Abstract
We investigated the mechanisms by which inhibitors of prostaglandin G/H synthase-2 (PGHS-2; known colloquially as COX-2) increase the incidence of myocardial infarction and stroke. These inhibitors are believed to exert both their beneficial and their adverse effects by suppression of PGHS-2–derived prostacyclin (PGI2) and PGE2. Therefore, the challenge remains to identify a mechanism whereby PGI2 and PGE2 expression can be suppressed while avoiding adverse cardiovascular events. Here, selective inhibition, knockout, or mutation of PGHS-2, or deletion of the receptor for PGHS-2–derived PGI2, was shown to accelerate thrombogenesis and elevate blood pressure in mice. These responses were attenuated by COX-1 knock down, which mimics the beneficial effects of low-dose aspirin. PGE2 biosynthesis is catalyzed by the coordinate actions of COX enzymes and microsomal PGE synthase-1 (mPGES-1). We show that deletion of mPGES-1 depressed PGE2 expression, augmented PGI2 expression, and had no effect on thromboxane biosynthesis in vivo. Most importantly, mPGES-1 deletion affected neither thrombogenesis nor blood pressure. These results suggest that inhibitors of mPGES-1 may retain their antiinflammatory efficacy by depressing PGE2, while avoiding the adverse cardiovascular consequences associated with PGHS-2–mediated PGI2 suppression.
Introduction
As prostaglandin G/H synthase-2 (PGHS-2) is not expressed in mature human platelets (1), the effects of platelet PGHS-1–derived thromboxane A2 (TXA2) and other endogenous factors that promote platelet activation, vasoconstriction, and vascular proliferation may be amplified by PGHS-2 inhibitors, affording a mechanism for their cardiovascular effects (2–4). Despite these observations, the mechanistic hypothesis to explain the cardiovascular hazard from these drugs remains controversial. Firstly, PGHS-2 inhibitors depress prostacyclin (PGI2) biosynthesis substantially, but incompletely in humans (5–7), so it is unclear whether mice entirely deficient in the PGI2 receptor (IP) would predict faithfully their effects. Secondly, it remains to be demonstrated that PGHS-2 inhibition or disruption — as opposed to IP deletion (8) — alters the response to thrombogenic stimuli in mice and whether this hazard is mitigated by inhibition of PGHS-1–derived TXA2. The cardiovascular hazard from PGHS-2 inhibitors has also fostered interest in microsomal PGE synthase-1 (mPGES-1) as a drug target (9). However, it is unknown whether such selective inhibition of PGE2 would replicate the cardiovascular consequences of PGHS-2 inhibition. Here we report that mPGES-1 deletion, unlike disruption, deletion, or inhibition of PGHS-2, fails to alter blood pressure or modulate the response to a thrombogenic stimulus. These observations raise the possibility that inhibitors of mPGES-1 may be less prone to adverse effects involving the cardiovascular system than are specific inhibitors of PGHS-2.
Results
Impact of PGHS inhibition, knock down, mutation, or knockout on eicosanoid biosynthesis.
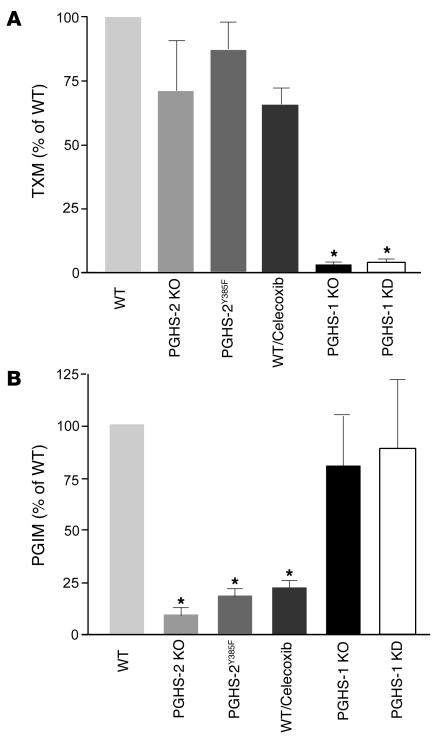
We addressed the relative contribution of the PGHS enzymes to indices of PGI2 and TX biosynthesis, urinary 2,3-dinor-6-keto PGF1α (PGIM) and 2,3-dinor-TXB2 (TXM), respectively (10), using mice deficient in either PGHS enzyme or treated with celecoxib or 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone (DFU), highly selective PGHS-2 inhibitors (11, 12). We also used a PGHS-1 knockdown (KD) mouse that mimics the effect of low-dose aspirin, achieving a more than 95% mean inhibition of platelet TX formation (13), and mutant PGHS-2Y385F mice, in which the COX, but not the peroxidase (pox), function of the enzyme is inactivated, thereby mimicking the effect of a selective PGHS-2 inhibitor (14). While TXM is markedly depressed by PGHS-1 knockout (KO) or KD, it is not altered significantly in PGHS-2 KO or PGHS-2Y385F mice or by treatment with the selective PGHS-2 inhibitors (Figure 1 and Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI27540DS1). PGIM, by contrast, is suppressed substantially by PGHS-2 inhibition or KO and in PGHS-2Y385F mice. Thus, PGHS-1 is the dominant source of TXM and PGHS-2 of PGIM in mice, as in humans (5, 15, 6).
Figure 1. PGHS enzymes and eicosanoid biosynthesis.
(A) Urinary excretion of TXM was decreased significantly (n = 6; *P < 0.001) from values in WT mice by PGHS-1 deletion (KO) or knock down (KD), but not in PGHS-2 KO or PGHS-2Y385F mice or those treated with 100 mg/kg/d of the PGHS-2 inhibitor celecoxib for 30 days on a mixed C57BL/6 × 129/Sv genetic background. (B) Urinary PGIM was depressed significantly in PGHS-2 KO and PGHS-2Y385F mice and by celecoxib, but not in PGHS-1 KO or KD mice.
IP deletion modulates vasoactivity, platelet aggregation, and thrombogenesis in gene/dose dependency.
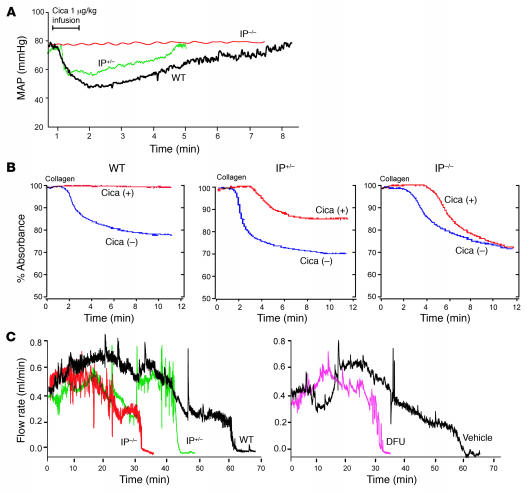
We studied the cardiovascular effects of deleting just 1 copy of the IP on cardiovascular function in vivo. The blood pressure response to an IP agonist, cicaprost (16), was reduced in a gene/dose-dependent manner (Figure 2A). A similar gene/dose-dependent effect of IP deletion was seen on the inhibitory impact of 10 nM cicaprost on platelet aggregation induced by 2 μg/ml collagen ex vivo (Figure 2B). The impact of IP deletion and PGHS-2 inhibition on the time to thrombotic carotid arterial occlusion after green laser activation of rose bengal and consequent free radical–catalyzed vascular injury was also gene/dose dependent. Inhibition of PGHS-2 also accelerated thrombogenesis, to a degree intermediate between that seen in IP+/– and that seen in IP–/– mice (Figure 2C).
Figure 2. Suppressive effects of IP deletion on vascular reactivity, platelet aggregation, and thrombogenesis are gene/dose dependent.
(A) The maximum decline (P < 0.0001) and duration (P < 0.005) of hypotension evoked in mean arterial pressure (MAP) by 1 μg/kg of IP agonist, cicaprost (Cica), was greater in WT than in IP+/– or IP–/– mice on a C57BL/6 background. (B) Platelet aggregation was initiated with 2 μg/ml collagen with (+) or without (–) pretreatment with 10 nM Cica. Inhibition of aggregation was not evident in IP–/– mice and averaged 86% of WT in IP+/– mice (P < 0.0001). (C) Carotid artery blood flow after photochemical injury. The time to complete occlusion after rose bengal dye injection fell from 66.3 ± 5.1 minutes in WT to 44.4 ± 7 minutes in IP+/– and to 29.7 ± 7.6 minutes in IP–/– mice (P < 0.006). The mean impact of the COX-2 inhibitor, DFU (10 mg/kg for 3 days), on time to occlusion (56.2% of WT value) was intermediate between that of IP+/– (68.1%) and IP–/– (45.5%) mice.
Impact of PGHS-2 disruption or inhibition on thrombogenesis and blood pressure regulation: PGHS-1 KD attenuates these effects in vivo.
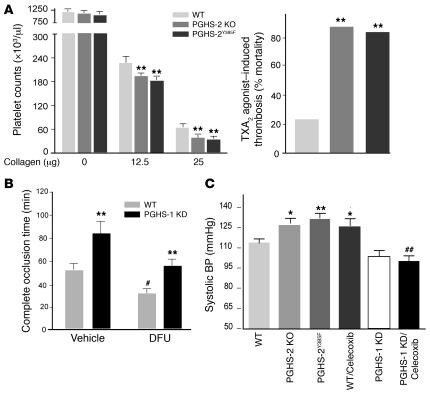
Both the fall in platelet count following injection of the platelet agonist, collagen — reflecting platelet consumption in a developing thrombus — and the frequency of sudden death induced by an i.v. dose of a TX receptor (TP) agonist, U46619, were augmented in PGHS-2 KO and PGHS-2Y385F mice (Figure 3A). Furthermore, the prolongation in bleeding time — an index of platelet–vessel wall interactions — induced by LPS administration (2 mg/kg i.p.) to WT mice (2.0 ± 0.2 minutes versus 8.9 ± 2.0 minutes, n = 14; P < 0.001) was abolished in PGHS-2Y385F mice (1.99 ± 0.27 minutes versus 2.3 ± 0.78 minutes, n = 6–7; P = NS). Genetic simulation of low-dose aspirin by PGHS-1 KD delayed the response to a thrombogenic stimulus. By contrast, acceleration of the time to thrombotic carotid vascular occlusion occurred with the PGHS-2 inhibitor DFU (Figure 3B), but this effect was attenuated by PGHS-1 KD. This suggests that the risk of thrombosis from selective inhibition of PGHS-2 would be attenuated — but not abolished — by low-dose aspirin.
Figure 3. PGHS-2 disruption or inhibition promotes thrombogenesis and hypertension and modulation effect of PGHS-1 KD.
(A) Circulating platelets before and 2 minutes after injection of collagen (12.5 and 25 μg/kg) plus epinephrine (15 μg/ml, 100 μl) into WT, PGHS-2Y385F, and PGHS-2 KO mice on a mixed C57BL/6 × 129/Sv background. Thrombocytopenia was more pronounced (**P < 0.01) in PGHS-2–disrupted mice. Sudden death was induced more commonly (**P < 0.01) within 15 minutes of U46619 injection in PHGS-2–deleted or –mutated mice. (B) The time to thrombotic carotid artery occlusion after photochemical injury was delayed in PGHS-1 KD mice (**P < 0.01) but accelerated by DFU treatment (#P < 0.05). The time to occlusion in DFU-treated animals was delayed in the PGHS-1 KD group compared with WT controls (**P < 0.01), while the time to occlusion in DFU-treated PGHS-1 KD mice did not differ significantly from that in vehicle-treated WT controls on a mixed C57BL/6 × 129/Sv background. (C) Systolic blood pressure, as measured by the tail cuff method, was elevated significantly in 3-month-old PGHS-2 KO, PGHSY385F, and celecoxib-treated (100 mg/kg/d for 30 days) mice as compared with WT mice on a mixed C57BL/6 × 129/Sv background (*P < 0.05; **P < 0.01). The hypertensive effect of celecoxib was attenuated in PGHS-1 KD mice compared with that in WT (##P < 0.01) mice.
The risk of hypertension on NSAIDs may relate frequently to inhibition of PGHS-2 and the selectivity with which it is attained (17). Hypertension has been reported in patients receiving both traditional NSAIDs (tNSAIDs) and those selective for inhibition of PGHS-2 (18, 19). Hypertension reported as an adverse event relates to dose in patients receiving either celecoxib or rofecoxib, with a more pronounced signal on the latter drug. While this reflects the relative degree of selectivity for inhibition of PGHS-2, it is confounded with the duration of drug exposure in this comparison (20). Hypertension was more common in patients taking PGHS-2–selective drugs than in those taking tNSAIDs in an observational study (21). Here, blood pressure was elevated by PGHS-2 deletion or mutation or by treatment with the PGHS-2 inhibitor celecoxib compared with that of WT controls on a regular chow diet. Blood pressure was unaltered in PGHS-1 KD mice, but the hypertensive effect of celecoxib was attenuated in these mice (Figure 3C). Thus, selective disruption or deletion of PGHS-2, just like deletion of the IP (22), can result in an elevation of blood pressure in mice, and this effect is attenuated by genetic mimicking of the impact of low-dose aspirin. This contrasts with the impact of TP deletion on the hypertensive response to IP deletion. While disruption of the TP prevents the consequent myocardial injury, it does not alter the rise in blood pressure in IP KO mice (22). These results suggest the importance of suppression of products additional to PGI2 (such as PGE2 acting via the E prostanoid receptor 2 (EP2) or PGD2 acting via the D prostanoid receptor 1) in the hypertensive response to PGHS-2 inhibition or disruption and/or the role of suppression of products additional to TXA2 (such as PGE2 acting via EP1 or PGF2α acting via the F prostanoid receptor) in the antihypertensive impact of PGHS-1 KD.
The major urinary metabolite of PGE2 and the role of PGHS and mPGES-1 enzymes in PGE2 biosynthesis.
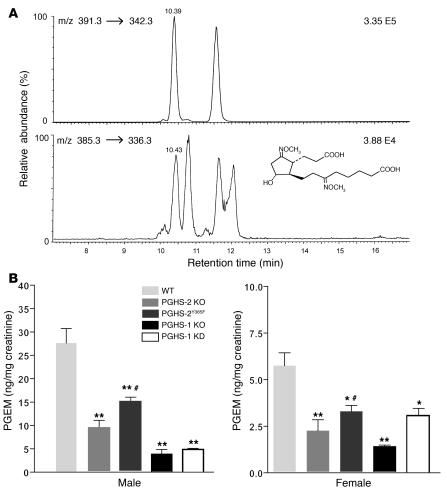
Unlike PGI2, both PGHS-1 and PGHS-2 each contribute substantially to PGE2 biosynthesis. Using a mass spectrometric assay (Figure 4A) for the major PGE metabolite 11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid (PGEM), we found that PGHS-1 KO or KD, as well as PGHS-2 KO or mutation or inhibition of PGHS-2 with DFU, depressed PGEM significantly (Figure 4B and Supplemental Figure 1). The mPGES-1 enzyme, which colocalizes with both PGHS enzymes (23, 24), is a major source of urinary PGEM. Urinary PGEM was significantly lower in both male mPGES-1 KO mice (82.1 ± 7.5 ng/mg creatinine in WT versus 18.8 ± 3.8 ng/mg creatinine in KO, n = 7–8 mice per group; P < 0.001) (Figure 5C) and female mPGES-1 KO mice (28.0 ± 2.2 ng/mg creatinine in WT versus 15.8 ± 2.2 ng/mg creatinine in KO, n = 11 mice per group; P < 0.001) than in WT mice.
Figure 4. The major urinary metabolite of PGE2 and the suppressive effect of PGHS-1 disruption or KD and PGHS-2 disruption or mutation on PGE2 biosynthesis.
(A) A selected ion-monitoring trace of the methoxime derivative of endogenous PGEM (9,15-dioxo-11α-hydroxy-2,3,4,5-tetranor-prostane-1,20-dioic-17,17,18,18,19,19-d6 acid) (bottom panel) and its hexadeuterated internal standard (top panel). (B) Urinary PGEM decreased significantly in both male and female PGHS-2 KO or PGHS-2Y385F mice compared with WT controls on a mixed C57BL/6 × 129/Sv genetic background (n = 5–6; *P < 0.05; **P < 0.001). PGEM was also significantly lower in PGHS-1 KD and PGHS-1 KO groups compared with WT mice of mixed C57BL/6 × 129/Sv genetic background (n = 5–6; *P < 0.05; **P < 0.001). PGEM was significantly higher in PGHS-2Y385F mice compared with PGHS-2 KO mice (#P < 0.05) on the same genetic background.
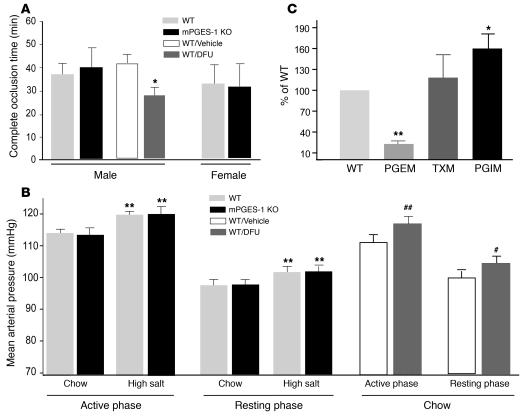
Figure 5. Impact of mPGES-1 disruption on thrombogenesis, blood pressure regulation, and eicosanoid biosynthesis.
(A) Deletion of mPGES-1 failed to alter the time to thrombotic carotid artery occlusion after photochemical injury, while it was accelerated by the PGHS-2 inhibitor DFU in mice on a DBA/11acJ background (*P < 0.05). (B) MAP exhibited diurnal variation in mPGES-1 KO and WT littermates on a mixed DBA/11acJ × C57BL/6 background. MAP was averaged over 4 days for 12 hours dark (active phase) and light (resting phase) periods. MAP was higher during active phase, and a high-salt diet elevated pressure similarly, a mean 6% in both groups (**P < 0.01). Oral DFU administration (10 mg/kg/d) for 21 days increased MAP in both the active (##P < 0.01) and resting (#P < 0.05) phases compared with vehicle-treated animals. (C) Urinary PGEM was lower (**P < 0.01) and PGIM was higher (*P < 0.05), while TXM was unaltered in male mPGES-1 KO mice compared with gender-matched WT littermates on a DBA/11acJ background.
Impact of mPGES-1 deletion on thrombogenesis, blood pressure regulation, and eicosanoid biosynthesis.
While deletion of the IP augments the response to thrombogenic or hypertensive stimuli, depression of PGE2 might also influence cardiovascular function. Thus, deletion of the EP2 receptor (25), like that of the IP (22, 26), increases salt sensitivity in mice. In addition to effects on blood pressure, low concentrations of PGE2 activate platelets via the EP3, while higher concentrations inhibit platelet function by ligating the IP (27). Unlike inhibition of PGHS-2, deletion of mPGES-1 failed to alter thrombogenesis in mice of either sex (Figure 5A). We examined the potential impact of mPGES-1 deletion by both tail cuff (Supplemental Figure 2) and telemetric approaches. Blood pressure was assessed continuously in male mice on both normal (0.6% NaCl) and high-salt (8% NaCl) diets. An impact of mPGES-1 deletion was not detected. Mean arterial blood pressure (MABP) rose significantly with activity on both dietary regimens and rose with high-salt feeding in both WT and mPGES-1 KO groups. As with the earlier experiments with celecoxib, 10 mg/kg/d DFU significantly augmented MABP in both active and resting phases in WT controls, when the experiments were performed in mice with a genetic background common with that of the mPGES-1 KO mice (Figure 5B).
Deletion (or inhibition) of a PG synthase enzyme, such as mPGES-1, may result in accumulation of the PGHS product PGH2, rendering it available for rediversion to other vasoactive PGs. Such a phenomenon has been reported in LPS-stimulated macrophages obtained from mPGES-1 KO mice, in which formation of both TXA2 and PGI2 was augmented, coincident with suppression of PGE2 (28). We have replicated these findings in murine macrophages ex vivo but find that, unlike in macrophages, only PGI2 formation is augmented in unstimulated mPGES-1–deleted VSMCs (E. Ricciotti et al., unpublished observations). Thus, the dominant product of PGH2 rediversion varies according to cell type. Here, we compared urinary excretion of PGIM and TXM — indices of total-body biosynthesis of the eicosanoids — between mPGES-1 KOs and WT controls. While urinary TXM was unaltered by mPGES-1 deletion (335.7 ± 43.4 ng/mg creatinine in WT versus 359.7 ± 52.5 ng/mg creatinine in KO, n = 14–17 mice per group; P = 0.98), PGI2 biosynthesis, reflected by urinary PGIM, was increased significantly (6.2 ± 1 ng/mg creatinine in WT versus 10 ± 1.3 ng/mg creatinine in KO, n = 8 mice per group; P < 0.05) (Figure 5C).
A marked sex difference was noted in PGEM (82.1 ± 7.5 ng/mg creatinine in males versus 20.1 ± 3.8 ng/mg creatinine in females, n = 7–12 per group; P < 0.001) in WT mice. However, deletion of mPGES-1 depressed PGEM in both sexes. Interestingly, a similar sex difference in TXM (335.7 ± 43.4 ng/mg creatinine in males versus 706.3 ± 86.7 ng/mg creatinine in females, n = 12–20 per group; P < 0.05), but not in PGIM (6.2 ± 1.0 ng/mg creatinine in males versus 5.5 ± 1.1 ng/mg creatinine in females, n = 8–12 per group; P = 0.68), was also noted in mice on this DBA/1lacJ background.
Discussion
Evidence has emerged for a cardiovascular hazard with specific inhibitors of PGHS-2 from placebo-controlled trials. This is consistent with the original prediction that such a complication would result from suppression of PGI2, but not TXA2, in patients treated with these drugs (5, 6). The plausibility of this argument rested heavily on integrating the biochemical measurements of PGI2 biosynthesis in humans with results obtained in mice lacking the IP. However, although PGI2 was indeed shown to modulate the cardiovascular effects of TXA2 in mice (2), concern was expressed as to the relevance of IP deletion to substantial but incomplete inhibition of PGI2 biosynthesis during treatment with PGHS-2 inhibitors.
Here, evidence derived from distinct pharmacological inhibitors and genetic models of PGHS inactivation indicates that PGHS-2 is indeed the dominant source of PGI2 in vivo, as is PGHS-1 of TXA2. Furthermore, inhibition, deletion, or inactivation of PGHS-2 augments the response to thrombogenic stimuli in 3 distinct experimental settings. In 1 such model, the thrombotic phenotype evoked by the PGHS-2 inhibitor is roughly intermediate between those resulting from hetero- and from homozygous deletion of the IP. Neither disruption of PGHS-2 nor IP deletion results in spontaneous thrombosis, but, rather, both augment the response to thrombogenic stimuli. Thus, an unrelated predisposition to thrombosis would facilitate detection of a cardiovascular hazard from PGHS-2 inhibitors, as has been observed in patients (29–31).
While inhibition of PGHS-2 accelerates thrombogenesis, this effect is attenuated, but not abolished, by PGHS-1 KD. Adequately powered clinical trials designed to determine whether aspirin mitigates the cardiovascular hazard from PGHS-2 inhibitors in humans have not been performed. Indeed, aspirin use has only been prespecified in 1 randomized trial of a PGHS-2 inhibitor, the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) (32). Here, the relative risk of myocardial infarction in patients receiving the PGHS-2 inhibitor lumiracoxib versus the tNSAID naproxen fell from 2.37 to 1.36 when low-dose aspirin was combined with the coxib.
The demonstration that mPGES-1 deletion is as effective as tNSAIDs in models of pain and inflammation, including collagen-induced arthritis (9), suggests that such inhibitors might represent alternatives to coxibs, potentially devoid of their cardiovascular hazard. However, while inhibition of PGE2-dependent platelet activation via EP3 may be desirable, inhibition of activation of the IP by PGE2 (27) is not. Similarly, suppression of PGE2, perhaps even more than PGI2, is thought to account for hypertension on NSAIDs, including those selective for PGHS-2. Here, although mPGES-1 is indeed a major, but not exclusive, source of PGE2, thrombogenesis is not accelerated in mPGES-1–/– mice. Blood pressure was also unaltered by mPGES-1 deletion, even in response to a high-salt diet. It is possible that this lack of a cardiovascular phenotype in the mPGES-1 KOs merely reflects the unimportance of PGE2 as a constraint on thrombogenic and hypertensive stimuli. However, it may be that accumulated PGH2 substrate in the setting of mPGES-1 deletion is redirected to augment PGI2 biosynthesis, thereby mitigating the loss of PGE2. Here, we show that urinary PGIM is significantly elevated in mPGES-1 KOs, without a significant alteration in urinary TXM. While this increase is modest — approximately 50% — we have also shown that loss of just 1 copy of the IP gene results in a detectable cardiovascular phenotype. Despite these findings, the profile of rediversion products formed and the consequent cardiovascular effects of mPGES-1 inhibition may differ when the relative predominance of different cell types varies in distinct clinical settings.
Similarly, we demonstrate contrasting effects on blood pressure of mPGES-1 deletion and PGHS-2 inhibition in mice sharing the same genetic background. However, it is well established that genetic background may influence manipulations of PG biosynthetic/response genes in mice (17, 33) and that the occurrence and severity of a hypertensive response to NSAIDs are quite variable in humans. Thus, these results do not preclude the possibility that interindividual variation in genetic modifiers might predispose some individuals to a cardiovascular response to mPGES-1 inhibitors.
Biosynthesis of PGE2, as reflected by excretion of urinary PGEM, differs markedly between males and females in mice, as in humans (34). While deletion of mPGES-1 depresses urinary PGEM in both sexes, the biological implications of this difference — and, indeed, sex-dependent differences in other prostanoid functions (35) — and the implications of these differences for therapy remain unexplored. However, a potential adverse outcome of inhibition of synthesis of PGE2 could be an increase in peptic ulceration.
In summary, inhibition of PGHS-2–derived PGI2 augments the response to thrombogenic stimuli in vivo and may contribute to hypertension on NSAIDs. Both of these effects are attenuated when the impact of low-dose aspirin is mimicked genetically. Deletion of mPGES-1, in contrast to PGHS-2 inhibition, deletion, or disruption, does not alter blood pressure or predispose to thrombosis. This may result from diversion of the PGH2 substrate to PGI synthase, augmenting the production of PGI2.
Methods
Animals
In all cases, transgenic mice deficient in the indicated gene were compared with appropriate strain-, age-, and sex-matched control animals. The investigator was unaware of the genotype throughout the experiment. All procedures were approved and animal husbandry was overseen by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Generation of PGHS-1 KD and PGHS-2Y385F mutant mice
PGHSs comprise a COX activity, which catalyzes the conversion of arachidonic acid to PGG2, and a POX activity, which reduces PGG2 to PGH2. Traditional NSAIDs target both PGHS-1 and PGHS-2 by inhibition of COX activity, leaving POX activity intact (36, 37). Similarly, NSAIDs selective for PGHS-2 do not affect POX activity. Others have shown that Tyr385 in PGHS (ovine PGHS-1 numbering) is critical for COX catalysis but is uninvolved in POX activity (36–38). We have generated PGHS-2Y385F mutant mice, replacing Tyr385 with phenylalanine using a homologous recombination strategy (14). All of these mice were maintained on a mixed C57BL/6 × 129/Sv genetic background, and the WT controls were generated from heterozygous PGHS-2Y385F mice.
Since insertion of a Neo within intronic sequences could generate a hypomorphic allele or “knock down” of gene expression (39), we used PGHS-1 KD mice with Neo insertion in the PGHS-1 intron 10 sequence as described previously (13).
IP KO and mPGES-1 KO mice
IP KO mice were backcrossed into a C57BL/6 genetic background (2). IP–/–, IP+/–, and WT littermates were identified in litters generated by the intercross of IP+/– animals by PCR analysis of genomic DNA isolated from tail biopsy samples. Southern blot analysis confirmed the IP gene copy number. mPGES-1 KO mice were kindly provided by Pfizer. They were generated and maintained on the DBA/1lacJ genetic background (9) or on a mixed DBA/1lacJ × C57BL/6 genetic background. Heterozygous animals were intercrossed, and the litters were screened by PCR analysis to identify both mPGES-1 KO and WT controls. Biochemical and functional analyses in each case were performed on mutant mice and WT littermate controls.
PGHS-2 inhibitors
For the selective PGHS-2 inhibitor treatment, mice were fed ad libitum a normal chow diet containing 800 ppm celecoxib as described in the figure legends. The selectivity of this dosage has been reported previously (40–42) and was confirmed in this study (Figure 1A). Another selective PGHS-2 inhibitor is DFU, which was prepared in 1% methylcellulose, and mice were treated with 10 mg/kg/d by oral administration as described in the figure legends. DFU is a highly selective PGHS-2 inhibitor in vitro (12). The selectivity of the dosage regimen that we used has been reported previously (43–45) and was confirmed in this study (Supplemental Figure 1).
Eicosanoid analyses
Urinary TXM and PGIM were measured in 24-hour urine collected in metabolic cages. The urinary 2,3-dinor-TXB2 metabolite (TXM) was measured using a stable isotope dilution reverse-phase (C18) HPLC-coupled tandem mass spectrometry assay (Finnigan Quantum Ultra AM; Thermo Electron Corp.) (2), whereas PGIM was measured by gas chromatography/mass spectrometry (Hewlett-Packard Co.) after extraction and purification by thin-layer chromatography (46). Urinary PGEM was measured by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS; Finnigan Quantum Ultra AM; Thermo Electron Corp.) as follows: First, 10 ng of hexadeuterated PGEM (9,15-dioxo-11α-hydroxy-2,3,4,5-tetranor-prostane-1,20-dioic-17,17,18,18,19,19-d6 acid; Cayman Chemical Co.) was added to 0.1 ml of mouse urine. Next, 50 μl of methoxamine HCl, 1 g/ml, was added, and the sample was mixed and allowed to stand at room temperature for 15 minutes. The sample was then diluted to 1.0 ml with water and loaded on a Strata-X solid-phase extraction (SPE) cartridge (Phenomenex). The cartridge was eluted with 1.0 ml of 5% acetonitrile in ethyl acetate, dried, dissolved in 200 μl 10% acetonitrile in water, and injected for LC/MS/MS analysis. Transitions monitored were m/z 385→336 for the endogenous PGEM and 391→342 for the internal standard.
Models of thrombogenesis
Photochemical vascular injury.
This model is an adaptation of one previously described (13, 47). Briefly, mice (12–16 weeks old) were anesthetized with sodium pentobarbital (80 mg/kg, i.p.). After a midline cervical incision, the left common carotid artery was isolated, and a Doppler flow probe (model 0.5 VB; Transonic Systems Inc.) was applied. The probe was connected to a flowmeter (model T105; Transonic Systems Inc.) and interpreted with a computerized data acquisition program (PowerLab; ADInstruments). Rose bengal (Fisher Scientific International) was diluted in PBS and then injected into the jugular vein in a volume of 0.12 ml at a concentration of 50 mg/kg. Just before injection, a 1.5-mW green light laser (540 nm) (Melles Griot) was applied to the desired site of carotid artery injury from a distance of 5 cm, and blood flow was monitored for 120 minutes or until stable occlusion occurred, after which the mice still showing blood flow were assigned a value of 120 minutes. Stable occlusion was defined as a blood flow of 0 ml/min for 3 minutes. To confirm occlusive thrombosis, carotid arterial segments subjected to injury were excised and embedded in paraffin. Sections were then stained with H&E.
Collagen-induced platelet consumption.
Briefly, mice (8 weeks old) were weighed and anesthetized with sodium pentobarbital (80 mg/kg). One hundred microliters of a mixture of collagen (250 μg/ml) and epinephrine (15 μg/ml) in 0.9% NaCl was injected rapidly into the tail vein. Blood was collected from the inferior vena cava after 2 minutes and anticoagulated with one-sixth volume of tripotassium EDTA. After thorough mixing, platelets were counted by automated multispecies hematology analyzers (CDC Technologies Inc.), as previously described (48).
U46619-induced sudden death.
Again this was based on a model established previously (49). Briefly, mice (3–4 months old) were anesthetized with sodium pentobarbital. Then they received a rapid i.v. injection of U46619 (0.2 mg/kg in PBS; Cayman Chemical Co.) via the tail vein. Heart rate was monitored for 15 minutes before sacrifice. The mice that did not die within this time period were recorded as survivors.
Tail bleeding time.
Two milligrams per kilogram LPS (Sigma-Aldrich) or saline vehicle was administered i.p. to mice (10–12 weeks old), and, 6 hours later, bleeding time was measured by the tail clip method (13). Briefly, mice were anesthetized i.p. with pentobarbital (80 mg/kg), and tails were transected 1 mm from the tip with a sterile scalpel blade. The remaining tail was immersed in 37°C saline, and the time until bleeding stopped for a period of at least 1 minute was observed and recorded. The experiment was terminated after 20 minutes, after which any mice still bleeding were assigned a value of 20 minutes for the purpose of statistical analysis.
Platelet aggregation assay.
Blood was isolated from the inferior vena cava of anesthetized 10- to 12-week-old mice (80 mg/kg sodium pentobarbital) using a heparinized syringe (15 U/ml blood). Two hundred fifty microliters of blood was mixed with 750 μl of sodium chloride at 37°C. Platelet aggregation was performed as previously described (50–52) using a 500 Whole Blood Lumi-Aggregometer System (Chrono-Log Corp.). Samples were pretreated with or without 10 nM cicaprost for 1 minute, and aggregation was initiated by addition of 2 μg/ml collagen (Chrono-Log Corp.).
Blood pressure measurements
Tail cuff measurement.
Resting systolic blood was measured in conscious mice (3–4 months old) using a computerized noninvasive tail cuff system (Visitech Systems Inc.). The validity of this system has been demonstrated previously (25, 53). Mice were adapted to the system for 14 days (measurements of sessions 25 minutes in length were done once each day between 1500 and 1800 hours). After that, blood pressure was recorded daily for 3 consecutive days in the same way. Data were collected and analyzed using updated BP-2000 software (Visitech Systems Inc., http://www.visitechsystems.com/).
Telemetry.
This approach is based on prior studies with minor modifications (54, 55). Briefly, male mice (3–4 months old) were anesthetized using ketamine (100 mg/kg, i.p.) and acepromazine (5 mg/kg, i.p.) and were subjected to surgery under strict sterile conditions. A horizontal incision (right blade to mid-scapular) was made on the back, and the telemetry probe (TA11-PA20; Transoma Medical Inc. — Data Sciences International) was inserted. The probe was secured by suturing of the 3 suture holes on the probe to the skin, along with an additional suture that ran through the muscle and looped around the body of the probe and through the first suture hole. This prevented the probe from sliding laterally down the side of the mouse. A vertical incision was then made on the neck, and the tips of fine hemostats were advanced underneath the skin to the incision on the back and externalized. The flexible tip of the transmitter catheter was gently grasped and pulled through, so that it protruded through the incision on the neck. The left common carotid artery was then isolated. The tip of the catheter was inserted into the common carotid lumen and advanced until the catheter notch reached the level of the carotid bifurcation. The transmitter signal was monitored with an AM radio tuned to the low end of the dial to verify the proper catheter placement. A pulsing tone indicated proper catheter placement. After surgery, mice were maintained on normal salt intake (0.6% NaCl; diet no. 8746; Harlan Teklad) for a 1-week period, after which the telemetry probes were turned on. The cage with the animal was placed on a receiver plate, and the signal was collected using the Dataquest LabPRO acquisition system (version 3.01; Transoma Medical Inc. — Data Sciences International). Mice were maintained on a 12-hour light/dark regimen, and in a sound-attenuated room. Ten-second waveforms of mean arterial pressure, diastolic arterial pressure, systolic arterial pressure, heart rate, and locomotor activity were sampled every 5 minutes during the 4-day monitoring periods, hourly averages and SD were calculated, and then all data were expressed as values averaged from daytime (resting phase) and nighttime (active phase) measurements. After this baseline data collection, the probes were then turned off, and the mice were fed a high-salt diet (8% NaCl; diet no. 5008; Harlan Teklad) for 1 week, after which the probes were turned on and the data were collected for an additional 4 days.
Direct measurement of blood pressure.
Mice were anesthetized (100 mg/kg ketamine, 5 mg/kg acepromazine) and placed on a temperature-controlled panel. The right internal jugular vein and left carotid artery were cannulated with PE-10 tubing. The arterial catheter was connected to a Capto SP844 pressure transducer (GE Healthcare — Capto), and blood pressure was monitored continuously with a PowerLab/8SP system (ADInstruments), as previously described (56, 57). Blood pressure and heart rate were continuously monitored for 20–40 minutes until stable values were obtained. After the equilibration period and the recording of baseline blood pressure, mice were injected via the right internal jugular vein with cicaprost (1 μg/kg in 4 ml/kg saline) as a bolus, and the same volume of saline was injected before cicaprost administration to exclude volume-mediated blood pressure changes. The blood pressure was continuously recorded until it returned to pretreatment baseline.
Statistics
Statistical analyses were performed by 1-way ANOVA, followed by a pairwise comparison and/or adjustment for multiple comparisons, as appropriate and using a computerized software package (GraphPad Prism version 4.0). All values were expressed as mean ± SEM. A value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We wish to thank Ekaterina Kostetskaia, Ping Liu, Helen Zhou, Jinjin Fan, Margaret Lucitt, and Weili Yan for technical support. This study was supported by grants (HL62250, HL70128, and GM063130) from the NIH and the Canadian Institutes for Health Research (MOP-79459), by the Heart and Stroke Foundation of Ontario, and in part by a grant from Merck. G.A. FitzGerald is the Elmer Bobst Professor of Pharmacology, and C.D. Funk holds a Canada Research Chair in Molecular, Cellular and Physiological Medicine and is a Career Investigator of the Heart and Stroke Foundation of Canada. The mPGES-1 KO mice were kindly provided by L. Audoly at Pfizer.
Footnotes
Nonstandard abbreviations used: DFU, 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone; IP, prostacyclin (PGI2) receptor; KD, knockdown; LC/MS/MS, liquid chromatography/mass spectrometry/mass spectrometry; mPGES-1, microsomal PGE synthase-1; PGEM, 11α-hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid; PGHS, prostaglandin G/H synthase; PGI2, prostacyclin; PGIM, 2,3-dinor-6-keto PGF1α; POX, peroxidase; tNSAID, traditional NSAID; TP, thromboxane receptor; TX, thromboxane; TXM, 2,3-dinor-T2.
Conflict of interest: G.A. FitzGerald receives financial support for investigator-initiated research from Bayer, Merck, and Boehringer Ingelheim, all of which manufacture drugs that target COXs. G.A. FitzGerald is a member of the Steering Committee of the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) Study Program. This author also serves as a consultant for Johnson & Johnson, Bayer, Merck, GlaxoSmithKline, Novartis, Boehringer Ingelheim, and NiCox. The other authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:1391–1399 (2006). doi:10.1172/JCI27540.
Yan Cheng, Miao Wang, and Ying Yu contributed equally to this work.
References
- 1.Patrignani P., et al. COX-2 is not involved in thromboxane biosynthesis by activated human platelets. J. Physiol. Pharmacol. 1999;50:661–667. [PubMed] [Google Scholar]
- 2.Cheng Y., et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald G.A. Coxibs and cardiovascular disease. N. Engl. J. Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 4.Grosser T., Fries S., FitzGerald G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdam B.F., et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. U. S. A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catella-Lawson F., et al. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J. Pharmacol. Exp. Ther. 1999;289:735–741. [PubMed] [Google Scholar]
- 7.Cullen L., Kelly L., Connor S.O., Fitzgerald D.J. Selective cyclooxygenase-2 inhibition by nimesulide in man. J. Pharmacol. Exp. Ther. 1998;287:578–582. [PubMed] [Google Scholar]
- 8.Murata T., et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 9.Trebino C.E., et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan K.M., et al. Cyclooxygenases, thromboxane, and atherosclerosis: plaque destabilization by cyclooxygenase-2 inhibition combined with thromboxane receptor antagonism. Circulation. 2005;111:334–342. doi: 10.1161/01.CIR.0000153386.95356.78. [DOI] [PubMed] [Google Scholar]
- 11.Penning T.D., et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib). . J. Med. Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 12.Riendeau D., et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br. J. Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y., et al. Differential impact of prostaglandin H synthase 1 knockdown on platelets and parturition. J. Clin. Invest. 2005;115:986–995. doi: 10.1172/JCI200523683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y., Fan J., Chen X.S., FitzGerald G.A., Funk C.D. Genetic model of selective COX2 inhibition reveals novel heterodimer signaling. Nat. Med. 2005 doi: 10.1038/nm1412. In press. [DOI] [PubMed] [Google Scholar]
- 15.Catella-Lawson F., et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. . N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 16.Skuballa W., Schillinger E., Sturzebecher C.S., Vorbruggen H. Synthesis of a new chemically and metabolically stable prostacyclin analogue with high and long-lasting oral activity. J. Med. Chem. 1986;29:313–315. doi: 10.1021/jm00153a001. [DOI] [PubMed] [Google Scholar]
- 17.FitzGerald G.A. The choreography of cyclooxygenases in the kidney. J. Clin. Invest. 2002;110:33–34. doi: 10.1172/JCI200216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brater D.C. Anti-inflammatory agents and renal function. Semin. Arthritis Rheum. 2002;32:33–42. doi: 10.1053/sarh.2002.37216. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H.F., Harris R.C. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–530. doi: 10.1161/01.HYP.0000116221.27079.ea. [DOI] [PubMed] [Google Scholar]
- 20.FitzGerald G.A. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003;2:879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 21.Aw T.J., Haas S.J., Liew D., Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch. Intern. Med. 2005;165:490–496. doi: 10.1001/archinte.165.5.IOI50013. [DOI] [PubMed] [Google Scholar]
- 22.Francois H., et al. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab. 2005;2:201–207. doi: 10.1016/j.cmet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Pini B., et al. Prostaglandin E synthases in zebrafish. Arterioscler. Thromb. Vasc. Biol. 2005;25:315–320. doi: 10.1161/01.ATV.0000152355.97808.10. [DOI] [PubMed] [Google Scholar]
- 24.Schneider A., et al. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int. 2004;65:1205–1213. doi: 10.1111/j.1523-1755.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy C.R., et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat. Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H., et al. Effects of salt loading on blood pressure in mice lacking the prostanoid receptor gene. Circ. J. 2005;69:124–126. doi: 10.1253/circj.69.124. [DOI] [PubMed] [Google Scholar]
- 27.Fabre J.E., et al. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J. Clin. Invest. 2001;107:603–610. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trebino C.E., et al. Redirection of eicosanoid metabolism in mPGES-1-deficient macrophages. . J. Biol. Chem. 2005;280:16579–16585. doi: 10.1074/jbc.M412075200. [DOI] [PubMed] [Google Scholar]
- 29.Furberg C.D., Psaty B.M., FitzGerald G.A. Parecoxib, valdecoxib, and cardiovascular risk [editorial]. Circulation. 2005;111:249. doi: 10.1161/01.CIR.0000155081.76164.17. [DOI] [PubMed] [Google Scholar]
- 30.Crofford L.J., et al. Thrombosis in patients with connective tissue diseases treated with specific cyclooxygenase 2 inhibitors. A report of four cases. Arthritis Rheum. 2000;43:1891–1896. doi: 10.1002/1529-0131(200008)43:8<1891::AID-ANR28>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Westgate E.J., FitzGerald G.A. Pulmonary embolism in a woman taking oral contraceptives and valdecoxib. PLoS Med. 2005;2:e197. doi: 10.1371/journal.pmed.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farkouh M.E., et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364:675–684. doi: 10.1016/S0140-6736(04)16894-3. [DOI] [PubMed] [Google Scholar]
- 33.Yang T., et al. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am. J. Physiol. Renal Physiol. 2005;288:F1125–F1132. doi: 10.1152/ajprenal.00219.2004. [DOI] [PubMed] [Google Scholar]
- 34.Seyberth H.W., Sweetman B.J., Frohlich J.C., Oates J.A. Quantifications of the major urinary metabolite of the E prostaglandins by mass spectrometry: evaluation of the method’s application to clinical studies. Prostaglandins. 1976;11:381–397. doi: 10.1016/0090-6980(76)90160-x. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan J.C., Sasser J.M., Pollock D.M., Pollock J.S. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45:406–411. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- 36.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 37.Marnett L.J., Rowlinson S.W., Goodwin D.C., Kalgutkar A.S., Lanzo C.A. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 38.Shimokawa T., Kulmacz R.J., DeWitt D.L., Smith W.L. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J. Biol. Chem. 1990;265:20073–20076. [PubMed] [Google Scholar]
- 39.Meyers E.N., Lewandoski M., Martin G.R. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 40.Loftin C.D., Trivedi D.B., Langenbach R. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J. Clin. Invest. 2002;110:549–557. doi: 10.1172/JCI200214924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reese J., et al. Comparative analysis of pharmacologic and/or genetic disruption of cyclooxygenase-1 and cyclooxygenase-2 function in female reproduction in mice. Endocrinology. 2001;142:3198–3206. doi: 10.1210/endo.142.7.8307. [DOI] [PubMed] [Google Scholar]
- 42.Sigthorsson G., et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology. 2002;122:1913–1923. doi: 10.1053/gast.2002.33647. [DOI] [PubMed] [Google Scholar]
- 43.Takano T., et al. Inhibition of cyclooxygenases reduces complement-induced glomerular epithelial cell injury and proteinuria in passive Heymann nephritis. J. Pharmacol. Exp. Ther. 2003;305:240–249. doi: 10.1124/jpet.102.043604. [DOI] [PubMed] [Google Scholar]
- 44.Tunctan B., et al. Nitric oxide reverses endotoxin-induced inflammatory hyperalgesia via inhibition of prostacyclin production in mice. Pharmacol. Res. 2006;53:177–192. doi: 10.1016/j.phrs.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Shin J.W., Hwang K.S., Kim Y.K., Leem J.G., Lee C. Nonsteroidal antiinflammatory drugs suppress pain-related behaviors, but not referred hyperalgesia of visceral pain in mice. Anesth. Analg. 2006;102:195–200. doi: 10.1213/01.ane.0000184828.39754.a3. [DOI] [PubMed] [Google Scholar]
- 46.Pratico D., Cyrus T., Li H., FitzGerald G.A. Endogenous biosynthesis of thromboxane and prostacyclin in 2 distinct murine models of atherosclerosis. Blood. 2000;96:3823–3826. [PubMed] [Google Scholar]
- 47.Bodary P.F., Westrick R.J., Wickenheiser K.J., Shen Y., Eitzman D.T. Effect of leptin on arterial thrombosis following vascular injury in mice. Jama. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 48.Gresele P., Corona C., Alberti P., Nenci G.G. Picotamide protects mice from death in a pulmonary embolism model by a mechanism independent from thromboxane suppression. Thromb. Haemost. 1990;64:80–86. [PubMed] [Google Scholar]
- 49.Momi S., et al. Prevention of pulmonary thromboembolism by NCX 4016, a nitric oxide-releasing aspirin. Eur. J. Pharmacol. 2000;397:177–185. doi: 10.1016/s0014-2999(00)00223-5. [DOI] [PubMed] [Google Scholar]
- 50.Gresele P., et al. Dipyridamole inhibits platelet aggregation in whole blood. Thromb. Haemost. 1983;50:852–856. [PubMed] [Google Scholar]
- 51.Booth B.P., Fung H.L. Regulation of in vivo whole blood aggregation in rats by calcitonin gene related peptide. Can. J. Physiol. Pharmacol. 1998;76:811–813. doi: 10.1139/cjpp-76-7-8-811. [DOI] [PubMed] [Google Scholar]
- 52.Emery J.D., et al. Whole-blood platelet aggregation predicts in vitro and in vivo primary hemostatic function in the elderly. Arterioscler. Thromb. Vasc. Biol. 1995;15:748–753. doi: 10.1161/01.atv.15.6.748. [DOI] [PubMed] [Google Scholar]
- 53.Tilley S.L., et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J. Clin. Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlson S.H., Oparil S., Chen Y.F., Wyss J.M. Blood pressure and NaCl-sensitive hypertension are influenced by angiotensin-converting enzyme gene expression in transgenic mice. Hypertension. 2002;39:214–218. doi: 10.1161/hy0202.104267. [DOI] [PubMed] [Google Scholar]
- 55.Carlson S.H., Wyss J.M. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:E1–E5. doi: 10.1161/01.hyp.35.2.e1. [DOI] [PubMed] [Google Scholar]
- 56.Hui Y., et al. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110:3360–3366. doi: 10.1161/01.CIR.0000147775.50954.AA. [DOI] [PubMed] [Google Scholar]
- 57.Rocca B., et al. Directed vascular expression of the thromboxane A2 receptor results in intrauterine growth retardation. Nat. Med. 2000;6:219–221.. doi: 10.1038/72334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.