDNA 2010015J01 | XP_237111 | Arpc5L | 2 | 2 |
| beta-catenin | NP_445809 | Catnb | 2 | 2 |
| gelsolin | NP_001004080 | Gsn | 3 | 3 |
| type II keratin Kb1 | NP_001008802 | Kb1 | 9 | 4 |
| similar to cytokeratin | XP_215513 | Krt1-18 | 3 | 2 |
| keratin complex 1, acidic, gene 19 | NP_955792 | Krt1-19 | 7 | 6 |
| keratin complex 2, basic, gene 8 | NP_955402 | Krt2-8 | 3 | 2 |
| myosin regulatory light chain | NP_059039 | Mrlcb | 16 | 5 |
| myosin heavy chain 10, non-muscle | NP_113708 | Myh10 | 20 | 14 |
| myosin, heavy polypeptide 9 | NP_037326 | Myh9 | 69 | 31 |
| Myosin light polypeptide 6 | Q64119 | Myl6 | 17 | 6 |
| similar to Myosin VI | XP_236444 | Myo6 | 2 | 2 |
| unconventional myosin Myr2 I heavy chain | NP_075580 | Myr2 | 19 | 14 |
| beta-spectrin 3 | NP_062040 | Spnb3 | 5 | 5 |
| tubulin, alpha 1 | NP_071634 | Tuba1 | 6 | 4 |
| tubulin, beta 5 | NP_775125 | Tubb5 | 6 | 5 |
| Unclassified | ||||
| similar to RIKEN cDNA 5730469M10 | XP_341407 | 8 | 5 | |
| similar to DKFZP586A0522 protein | XP_235659 | 4 | 2 | |
| similar to Hypothetical protein KIAA0152 | XP_222228 | 5 | 3 | |
| similar to RIKEN cDNA 1500009M05 | XP_215704 | 4 | 2 | |
| aldehyde reductase 1 | NP_036630 | Aldr1 | 7 | 3 |
| crystallin, alpha B | NP_037067 | Cryab | 4 | 4 |
| hydroxysteroid (17-beta) dehydrogenase 12 | NP_114455 | Hsd17B12 | 2 | 2 |
| heat shock protein 8 | NP_077327 | Hspa8 | 5 | 4 |
| heat shock 90kDa protein 1, beta | NP_001004082 | Hspcb | 4 | 4 |
| similar to pM5 protein | XP_341862 | Nomo1 | 4 | 3 |
| similar to CG8014-PA | XP_236584 | Rme-8 | 6 | 5 |
| Mitochondrial | ||||
| ATP synthase, H+ transporting, mitochondrial F1 complex, beta subunit | NP_599191 | Atp5B | 3 | 3 |
| voltage-dependent anion channel 1 | NP_112643 | Vdac1 | 4 | 3 |
| voltage-dependent anion channel 2 | NP_112644 | Vdac2 | 4 | 3 |
| Extracellular (secreted) | ||||
| collagen, type XVIII, alpha 1 | XP_241632 | Col18A1 | 27 | 4 |
| fibronectin 1 | NP_062016 | Fn1 | 3 | 3 |
| similar to Basement membrane-specific heparan sulfate proteoglycan core protein precursor | XP_233606 | Hspg2 | 3 | 2 |
Immunoblotting
Immunoblotting was performed as described (14). Briefly, 10–20 μg protein was resolved by SDS-PAGE gel electrophoresis on 10–12% polyacrylamide gels and transferred electrophoretically onto nitrocellulose membranes. The membranes were then blocked with 5% nonfat dry milk in immunoblot wash buffer (42 mM Na2HPO4, 8 mM NaH2PO4, 150 mM NaCl, and 0.05% Tween 20, pH 7.5), rinsed and probed with primary antibody overnight at 4°C. After washing, blots were incubated with species-specific secondary antibody conjugated to horseradish peroxidase. After the final wash, antibody binding was visualized by chemiluminescence (LumiGLO; KPL, Gaithersburg, MD) and autoradiography.
Immuno-electron microscopy
Vesicle suspensions were prepared by eluting the immunoisolated vesicles from magnetic beads with PBS titrated to pH 3 with 50 mM HCl. Then the suspension was titrated back to pH 7.5 with 50 mM NaOH. Vesicle suspensions were mixed 1:1 with 4% paraformaldehyde then applied to 200 mesh nickel grids. After blocking with 1% BSA and washing, the grid was incubated with primary antibody for 45 min at room temperature. Grids were exposed to primary antibodies recognizing AQP2, Rab5, Rab7, or Rab11 followed by exposure to species-specific anti-IgG antibodies conjugated to colloidal gold particles (6 nm or 12 nm, Jackson ImmunoResearch Laboratories, West Grove, PA). After washing, vesicles underwent negative staining with 1% uranyl acetate. After drying, the grids were examined with a JOEL 1200 EX electron microscope operated at 60 kV. Control labeling was performed identically, but non-immune IgG was substituted for the primary antibody.
Antibodies
Aside from the chicken anti-AQP2 antibody described above, primary antibodies were obtained from either commercial sources or from independent investigators. The affinity-purified rabbit polyclonal aquaporin-2 (14) and VAMP-2 (15) antibodies were produced in our laboratory. The ARF6 antibody was kindly provided by Dr. J. Donaldson (NHLBI, Bethesda). The anti-myosin IIA rabbit polyclonal was a gift of Dr. Robert Adelstein (NHLBI, Bethesda, MD). The myosin 1C rabbit polyclonal (M3567) and myosin VI mouse monoclonal (M0691) antibodies were obtained from Sigma-Aldrich. The following rabbit polyclonal antibodies were purchased from Santa Cruz Biotechnology: Rab7 (H-50, sc-10767), Rab4 (D-20, sc-312), and Rab5a (A-20, sc-598). The Rab11 mouse monoclonal antibody (610656) was obtained from BD Transduction Laboratories. The Rab3a mouse monoclonal antibody (107 011) was obtained from Synaptic Systems. Stressgen supplied the following mouse monoclonal antibodies: Syntaxin 13 (VAM-SV026), rSec8 (VAM-SV016), and rSec6 mouse (VAM-SV021). The ubiquitin mouse monoclonal antibody (P4D1) was obtained from Santa Cruz Biotechnology.
Infusion of the vasopressin analog dDAVP in Brattleboro rats
Male Brattleboro rats (180–230 g body wt; Harlan-Sprague Dawley, Indianapolis, IN) received an infusion of the V2R-selective vasopressin analog dDAVP (Rhone-Poulenc Rorer, Collegeville, PA) at 5 ng/h by subcutaneous osmotic minipumps (model 2001; Alzet, Palo Alto, CA). Control rats received minipumps delivering isotonic saline solution. Rats were maintained in metabolic cages in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle. They had free access to water and regular pelleted rat chow. After 24 hours, the rats were killed by rapid decapitation, and inner medullas were rapidly isolated for immunoisolation of AQP2 vesicles as described above.
Results
An anti-AQP2 antibody was prepared in chickens and biotinylated for these studies. The ability of the biotinylated chicken antibody to recognize AQP2 was tested by probing an immunoblot prepared from a homogenate of rat renal inner medulla (Figure 2A). The biotinylated chicken anti-AQP2 antibody (left lane) labeled the same bands as did a previously characterized rabbit anti-AQP2 antibody (right lane).
Figure 2. A) Immunoblot characterization of biotinylated chicken anti-AQP2 antibody.

The biotinylated chicken anti-AQP2 (right panel) recognized nonglycosylated AQP2 at 29 kDa and glycosylated AQP2 at 35–40 kDa, as seen with previously characterized (13) rabbit anti-AQP2 antibody (left panel). Immunoblot using biotinylated chicken antibody utilized streptavidin-horseradish peroxidase (HRP) conjugate in lieu of HRP-conjugated secondary antibody. B) Surface biotinylation showing relative absence of plasma membrane proteins in low-density membrane fraction. Surface biotinylation of inner medullary collecting duct (IMCD) suspension was performed (see Methods). The IMCD cells were homogenized and then subjected to differential centrifugation to obtain 17,000 Xg pellet (high-density membrane fraction) and 200,000 Xg pellet (low-density membrane fraction). Detection of biotinylated proteins in these two fractions was accomplished by SDS-PAGE followed by electroblotting and probing the blots with a streptavidin-horseradish peroxidase (HRP) conjugate. Signal was detected through use of chemiluminescence and light-sensitive film. Three μg of protein was loaded in both lanes. The two lanes shown are from the same exposure of the same blot.
The biotinylated chicken anti-AQP2 antibody was used to immunoisolate AQP2-containing intracellular vesicles from a low-density membrane fraction of rat inner medulla (200,000 Xg spin of the 17,000 Xg supernatant). By immunoblotting with antibodies to plasma membrane marker proteins, we previously showed that this low-density membrane fraction is virtually devoid of plasma membranes (10) (11). To confirm that conclusion, we have carried out surface biotinylation of rat IMCD cells in suspension and used these cells as input for the same differential centrifugation procedure (Figure 2B). Although the high-density membrane fraction (17,000 xg pellet) contained abundant quantities of biotinylated (plasma membrane) proteins, the low-density membrane fraction (200,000 Xg pellet) contained only very small amounts of biotinylated proteins. Hence, we conclude that the low-density membrane fraction used for AQP2-vesicle isolation does not contain substantial amounts of plasma membranes.
Intracellular membrane vesicles containing AQP2 were immunoisolated from the low-density membrane fraction of rat inner medullas using the chicken anti-AQP2 antibody and the associated proteins were separated initially by 1-D SDS-PAGE (Figure 3A). A control sample was obtained by carrying out the same procedure with substitution of non-immune chicken IgY for the anti-AQP2 antibody. The Coomassie-stained gels were sliced into blocks designated A through I at points defined by the molecular weight markers, and then each block was further cut into 1–2 mm thick slices, each of which was subjected to in-gel trypsinization and elution of the resulting peptides. To confirm the specificity of the AQP2-vesicle immunoisolation, an immunoblot was performed using a rabbit anti-AQP2 antibody (Figure 3B). AQP2 band density was approximately 50-fold greater in AQP2-immunoisolated vesicle sample than in the control sample. Figure 3C shows immuno-gold labeling of AQP2 in the immunoisolated vesicles, confirming that AQP2 is associated with discrete vesicular structures.
Figure 3.

(A) Coomassie-stained gels of immunoisolated proteins and proteins isolated nonspecifically in non-immune IgY control. 1-D SDS-PAGE using 12% polyacrylamide. Heavy band in control lane is BSA. Gel was sliced as indicated to obtain 35 gel pieces for in-gel trypsinization and LC-MS/MS analysis. (B) Immunoblot of AQP2 in the whole renal inner medulla homogenate, AQP2-immunoisolated vesicles, and control sample. Each lane was loaded with an equal amount of total protein. The immunoblot was probed with a rabbit anti-AQP2 antibody. Film was deliberately overexposed to visualize weak AQP2 signal in control lane. AQP2 was markedly enriched in AQP2-immunoisolated vesicles as compared to the control sample. (C) Immuno-gold electron microscopy of immunoisolated vesicles. Primary antibody was affinity purified rabbit anti-AQP2.
Following trypsinization and extraction from each gel slice (Figure 1), the peptides were analyzed by LC-MS/MS. The associated proteins were identified by comparison with the rat protein databases (standard database from NCBI and ab initio database from Ensembl) using BioWorks Version 3.1 software. Figure 4 and Supplementary Table 1 report the 181 proteins identified for which at least two distinct peptides were found. Table 1 shows a summary of the proteins identified in the trafficking and cytoskeletal/motor protein categories, including both the proteins from Supplementary Table 1 and proteins identified based on a single peptide with manual inspection of the spectra. The identification of Rab GTPases 4, 5, 18, and 21 (associated with early endosomes), Rab7 (associated with late endosomes), and Rab11 and 25 (associated with recycling endosomes) points to the conclusion that a large component of intracellular AQP2 is located in endosomes (16). In addition, several endosome-associated SNARE proteins were identified including syntaxin-7 (present in late endosomes (17)), syntaxins 12 and -13 (present in recycling endosomes (18)), Vti1a (present in TGN (19)), and VAMP3. Additionally, we identified actin and several actin-related proteins as well as actin-based motor proteins (myosin 1C, non-muscle myosin IIA&B, and myosin VI), annexins, tetraspan proteins, coat proteins, adaptor proteins, and heterotrimeric G-proteins as summarized in Table 1.
Figure 4. Classification of proteins identified with two or more unique peptides in immunoisolated AQP2-vesicles by LC-MS/MS analysis.
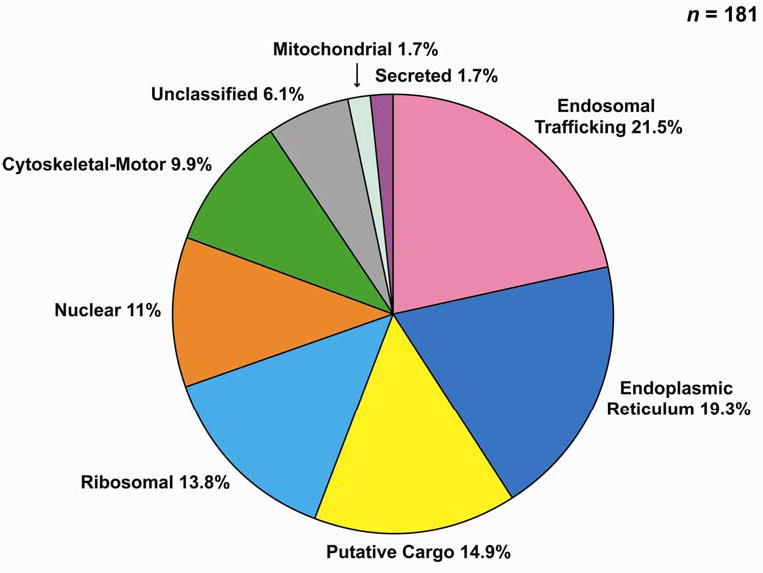
Protein classification utilized Rat Genome Database (http://rgd.mcw.edu/) and Harvester (EMBL, http://harvester.embl.de/).
Table 1.
Summary of trafficking and cytoskeletal proteins identified in association with intracellular AQP2 vesicles in inner medullary collecting duct (see Supplementary Tables for full list)
| Small GTP-binding proteins |
| ARF1 |
| ARF2 |
| ARF3 |
| ARF4 |
| ARF5 |
| ARF6 |
| ARFd1 |
| Rab1 |
| Rab2 |
| Rab4B |
| Rab5A,B,C |
| Rab7 |
| Rab10 |
| Rab11A,B |
| Rab14 |
| Rab18 |
| Rab21 |
| Rab25 |
| Rac1 |
| RalA (immunoblot only) |
| RalB |
| SNARES and related proteins |
| pantophysin |
| reticulon 4 |
| α-SNAP |
| synaptotagmin-13 |
| syntaxin-5A |
| syntaxin-7 |
| syntaxin-12 |
| syntaxin-13 |
| syntaxin-16 |
| VAMP2 |
| VAMP3 |
| VapA,B |
| Vti1A |
| Annexins |
| Annexin I |
| Annexin II |
| Annexin IV |
| Annexin V |
| Tetraspan proteins |
| synaptogyrin-2 |
| SCAMP1 |
| SCAMP2 |
| SCAMP3 |
| Coat proteins |
| clathrin heavy chain |
| α-COP |
| β-COP |
| β′-COP |
| Clathrin adaptors |
| AP1 β-1 subunit |
| AP2 (several subunits) |
| Exocyst |
| Sec6 (immunoblot only) |
| Sec8 (immunoblot only) |
| Actin cytoskeleton |
| β-actin |
| γ-actin |
| ARP 2/3 (several subunits) |
| destrin |
| gelsolin |
| β-spectrin-3 |
| α-tropomyosin |
| Myosins and associated proteins |
| myosin IC |
| myosin IIA |
| myosin IIB |
| myosin VI |
| myosin IXB |
| myosin light chain 3 |
| myosin regulatory light chain – B |
| myosin regulatory light chain - C |
| Microtubule proteins |
| α-tubulin |
| β-tubulin |
| Microtubule-based motor proteins |
| none |
| Intermediate filament proteins |
| cytokeratin 8 |
| cytokeratin 18 |
| cytokeratin 19 |
| Heterotrimeric G-proteins |
| Gαi2 |
| Gαi3 |
| Gαq |
| Gβ1,2,3 |
| Miscellaneous |
| 14-3-3 theta |
| 14-3-3 zeta |
| β-catenin |
| choroidermia |
| dynamin-II |
| gp250 |
| mannose 6-phosphate receptor (CD) |
| myoferlin |
| Sec23A |
| sortilin 1 |
| ubiquitin |
| ubiquitin-conjugating enzyme E2N |
| vacuolar proton pump, subunit a |
| vacuolar proton pump, subunit d |
| valosin-containing protein |
Table 2 shows a summary of putative cargo proteins that were identified in association with the immunoisolated AQP2 vesicles. For the purposes of this paper we define putative cargo as “proteins that are putatively carried in or attached to vesicles, but are not part of the trafficking apparatus”. In most cases, these are proteins that function as components of the plasma membrane. Furthermore, there were several membrane receptors, adhesion molecules, cell-surface enzymes, and transport proteins including AQP2. These studies, however, do not demonstrate which intracellular subcompartment these proteins are present in.
Table 2.
Summary of putative cargo proteins associated with immunoisolated AQP2 vesicles (see Supplementary Tables for fully annotated list)
| Transporters/channels |
| Adenine nucleotide transporter |
| Aquaporin-1 |
| Aquaporin-2 |
| Claudin 4 |
| ClC-K chloride channel |
| Clp55 |
| FXYD-2a and -2b (Na/K-ATPase, γ subunit) |
| FXYD-4 (CHIF) |
| Na/K-ATPase, α1 subunit |
| Na/K-ATPase, α2 subunit |
| Na/K-ATPase, β1 subunit |
| Na channel, voltage gated (rSkM1) |
| Enzymes |
| Aminopeptidase N |
| Ceruloplasmin |
| Insulin-regulated aminopeptidase (IRAP) |
| Adhesion molecules |
| Cadherin-16 |
| Integrin α2 |
| Integrin α3 |
| Integrin α5 |
| Integrin β1 |
| Receptors |
| Insulin-like growth factor receptor-2 |
| Lutheran blood group antigen (laminin receptor) |
| Membrane progesterone receptor |
| Scavenger receptor class B, member 2 |
| Miscellaneous |
| Agrin |
| Apolipoprotein E |
| CD59 (Protectin) |
| Defender-against-cell-death |
| Endomucin |
| Lysosomal membrane glycoprotein 1 and 2 |
| Membrane bound C2 domain containing protein |
| Plasmalemma vesicle associated protein |
| RAP1 |
| SPFH domain family, member 1 and 2 |
| Transmembrane emp24 protein |
| Transmembrane protein 4 |
| Tumor-associated calcium signal transducer 1 |
“Cargo” is defined here as proteins that are putatively carried in or attached to vesicles but are not part of the trafficking apparatus.
The immunoisolated AQP2-vesicles also contained many ribosomal and ER resident proteins (Figure 4 and Supplementary Table 1) consistent with the presence of a substantial amount of intracellular AQP2 in the rough endoplasmic reticulum. Thus, we conclude that AQP2-containing intracellular vesicles are heterogeneous and include rough endoplasmic reticulum as well as various endosomal compartments.
The protein yield for the control isolation with non-immune chicken IgG was much lower than the yield for the AQP2-specific isolation (Figure 3). Much of the protein isolated appears to be BSA used for blocking the beads. LC-MS/MS analysis of proteins eluted from the control beads did not recapitulate the protein list found in analysis of those isolated with the AQP2 antibody (Supplementary Table 4). Nevertheless, a few abundant proteins did appear on both lists including α-B crystallin, aldehyde reductase 1, α-1 and α-2 Na/K-ATPase, annexin A2, myosin IC, Rab1, valosin-containing protein, hydroxysteroid 11-β-dehydrogenase 1, β- and γ -actin, band 7 protein, and GTP-binding protein (Gαi2). The association of these proteins with AQP2 intracellular vesicles, therefore, must be held as uncertain. Among all proteins identified from the control sample (no AQP2 antibody), only 15 proteins were identified from distinct spectra corresponding to 2 or more peptides. All others were based on single peptides, and most of these spectra were of relatively low quality, and did not in general satisfy the acceptance criteria applied for the AQP2-immunoisolated proteins.
We carried out immunoblotting of additional immunoisolated AQP2-vesicle preparations to confirm the presence of selected proteins identified by LC-MS/MS. Figure 5A shows confirmatory immunoblots of Rab GTPases associated with endosomes including Rab4 (early endosomes), Rab5 (early endosomes and clathrin-coated endocytic vesicles), Rab7 (late endosomes), and Rab11 (recycling endosomes). All of these Rab proteins were enriched in the AQP2 vesicle sample relative to the whole IM sample and were of decreased abundance in the control sample. These results lend further support to the conclusion that substantial quantities of AQP2 are present in endosomal subcompartments including early endosomes, late endosomes, recycling endosomes, as well as the TGN (see Discussion).
Figure 5. Confirmatory immunoblots for Rab GTPases.
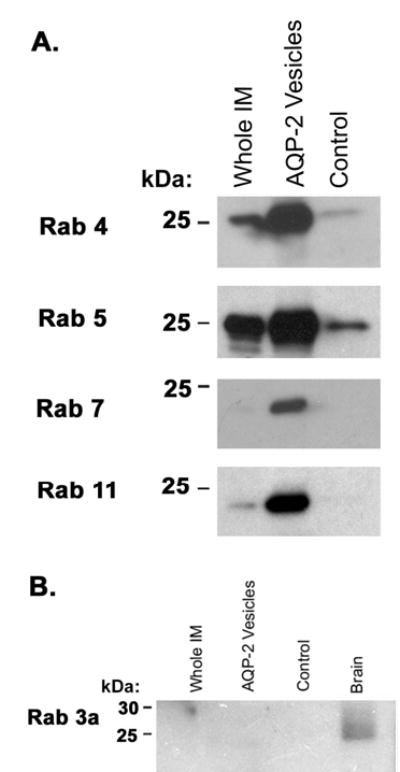
(A) Immunoblots for endosome-associated Rab GTPases in AQP2-immunoisolated vesicles: Rab4 (present in early endosomes), Rab5 (present in early endosomes and clathrin-coated endocytic vesicles), Rab7 (present in late endosomes), and Rab11 (present in recycling endosomes). (B) Immunoblot comparing abundance of Rab3a in the whole renal inner medulla homogenate, AQP2-immunoisolated vesicles, non-immune IgY control sample, and whole brain homogenate.
Secretory vesicles typically contain a different Rab protein, namely Rab3. The LC-MS/MS analysis did not identify Rab3 isoforms in AQP2 vesicles. To look further, we carried out immunoblotting for Rab3, which has been previously reported to be present in rat inner medullary intracellular vesicles (20) (Figure 5B). Although a clearcut band was seen in the whole brain homogenate, no evidence of Rab3a was found in inner medulla or AQP2 isolated vesicles. Thus, Rab3a-positive vesicles do not appear to be abundantly represented among the AQP2-containing intracellular membrane compartments in the IMCD.
The presence of Rab5, Rab7, and Rab11 in AQP2 vesicles was further confirmed by immuno-gold labeling (Figure 6). Figure 6A shows labeling for Rab5 with double labeling for Rab5 (small gold particles) and AQP2 (large particles) in the inset. Figure 6B shows double labeling for Rab7 (small gold particles) and AQP2 (large particles). Single immunolabeling is shown for Rab11 in Figure 6C. In general, each of these three Rab proteins appears to be present in a subset of AQP2-immunoisolated vesicles.
Figure 6. Electron micrographs showing immuno-gold labeling of AQP2-immunoisolated vesicles.
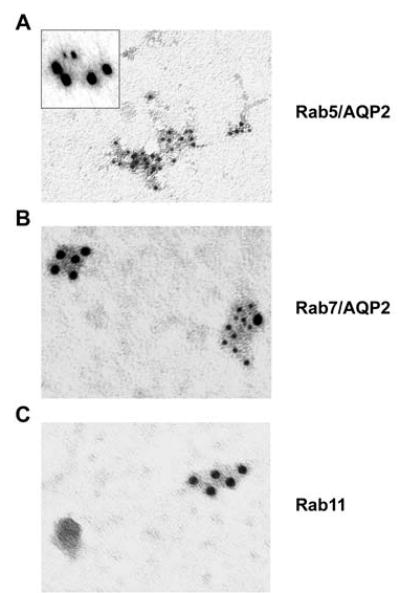
(A) Rab5 (inset, Rab5 [small particles] and AQP2 [large particles], (B) Rab7 (small gold particles) and AQP2 (large gold particles), (c) Rab11 (single labeling only).
Several members of the ARF (ADP ribosylation factor) family of small GTP-binding proteins were also identified by mass spectrometry of immunoisolated AQP2 membranes, viz. ARF 1–6. These proteins are important in clathrin-mediated vesicle budding, but have other roles in membrane trafficking (21). Figure 7 depicts a confirmatory immunoblot for ARF 6, showing a single band at approximately 18 kDa in both the IM and AQP2 immunoisolated samples, but not the control sample.
Figure 7.

Immunoblot using anti-ARF 6 confirming presence of ARF 6 in AQP2-immunoisolated vesicles.
Immunoblotting was also carried out for the SNARE proteins syntaxin 13, VAMP2 (synaptobrevin II), and VAMP3 (cellubrevin), all of which were identified by LC-MS/MS analysis in AQP2 immunoisolated vesicles (Figure 8). All of these SNARE proteins were enriched in the AQP2 isolated sample relative to the whole IM sample and were of decreased abundance in the control sample. Syntaxin 13 was previously shown to be localized to early and recycling endosomes (18) (22) (23). VAMP2 (synaptobrevin-2) has been previously shown to be associated with endosomal vesicles (24). It has been shown to be present in intracellular vesicles from rat inner medulla (15) and to colocalize with AQP2-labeled intracellular vesicles via immuno-electron microscopy (25). VAMP3 has also been previously been shown to be associated with AQP2 vesicles (26). Again, these data support the idea that the AQP2 immunoisolation procedure yields intracellular vesicles that are largely of endosomal origin.
Figure 8.
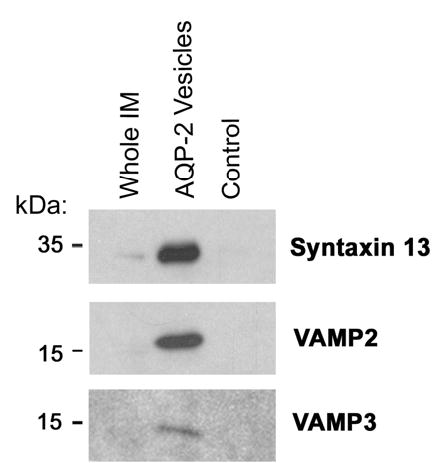
Immunoblots confirming presence of SNARE proteins syntaxin 13, VAMP2 (synaptobrevin II), and VAMP3 (cellubrevin) in AQP2-immunoisolated vesicles.
The myosin isoforms identified by LC-MS/MS included myosin regulatory light chain, conventional non-muscle myosins IIA and IIB as well as unconventional myosins 1C, VI, and myosin IXB (Table 1). Figure 9 shows confirmatory immunoblots for myosin 1C, myosin IIA, and myosin VI, all of which were identified in the AQP2 immunoisolated sample. Note the modest decrease in mobility in myosin IIA and myosin VI proteins attached to AQP2 vesicles, suggesting the presence of an unknown post-translational modification.
Figure 9.

Immunoblots confirming presence of myosin 1C, myosin IIA, and myosin VI in AQP2-immunoisolated vesicles.
Ubiquitination is a signal for endocytosis and/or degradation of plasma membrane proteins. Ubiquitin was identified by LC-MS/MS in the SDS-PAGE gel blocks representing molecular masses greater than 75 kDa (Supplementary Table 2). Immunoblotting with an anti-ubiquitin antibody confirmed the presence of ubiquitin in the AQP2-immunoisolated vesicles (Figure 10). The anti-ubiquitin antibody labeled proteins throughout the molecular weight range from 25 kDa to over 250 kDa. The broad smear suggests that ubiquitin is attached to a variety of proteins present in the AQP2 vesicles.
Figure 10.

Immunoblot using anti-ubiquitin antibody. Note smear over a molecular weight range from 25 kDa to over 250 kDa, suggesting the presence of a variety of ubiquitinated proteins in AQP2-immunoisolated vesicles.
Proteins initially targeted to the basolateral plasma membrane via the exocyst complex may travel to the apical plasma membrane by transcytosis (8). In the LC-MS/MS analysis, we did not identify any components of the exocyst complex. Since a negative result using mass spectroscopy does not necessarily imply the absence of a particular protein, we tested for exocyst complex components by immunoblotting (Figure 11). Two components, Sec 6 and Sec 8, were found to be associated with AQP2 vesicles. Furthermore, a small GTP-binding protein, known to be associated with the exocyst complex, Ral A, was also identified in the AQP2 vesicles. The exocyst is known to function as an effector for the Rab-like small GTPase Sec4 in yeast (16). The mammalian Rab with the highest similarity to Sec4 is Rab8 (BLAST analysis), which was not identified in AQP2-vesicles by mass spectrometry. These results give preliminary support to the view that AQP2 is present in an intracellular structure that contains the exocyst complex, possibly recycling endosomes or TGN (16).
Figure 11.

Immunoblots identifying exocyst complex proteins Sec 8, Sec 6, and Ral A in AQP2-immunoisolated vesicles.
To investigate whether vasopressin may affect the ability to detect Rab proteins in AQP2-vesicles, we carried out the studies shown in Figure 12. Here, we infused vasopressin-deficient Brattleboro rats with either vehicle or dDAVP for 24 hours and immunoblotted inner medullary samples for markers of the early endosomes (Rab4 and 5), late endosomes (Rab7), recycling endosomes (Rab11), and secretory vesicles (Rab 3). Again Rab4, Rab5, Rab7, and Rab11 were readily detectable and we could find no evidence for Rab3 under either condition. dDAVP infusion appeared to increase the amount of Rab11 (recycling endosomes) while decreasing the amount of Rab7 (late endosomes) associated with AQP2-immunoisolated intracellular membranes.
Figure 12. Immunoblots showing relative abundance of various Rab GTPases in AQP2-immunoisolated intracellular vesicles from Brattleboro rats treated with either dDAVP or vehicle.
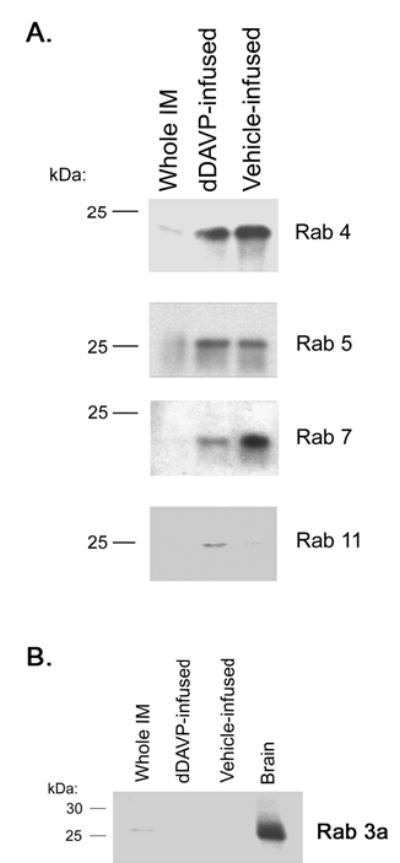
(A) Endosome-associated Rab GTPases, including Rab4 and 5 (early endosomes), Rab7 (late endosomes), and Rab11 (recycling endosomes). (B) Rab3a (normally associated with secretory vesicles).
Discussion
Advances in proteomics and mass spectrometry applied to proteins have enabled large-scale identification of proteins in specific tissues, cells, or subcellular fractions (27) (28). Here we have utilized tandem mass spectrometry coupled to high performance liquid chromatography (LC-MS/MS) to enumerate proteins in inner medullary collecting duct cells that are contained in or attached to AQP2-bearing intracellular membranes. These “AQP2 vesicles” were purified by differential centrifugation followed by immunoisolation using a biotinylated chicken anti-AQP2 antibody. One-dimensional SDS-PAGE was used to pre-fractionate the proteins prior to trypsinization and one-dimensional LC-MS/MS.
Recent advances in cell biology have led to identification of numerous proteins involved in membrane trafficking including those responsible for budding, fusion, and cytoskeletal interactions. Many of these proteins were identified in this analysis (Figure 4 and Table 1). Of particular interest are proteins known to be associated with distinct subcellular membrane domains, which may serve as markers for these discrete membrane compartments. One family of proteins, the Rab family of small GTPases, is an example. Our mass spectrometric analysis identified a number of Rab family members. The Rab GTPases and their effectors orchestrate vesicular trafficking between disparate membrane subdomains in both the endocytic and exocytic trafficking pathways (16). Thus, it is recognized that specific Rab proteins are associated with secretory vesicles (Rab3 isoforms), with recycling endosomes (Rab11 and Rab25), with early endosomes (Rab4, Rab5, Rab18, and Rab21), and with late endosomes and multivesicular bodies (Rab7). Our identification of Rab4, Rab5, Rab7, Rab11, Rab18, Rab21, and Rab25 supports the conclusion that a substantial component of intracellular AQP2 is contained in endosomal membranes. The presence of Rab5, Rab7, and Rab11 was also confirmed by immunoblotting. Immuno-electron microscopy further confirmed the presence of these proteins and also showed that individual Rab proteins are present in some, but not all immunoisolated vesicles.
SNARE proteins, mediators of membrane fusion between vesicular and target membranes, have been shown to colocalize with markers of distinct membrane domains (29) and can be used as independent markers of specific subcellular compartments. The identification of endosomal syntaxins 7, 12, and 13, as well as VAMP2 and VAMP3 in immunoisolated AQP2 vesicles brings further support to the conclusion that a substantial fraction of intracellular AQP2 is contained in various endosomal compartments.
Although it appears clear that intracellular AQP2 is present in endosomes, analysis of immunoisolated AQP2 vesicles from the IMCD also revealed a large number of ribosomal and endoplasmic reticulum-resident proteins. This result demonstrates that in addition to endosomes, intracellular “AQP2 vesicles” also include the rough endoplasmic reticulum (RER). Obviously, AQP2 and other integral membrane proteins are translated at the RER and the presence of AQP2 in RER membranes implies that new AQP2 that is being produced has a sufficient residence time in the RER to manifest itself in this analysis. The presence of AQP2 in both endosomes and RER raises doubt about the interpretation of experiments that depend on differential centrifugation alone to assess the distribution of AQP2 between endosomal compartments and the plasma membrane through the determination of the ratio of AQP2 in low-density (LD) membrane to AQP2 in high-density (HD) membranes (10) (12). For example, stimuli that increase the production of AQP2 may increase the abundance of AQP2 in the low-density membrane fraction of IMCD and result in a reduced HD:LD ratio without any change in trafficking to and from the plasma membrane.
One potential route of AQP2 trafficking from the intracellular compartment to the plasma membrane could be via recycling endosomes. AQP2 can hypothetically move from the trans-Golgi network (TGN) directly into recycling endosomes. An additional possibility is that AQP2 can be translocated directly from the TGN to plasma membrane via secretory vesicles as is seen with synaptic vesicles. We did not identify Rab3, a secretory vesicle marker, by either mass spectrometry or immunoblotting. However, this result does not rule out some role for the secretory pathway in AQP2 trafficking to the plasma membrane. A negative result could be due to the fact that the secretory vesicles may move very rapidly from the TGN to the plasma membrane, as demonstrated by Lippincott-Schwartz et al (30), so that a large AQP2 flux could occur despite a low abundance of secretory vesicles. Indeed, Rab3a appears to be present in whole inner medullary homogenates (Figure 12) and previous reports have demonstrated that when sufficient amounts of intracellular membranes are loaded on an immunoblot, Rab3 is indeed detectable in inner medulla (20).
Another pathway for translocation of proteins from the TGN to the apical plasma membrane of epithelial cells has recently been proposed, viz. membrane proteins may be initially targeted to the basolateral plasma membrane via the exocyst complex and may travel to the apical plasma membrane by transcytosis (8). Our LC-MS/MS analysis did not identify any exocyst component in AQP2 vesicles. However, immunoblotting demonstrated the presence of both Sec6 and Sec8, two exocyst complex proteins in intracellular AQP2 vesicles (Figure 11). In addition, we found Ral A, an exocyst-associated small GTPase, in the immunoisolated membranes, further supporting the conclusion that some subset of AQP2 intracellular vesicles contains the exocyst complex. This complex, which is associated with trafficking to the lateral plasma membrane (31), is also present in recycling endosomes and TGN (16). Polishchuk et al (8) propose that some apically targeted proteins move to the apical plasma membrane by transcytosis after exocyst-associated basolateral targeting, and that the transcytosis is initiated by internalization via caveolae. It appears possible that this pathway could be involved in AQP2 trafficking. Conceivably, this indirect targeting model may provide an explanation to the finding in IMCD (2) (32) and earlier parts of the collecting duct system (33) that a substantial fraction of total cellular AQP2 is “mistargeted” to the basolateral plasma membrane.
Ubiquitin was readily detectable in AQP2 immunoisolated vesicles both by mass spectrometry (Table 1) and immunoblotting (Figure 10). Ubiquitin was present throughout most of the molecular weight range investigated, suggesting that ubiquitinated proteins were present in most fractions. Mono-ubiquitination is recognized as a signal that targets cargo proteins from the plasma membrane to the endosomal pathway whereas polyubiquitination targets proteins to the proteasome (34).
Another class of proteins that is relatively well represented in immunoisolated AQP2 vesicles was the myosins (Table 1). We identified nonmuscle myosin IIA and IIB as well as the unconventional myosins 1C, VI, and IXB in association with AQP2 immunoisolated vesicles. Nonmuscle myosin II is involved with organization of actin microtubules in cells and with cell shape changes. A role of nonmuscle myosin II has been proposed in vesicle budding at the TGN along with heterotrimeric G-proteins (also identified in this study) (35) (36) (37). Recent studies have implicated nonmuscle myosin II in AQP2 trafficking (38).
LC-MS/MS analysis also identified a number of heterotrimeric G-protein subunits including Gαi2, Gαi3, Gαq, Gβ1, Gβ2, and Gβ3 (Table 1). Previous studies have implicated heterotrimeric G proteins in vesicle fusion events in pancreatic zymogen granules (39) and GLUT4 vesicles (40). Additionally, Donaldson et al showed that heterotrimeric G proteins may be involved in the regulation of β-COP and ARF binding to Golgi membranes (41). Although the presence of heterotrimeric G-proteins in the collecting duct cytoplasm has been previously reported (42), a role for heterotrimeric G proteins in vasopressin-induced AQP2 trafficking, beyond their roles in G-protein coupled receptor signaling, has undergone limited investigation. Valenti et al. (43) found that Gαi may play a role in vasopressin-induced insertion of AQP2 into the apical membrane of CD8 RC.SV3 rabbit cortical collecting duct cells. However, the nature of this involvement remains unclear.
Conclusion
This study provides the first step toward establishment of the feasibility of a new approach to the investigation of membrane trafficking in cells involving a combination of ‘immunodissection’ of individual membrane compartments and large-scale protein identification by LC-MS/MS. Immunoisolation using an antibody to AQP2 yielded a heterogeneous mixture of membrane compartments, which includes both endosomes and the RER. A logical next step would be to use antibodies to marker proteins associated with specific membrane compartments to selectively identify their respective proteomes.
Acknowledgments
This work was supported by the intramural budget of NHLBI (Z01-HL-01282-KE). Maria Barile was supported by the Howard Hughes Research Scholars Program. Trairak Pisitkun was supported by an International Society of Nephrology Fellowship Award. We thank Drs. Martha Vaughan and Julie Donaldson (NHLBI) for advice and antibodies.
Footnotes
Note to referees: Supplementary Table 1 is included in this PDF document. Supplementary Table 2 is a full list of verified identifications. Supplementary Tables 3–4 are extensive lists of raw data. Supplementary Tables 2–4 can be accessed at URL: http://dir.nhlbi.nih.gov/reviews/lkem/pisitkun1.asp (login: lkem; password: reviewdata).
References
- 1.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Proceedings of the National Academy of Sciences, USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Proceedings of the National Academy of Sciences, USA. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Balkom BW, Graat MP, van Raak M, Hofman E, van der SP, Deen PM. AmJPhysiol Cell Physiol. 2004;286:C372–C379. doi: 10.1152/ajpcell.00271.2003. [DOI] [PubMed] [Google Scholar]
- 4.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. AmJPhysiol Renal Physiol. 2004;286:F233–F243. doi: 10.1152/ajprenal.00179.2003. [DOI] [PubMed] [Google Scholar]
- 5.Asai T, Kuwahara M, Kurihara H, Sakai T, Terada Y, Marumo F, Sasaki S. Kidney Int. 2003;64:2–10. doi: 10.1046/j.1523-1755.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WJ, Yeaman C. Trends Cell Biol. 2001;11:483–486. doi: 10.1016/s0962-8924(01)02145-6. [DOI] [PubMed] [Google Scholar]
- 7.Maxfield FR, McGraw TE. NatRevMolCell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 8.Polishchuk R, Di Pentima A, Lippincott-Schwartz J. NatCell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- 9.Sands JM, Nonoguchi H, Knepper MA. American Journal of Physiology. 1987;253:F823–F832. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- 10.Marples D, Knepper MA, Christensen EI, Nielsen S. American Journal of Physiology. 1995;269:C655–C664. doi: 10.1152/ajpcell.1995.269.3.C655. [DOI] [PubMed] [Google Scholar]
- 11.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. American Journal of Physiology: Renal Physiology. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Terris J, Ecelbarger CA, Chou CL, Nielsen S, Knepper MA. American Journal of Physiology. 1999;276:F559–F566. doi: 10.1152/ajprenal.1999.276.4.F559. [DOI] [PubMed] [Google Scholar]
- 13.Chou CL, DiGiovanni SR, Luther A, Lolait SJ, Knepper MA. American Journal of Physiology. 1995;268:F78–F85. doi: 10.1152/ajprenal.1995.269.1.F78. [DOI] [PubMed] [Google Scholar]
- 14.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Proceedings of the National Academy of Sciences, USA. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandon B, Nielsen S, Kishore BK, Knepper MA. American Journal of Physiology. 1997;273:F718–F730. doi: 10.1152/ajprenal.1997.273.5.F718. [DOI] [PubMed] [Google Scholar]
- 16.Zerial M, McBride H. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 17.Mullock BM, Smith CW, Ihrke G, Bright NA, Lindsay M, Parkinson EJ, Brooks DA, Parton RG, James DE, Luzio JP, Piper RC. Mol Biol Cell. 2000;11:3137–3153. doi: 10.1091/mbc.11.9.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prekeris R, Klumperman J, Chen YA, Scheller RH. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreykenbohm V, Wenzel D, Antonin W, Atlachkine V, von Mollard GF. Eur J Cell Biol. 2002;81:273–280. doi: 10.1078/0171-9335-00247. [DOI] [PubMed] [Google Scholar]
- 20.Liebenhoff U, Rosenthal W. FEBS Letters. 1995;365:209–213. doi: 10.1016/0014-5793(95)00476-p. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson JG, Jackson CL. Curr Opin Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 22.Hirling H, Steiner P, Chaperon C, Marsault R, Regazzi R, Catsicas S. Eur J Neurosci. 2000;12:1913–1923. doi: 10.1046/j.1460-9568.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Kuo YM, Gitschier J. Nat Genet. 1999;23:329–332. doi: 10.1038/15507. [DOI] [PubMed] [Google Scholar]
- 24.Grote E, Kelly RB. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen S, Marples D, Mohtashami M, Dalby NO, Trimble W, Knepper M. Journal of Clinical Investigation. 1995;96:1834–1844. doi: 10.1172/JCI118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franki N, Macaluso F, Schubert W, Gunther L, Hays RM. American Journal of Physiology. 1995;269:C797–C801. doi: 10.1152/ajpcell.1995.269.3.C797. [DOI] [PubMed] [Google Scholar]
- 27.Pandey A, Mann M. Nature. 2000;405:837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- 28.Lin D, Tabb DL, Yates JR., III Biochim Biophys Acta. 2003;1646:1–10. doi: 10.1016/s1570-9639(02)00546-0. [DOI] [PubMed] [Google Scholar]
- 29.Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- 30.Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 32.Christensen BM, Wang W, Frokiaer J, Nielsen S. Am J Physiol Renal Physiol. 2003;284:F701–F717. doi: 10.1152/ajprenal.00234.2002. [DOI] [PubMed] [Google Scholar]
- 33.Jeon US, Joo KW, Na KY, Kim YS, Lee JS, Kim J, Kim GH, Nielsen S, Knepper MA, Han JS. Nephron Exp Nephrol. 2003;93:e36–e45. doi: 10.1159/000066651. [DOI] [PubMed] [Google Scholar]
- 34.Katzmann DJ, Odorizzi G, Emr SD. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 35.Musch A, Cohen D, Rodriguez-Boulan E. J Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikonen E, de Almeid JB, Fath KR, Burgess DR, Ashman K, Simons K, Stow JL. J Cell Sci. 1997;110 ( Pt 18):2155–2164. doi: 10.1242/jcs.110.18.2155. [DOI] [PubMed] [Google Scholar]
- 37.Fath KR, Trimbur GM, Burgess DR. J Cell Biol. 1997;139:1169–1181. doi: 10.1083/jcb.139.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou, C. L., Christensen, B. M., Frische, S., Vorum, H., Desai, R. A., Hoffert, J. D., de Lanerolle, P., Nielsen, S., and Knepper, M. A. (2004) J.Biol.Chem. [DOI] [PubMed]
- 39.Sattar AA, Boinpally R, Stromer MH, Jena BP. J Biochem (Tokyo) 2002;131:815–820. doi: 10.1093/oxfordjournals.jbchem.a003170. [DOI] [PubMed] [Google Scholar]
- 40.Ferrara CM, Cushman SW. Biochem J. 1999;343(Pt 3):571–577. [PMC free article] [PubMed] [Google Scholar]
- 41.Donaldson JG, Kahn RA, Lippincott-Schwartz J, Klausner RD. Science. 1991;254:1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- 42.Stow JL, Sabolic I, Brown D. Am J Physiol. 1991;261:F831–F840. doi: 10.1152/ajprenal.1991.261.5.F831. [DOI] [PubMed] [Google Scholar]
- 43.Valenti G, Procino G, Liebenhoff U, Frigeri A, Benedetti PA, Ahnert-Hilger G, Nurnberg B, Svelto M, Rosenthal W. J Biol Chem. 1998;273:22627–22634. doi: 10.1074/jbc.273.35.22627. [DOI] [PubMed] [Google Scholar]


