Abstract
CCN2, (connective tissue growth factor, CTGF) is a matricellular factor associated with fibrosis that plays an important role in the production and maintenance of fibrotic lesions. Increased collagen deposition and accumulation is a common feature of fibrotic tissues. The mechanisms by which CCN2 /CTGF contributes to fibrosis are not well understood. Previous studies suggest that CTGF exerts some of its biological effects at least in part by integrin binding, though this mechanism has not been previously shown to contribute to fibrosis. Utilizing full length CCN2/CTGF, CCN2/CTGF fragments, and integrin neutralizing antibodies, we provide evidence that the effects of CCN2/CTGF to stimulate extracellular matrix deposition by gingival fibroblasts are mediated by the C-terminal half of CCN2/CTGF, and by α6 and β1 integrins. In addition, a synthetic peptide corresponding to a region of CCN2/CTGF domain 3 that binds α6β1 inhibits the collagen deposition assay. These studies employed a new and relatively rapid assay for CCN2/CTGF-stimulated collagen deposition based on Sirius Red staining of cell layers. Data obtained support a pathway in which CCN2/CTGF could bind to α6β1 integrin and stimulate collagen deposition. These findings provide new experimental methodologies applicable to uncovering the mechanism and signal transduction pathways of CCN2/CTGF mediated collagen deposition, and may provide insights into potential therapeutic strategies to treat gingival fibrosis and other fibrotic conditions.
Keywords: Collagen deposition, Connective tissue growth factor, Integrins, Gingival overgrowth, Fibrosis
The CCN family of proteins consists of six multifunctional members including CCN1 (Cyr61), CCN2 (connective tissue growth factor, CTGF), CCN3 (Nov), CCN4 (WISP1), CCN5 (WISP2), and CCN6 (WISP3) [Brigstock et al., 2003; Perbal, 2004]. These factors are conserved multi-domain cysteine rich proteins with diverse functions including regulation of angiogenesis, cell adhesion, proliferation, skeletal development, tooth development, apoptosis, and in the case of CCN2/CTGF promotion of fibrosis [Brigstock et al., 1997; French et al., 2004; Hishikawa et al., 2000; Hishikawa et al., 1999; Kireeva et al., 1998; Leu et al., 2003; Nishida et al., 2004; Oemar and Luscher, 1997; Shimo et al., 1999; Shimo et al., 2002; Takigawa et al., 2003]. These factors are matricellular proteins, meaning that they function in collaboration with other factors and extracellular matrix components [Bornstein, 2000; Yang et al., 2000], and also bind receptors including low density lipoprotein related protein and integrins [Gao and Brigstock, 2003; Gao and Brigstock, 2004; Leu et al., 2003]. Proteolytic products of these proteins containing one or two domains or modules retain biological activities [Brigstock et al., 1997; Perbal, 2004]. Although it is well known that CCN2/CTGF is expressed in fibrotic tissues and contributes to fibrosis, the mechanisms by which this occurs remains largely unknown.
Drug-induced gingival overgrowth is a side effect of three classes of medications: phenytoin is an anti-seizure drug, nifedipine is a calcium channel blocker, and cyclosporine A is an immunosuppressant. Our laboratory has found that CCN2/CTGF is highly expressed in phenytoin induced gingival overgrowth, whereas it is not expressed in cyclosporine A induced overgrowth [Hong et al., 1999; Uzel et al., 2001]. CCN2/CTGF is found at intermediate levels in nifedipine induced gingival overgrowth [Uzel et al., 2001]. As phenytoin induced lesions are the most fibrotic, and cyclosporine induced lesions are not fibrotic but highly inflamed, we reasoned that CCN2/CTGF likely contributes to fibrosis in phenytoin induced lesions. At the same time, we have found no effect of CCN2/CTGF on collagen mRNA levels in gingival fibroblast cultures, whereas CCN2/CTGF effectively increased collagen deposition in these cultures [Hong et al., 1999]. The major goal of the present study, therefore, was to investigate structure/function relationships of CCN2/CTGF in the stimulation of collagen deposition. In addition, we investigated the role of several integrins in mediating effects of CCN2/CTGF on collagen deposition. In order to accomplish these goals we developed a relatively rapid assay for collagen deposition in gingival fibroblasts. These findings provide new insights into the mechanisms by which CCN2/CTGF contributes to fibrosis in gingival tissues, and may in addition ultimately provide new therapeutic strategies to address fibrotic disease in other tissues as well.
MATERIALS AND METHODS
Human recombinant CTGF/CCN2 was kindly provided by FibroGen Corporation, South San Francisco, and was produced in a baculovirus expression system. The N-terminal half of CTGF/CCN2 (containing module 1 & 2) and the C-terminal half (containing module 3 & 4) and affinity purified goat polyclonal antibodies recognizing these portions of CTGF/CCN2 were also generously provided. The N-terminal and C-terminal halves of CTGF were affinity purified following partial digestion of full-length CTGF with chymotrypsin, which specifically cleaves the molecule between module 2 and module 3. The polyclonal antibody against full-length recombinant human CTGF was purified by affinity chromatography. N-terminal or C-terminal specific polyclonal antibodies were prepared from the affinity purified polyclonal antibody by purification on affinity columns made from C-terminal or N-terminal halves, respectively. Specificity of the purified polyclonal antibodies for the N-terminal or C-terminal half fragments were confirmed by Western blotting. Human recombinant TGF-β1 was purchased from Peprotech, Rocky Hill, NJ. Sirius Red powder was obtained from Chroma, Münster, Germany. Anti- integrin monoclonal neutralizing antibodies were purchased from Chemicon, Temecula, CA: anti-β1 (catalogue MAB2253Z, clone B44), anti-β3 (catalogue # MAB2023Z, cloneB3A), and αM (catalogue # MAB1380, clone ICRF44), and the anti-α6 integrin neutralizing antibody clone GoH3 (catalogue # 0796) was purchased from Immunotech, Coulter, France. The anti-integrin αIIb antibody was purchased from Santa Cruz Biotechnology, Santa Cruz, CA (catalog # sc19963). If antibody formulations contained azide, these samples were thoroughly dialyzed against cold PBS prior to use. All other reagents were purchased from Sigma Invitrogen unless otherwise indicated.
Cell Culture
Early passage human gingival fibroblasts were grown from gingival tissue explants [Piche et al., 1989] obtained from two adult subjects undergoing routine periodontal treatments and who did not have any form of gingival overgrowth. Human subject protocols were fully approved by a Boston University Medical Center IRB committee. Subject 1 (N5 cells) was a 32 year old female, subject 2 (HCT11 cells) was a 42 year old man. Cells were grown from frozen stocks at passage 5 in 100 mm cell-culture plates and cultured at 37 °C in a 5 % CO2 atmosphere in DMEM (Dulbecco’s Modifiered Eagle’s Medium) containing 10 % Newborn Bovine Serum (NBS), 0.1 mM non-essential amino acids and antibiotics (penicillin/ streptomycin). Cells were re-fed every two or three days. The fibroblasts grown from frozen stocks were passaged twice for expansion, before being plated for experimental treatments at an initial concentration of 50,000 cells per well in 6-well plates or 25,000 cells per well in 12-well culture plates. The cells were grown to visual confluence, and were grown for an additional seven days before initiation of the cell treatment protocols. Synthetic CTGF/CCN2 peptide RANCLVQTTEWSACSKT is a custom-made peptide and was purchased from SynPep Corporation, Dublin, CA.
Treatment of Cells
Cells were cultured in media described above in the additional presence of ascorbate (0.05 mg/mL) beginning on day 0 of treatment protocols. In addition, TGF-β1 (10 ng/ml), CTGF/CCN2 (100 ng/mL), N-terminal CTGF/CCN2 (50 or 100 ng/mL), C-terminal CTGF/CCN2 (50 or 100 ng/mL) or anti-CTGF/CCN2 antibody (10 μg/mL) with CTGF/CCN2 (100 ng/mL) were used in experiments. The total volume of PBS (Dulbecco’s buffered saline solution) added to media did not vary between plates within each experiment and did not exceed 5% of the total volume of media. After the cells were grown to full confluence, the fibroblasts were cultured in the presence of one of the solutions for 7 days, with 3 media changes, or 6 days, with 2 media changes, each in the continuous presence of ascorbate, CTGF/CCN2 proteins and anti-CCN2/CTGF antibodies. In every set of experiments, TGF-β1 (10 ng/ml) was used as a positive control, and 2 sets of untreated cell controls were also grown as an additional check of reproducibility of data. Each treatment condition consisted of six wells (n=6) to provide sufficient statistical power for these studies. In treating with antibodies against CCN2/CTGF, antibodies (4 μg/ml) were preincubated for 15 minutes 37° C in media containing all other components including CCN2/CTGF before adding to the confluent cell cultures to allow for antibody binding to CCN2/CTGF. On the other hand, antibodies against integrins were added into each well 15 minutes and incubated under 37° C prior to adding CCN2/CTGF in order to allow antibody-integrin binding.
Fixation and Sirius Red Assay
The Sirius Red dye-binding assay for measuring collagen accumulation in gingival fibroblasts was adapted from a previous study done in osteoblasts [Tullberg-Reinert and Jundt, 1999]. Following the 7 day treatment period media were removed and the cell layers washed three times with PBS. The cell layers were then fixed with Bouin’s solution for 1 hour at room temperature. The solution was removed and plates were washed in running tap water until the yellow stain was removed. The plates were then air-dried in a fume hood overnight. Sirius red dye solution (1 mg/ml in picric acid) was added to each well for 1 hour and placed under mild shaking. For 12 well plates, 1 ml of dye solution was used; for 6-well plates 2 ml per well was used. The dye solution was then removed and each well was washed four times with 2 ml aliquots of 0.01 N of HCl to remove unbound dye. The bound dye in each well was eluted with 1 ml of 0.1 N NaOH under mild shaking for 30 min. Optical density was then measured at 550 nm using 0.1 N NaOH as blank. Multi-well plates without fibroblasts treated identically were used as the background control.
Crystal Violet Assays
A Crystal Violet dye-binding assay was used to determine the relative DNA content of each well [Kostenuik et al., 1997]. After the Sirius Red elution was complete, the plates were rinsed with water and air-dried. Then, 0.1 % of Crystal Violet dye solution was added to each well and placed under mild shaking for 30 min. The unbound dye was removed thoroughly by rinsing thoroughly under running water until the washes were colorless. The plates were again air-dried. After air-drying overnight, the bound dye was eluted with 10% acetic acid under mild shaking for 1 hour. The elution was collected and absorbance at 590 nm was determined using 10% acetic acid as blank. Samples were diluted in 10% acetic acid as required to obtain accurate readings. Data were recorded as total absorbance units per well if all dye were eluted in 1 ml. Culture plates without fibroblasts were used as the background control.
Hydroxyproline assays
Cells were grown and treated with CCN2/CTGF (100 ng/ml), TGF-β1 (10 ng/ml, positive control), or no additions (negative control) for seven days with media changes as described in Materials and Methods. Cell layers were rinsed three times with PBS, and then scraped and collected in microcentrifuge tubes. Samples were hydrolyzed in 6 N HCl at 110° C for 24 hours, and then vacuum dried. Samples were then subjected to colorimetric hydroxyproline analyses [Edwards and O'Brien, 1980].
Statistics
Student t test with equal variance was used to compare the data from control cultures to experimental groups, and p< 0.05 was used to declare statistical significance.
RESULTS
CCN2/CTGF is expressed at elevated levels in fibrotic tissues, and contributes in some way to fibrosis [Moussad and Brigstock, 2000; Oemar and Luscher, 1997; Yokoi et al., 2004]. The mechanisms by which CCN2/CTGF contributes to increased extracellular matrix production or deposition are not well understood. This may stem largely from the lack of a well defined and reproducible in vitro assay to measure effects of CCN2/CTGF on extracellular matrix deposition. We, therefore, first developed a rapid assay to determine CCN2/CTGF stimulated collagen deposition in gingival fibroblasts, adapted from a Sirius red dye-binding assay developed to measure collagen deposition in osteoblast cultures [Tullberg-Reinert and Jundt, 1999]. The experimental approach taken was to culture fully confluent gingival fibroblasts in the continuous presence of ascorbate and increasing concentrations of recombinant human CCN2/CTGF for seven days, fix, and then stain cell layers with Sirius red. The seven day time point was chosen based on our previous studies measuring collagen deposition by gingival fibroblasts by conventional hydroxyproline analyses [Hong et al., 1999]. Bound dye was eluted and quantitated by spectrophotometry as described in Methods and Materials. TGF-β1 treated cultures served as positive controls. Data in Figure 1A show that 50 – 125 ng/ml CCN2/CTGF significantly increased Sirius red dye binding (p< 0.05), whereas 10 and 25 ng/ml CCN2/CTGF were unable to stimulate Sirius red dye binding to cell layers. TGF-β1 strongly and significantly stimulated Sirius red binding. These data suggest that CCN2/CTGF stimulates collagen deposition at 50 ng/ml and higher, and that the effect of CCN2/CTGF is weaker than that of TGF-β1. Staining of the same cell layers with the DNA dye crystal violet followed by elution and spectrophotometric quantitation [Kostenuik et al., 1997] did not reveal consistent significant increases induced by CCN2/CTGF indicating that cell number was not increased by CCN2/CTGF treatment (Table I). By contrast TGF-β1 increased crystal violet binding to cell layers as expected, as TGF-β1 is a potent mitogenic factor for human fibroblasts cultured under these conditions (Table I) [Clark et al., 1997]. Thus, CCN2/CTGF increases collagen deposition without significantly stimulating growth of gingival fibroblast cultures.
Figure 1.
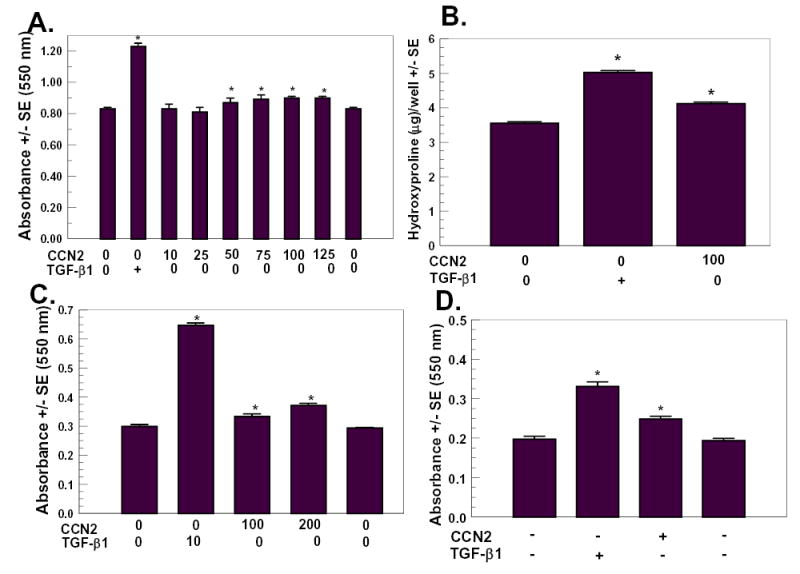
Collagen deposition stimulated by CTGF determined by Sirius Red dye binding assay and confirmed by hydroxyproline assays. Human gingival fibroblasts from subject 1 (A–C) and subject 2 (D) were cultured and treated with CTGF/CCN2 in the amounts indicated in ng/ml, or with TGF-β1 (10 ng/ml), or no additions (control) as for seven days with media changes as described in Methods and Materials. Cell layers were fixed and stained with Sirius Red, and eluted dye was quantitated by spectrophotometry at 550 nm (A, C, and D). (A) dose response study; B, hydroxyproline measurements confirming increased collagen deposition by CCN2/CTGF, (C) dose response study with different serum batch, (D) collagen deposition stimulated by CCN2/CTGF in human gingival fibroblasts from subject 2. In B, cell layers were scraped and hydrolyzed, and subjected to hydroxyproline determinations as described in Materials and Methods. *, p< 0.05 compared to untreated controls.
Table I.
Crystal violet assay for relative DNA content of cell layers from CTGF and TGF-β1 treated human gingival fibroblast cultures.
| Cell Culture Sample | Absorbance +/− SE (590 nm) |
|---|---|
| Untreated Control | 5.31 +/− 0.46 |
| TGF;β1 (10 ng/ml) | 7.61* +/− 0.50 |
| CTGF (10 ng/ml) | 5.29 +/− 0.30 |
| CTGF (25 ng/ml) | 4.48* +/− 0.27 |
| CTGF (50 ng/ml) | 4.85 +/− 0.19 |
| CTGF (75 ng/ml) | 5.01 +/− 0.22 |
| CTGF (100 ng/ml) | 4.68* +/− 0.39 |
| CTGF (125 ng/ml) | 5.36 +/− 0.18 |
Human gingival fibroblasts were cultured and then treated for seven days as described in “Materials and Methods”. Cell layers were fixed and subjected to the Sirius Red assay and data presented in Figure 1. Cell layers were then stained with crystal violet for DNA content, and dye was eluted and quantitated as described in “Materials and Methods”.
p<0.05. Data show no dose-dependent effect of CTGF on cell layer DNA content, whereas TGF-β1 significantly increased cell layer DNA content, as expected.
In order to independently confirm that collagen deposition is increased by CCN2/CTGF, we cultured confluent cells as before in the constant presence of 10 ng/ml TGF-β1 or 100 ng/ml CCN2/CTGF, or no additions for seven days. Cell layers were collected as described in “Methods and Materials” and were then hydrolyzed in 6 N HCl for 24 hours, and residues were analyzed for hydroxyproline levels. Results in Figure 1B show that TGF-β1 and CCN2/CTGF increased hydroxyproline levels by 41.7% and 16.1%, respectively.
Collagen deposition assays were reproducible between experiments, and CCN2/CTGF always increased Sirius Red staining of cell layers in all experiments, and more than 20 experiments have been conducted. CCN2/CTGF stimulation of collagen deposition varied between 10% and 25% in different experiments, and collagen deposition was consistently stimulated by CCN2/CTGF. Changing serum lots affected the absolute value of Sirius Red staining, but did not change the finding that CCN2/CTGF stimulated collagen deposition. Data in Figure 1C done with the same cells as Figures 1A and B but with a different lot of newborn calf serum showed that CCN2/CTGF still stimulated collagen deposition, and this effect was dose-dependent.
Studies in Figure 1A–C were performed with gingival fibroblasts cultured from one individual. In order to determine that these experiments are representative of normal human gingival fibroblasts we measured CCN2/CTGF stimulated collagen deposition in a culture derived from a different donor. As seen in Figure 1D, CCN2/CTGF stimulated collagen deposition as determined by the Sirius red assay, and consistent with previous studies by our laboratory [Hong et al., 1999].
Structure/function studies
The N-terminal half of CCN2/CTGF stimulates collagen synthesis, whereas the C-terminal half of CCN2/CTGF stimulated cell proliferation in a rat kidney cell line [Grotendorst and Duncan, 2005; Grotendorst et al., 2001]. Based on antibody inhibition studies in vivo, the active portion of CCN2/CTGF in stimulating tooth development resides in the N-terminal half of CCN2/CTGF [Shimo et al., 1999]. By contrast, adhesion of endothelial cells and activated stellate cells depends on module 3 in the C-terminal half of CCN2/CTGF [Gao and Brigstock, 2003; Gao and Brigstock, 2004; Leu et al., 2003]. Because collagen deposition is often uncoupled from collagen synthesis [Franceschi and Iyer, 1992; Gerstenfeld et al., 1993; Hong et al., 2004; Pischon et al., 2004; Quarles et al., 1992], and functional collagen is insoluble [Prockop and Kivirikko, 1995], the present study investigates what portions of CCN2/CTGF stimulate collagen deposition by human gingival fibroblasts. Sirius Red collagen deposition assays were performed with CCN2/CTGF preincubated with 10 μg/ml affinity purified polyclonal antibody against, respectively, full length CCN2/CTGF, N-terminal half of CCN2/CTGF, or C-terminal half of CCN2/CTGF. Data (Figure 2) show that antibody against full length CCN2/CTGF inhibited the collagen deposition assay, whereas the antibody against the N-terminal half of CTGF failed to inhibit. Most, important, the antibody against the C-terminal half of CCN2/CTGF inhibited CCN2/CTGF stimulated collagen deposition. Data suggest that the C-terminal half of CCN2/CTGF contains domains that are responsible for stimulating collagen deposition by primary human gingival fibroblasts.
Figure 2.
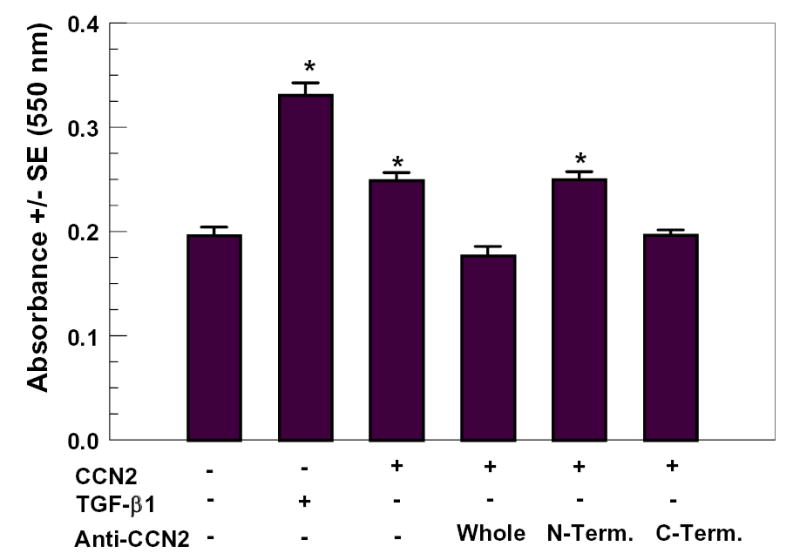
Inhibition of CCN2/CTGF stimulated collagen deposition with region-specific CCN2/CTGF polyclonal antibodies. Media containing 100 ng/ml CCN2/CTGF and affinity purified antibodies (10 μg/ml) recognizing, respectively, full-length CCN2/CTGF, the N-terminal half of CCN2/CTGF, or the C-terminal half of CCN2/CTGF were pre-incubated for 15 minutes at 37° C and then fed to confluent gingival fibroblast cells as described in Methods and Materials. This protocol was repeated twice during the seven day experimental period to refresh media. Cell layers were then fixed and the Sirius Red dye binding assay for collagen deposition was performed. TGF-β1 (10 ng/ml) was a positive control. *, p< 0.05, compared to untreated controls.
Although the antibody experiment indicated that the C-terminal half of CCN2/CTGF is required for collagen deposition by CCN2/CTGF, it did not determine whether the C-terminal half of CCN2/CTGF in the absence of the N-terminal half is sufficient to stimulate collagen deposition when added to gingival fibroblast cultures. To address this question, we determined activity of each half of CCN2/CTGF to stimulate cell layer collagen accumulation using the Sirius red assay. Data show that full length CCN2/CTGF stimulated collagen deposition, as expected, and that the N-terminal half of CCN2/CTGF was unable to stimulate collagen deposition. The C-terminal half of CCN2/CTGF did stimulate collagen deposition, and this effect was dose-dependent and similar in magnitude to full length CCN2/CTGF. Thus, antibody inhibition studies and data from assays of each half of CCN2/CTGF taken together indicate that the C-terminal half of CCN2/CTGF is sufficient to stimulate collagen deposition in human gingival fibroblast cultures.
Integrin antibody inhibition studies
CCN family members including CCN2/CTGF (CTGF) and the closely related CCN1/Cyr61 interact with integrins, which then mediate biological activities [Babic et al., 1999; Chen et al., 2001; Crean et al., 2002; Grzeszkiewicz et al., 2002; Jedsadayanmata et al., 1999; Kireeva et al., 1998; Schober et al., 2002]. We wished to investigate the potential role of selected integrins in mediating CCN2/CTGF stimulated collagen deposition by gingival fibroblasts. Gingival fibroblasts express several integrins, including all of those investigated in the present study [Palaiologou et al., 2001; van der Pauw et al., 2002]. The collagen deposition assay was therefore performed in the presence of integrin neutralizing antibodies. In these experiments collagen deposition was measured in the constant presence of anti-intgrin antibody both in the presence and absence of CCN2/CTGF. Effective inhibition of collagen deposition was declared if there was no significant difference in Sirius Red values between antibody alone and antibody plus CCN2/CTGF treated cultures (n=6, p>0.05). An additional control of CCN2/CTGF without any added antibodies was included to confirm that CCN2/CTGF stimulated deposition was occurring consistent with previous experiments. Data in Figure 4 show that anti-α6, but not anti-αM or anti-αIIb inhibited CCN2/CTGF-induced collagen deposition. Anti-β1, but not anti-β3 antibodies inhibited CCN2/CTGF-induced collagen deposition. Each integrin antibody alone in the absence of CCN2/CTGF did not alter collagen deposition or cell accumulation determined by the Sirius red and crystal violet assays, respectively. Thus, the hypothesis is developed that α6β1 integrin could mediate effects of CCN2/CTGF on collagen deposition.
Figure 4.
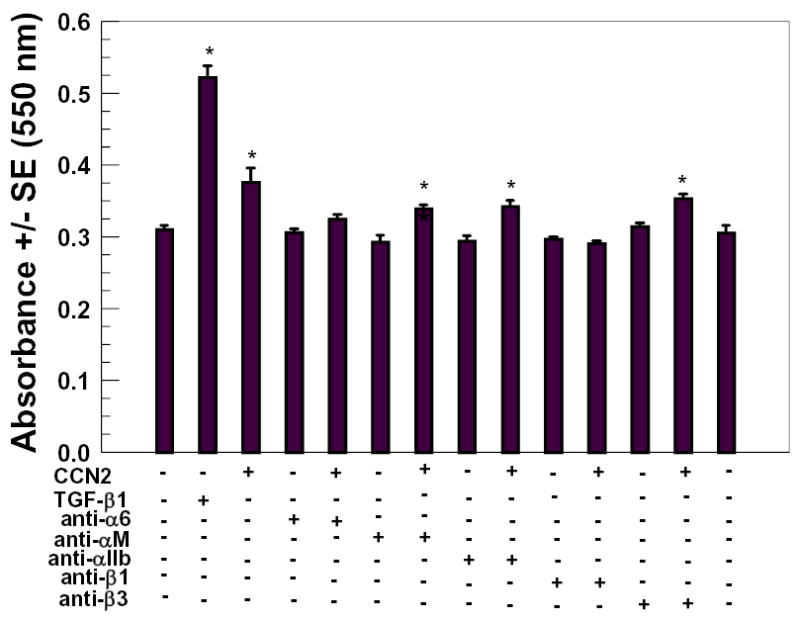
Integrin antibody inhibition of CCN2/CTGF-stimulated collagen deposition. Confluent gingival fibroblast cultures were preincubated with 4 μg/ml integrin antibodies or no antibody (controls) in media for 15 minutes at 37° C, followed by addition of CCN2/CTGF (or no CCN2/CTGF, controls) to a final concentration of 200 ng/ml. Cultures were refed twice using the same protocol and media additions during a seven day period. Cells layers were then fixed and subjected to the Sirius Red assay to determine relative deposition of collagen. *, p< 0.05, compared to untreated controls.
CCN1/Cyr61 mediates attachment of endothelial cells and skin fibroblasts via α6β1 integrin [Chen et al., 2000]. A binding site on CCN1 for α6β1 has been identified and is located in the C-terminal half of domain 3, and is approximately 80% identical to the corresponding domain 3 sequence in CTGF [Leu et al., 2003]. A 17 amino acid long CCN1 peptide encompassing the α6β1 binding region was found to inhibit skin fibroblast attachment to α6β1 coated cell culture plates. Thus, we synthesized the corresponding CCN2/CTGF peptide (residues 199 – 215) to test its ability to inhibit CCN2/CTGF stimulated collagen deposition. Data in Figure 5 show that the synthetic peptide alone does not affect collagen deposition. The peptide does, however, inhibit CCN2/CTGF-stimulated collagen deposition. These findings further support the notion that CCN2/CTGF mediates collagen deposition by domain 3 of CCN2/CTGF binding to α6β1 integrin.
Figure 5.
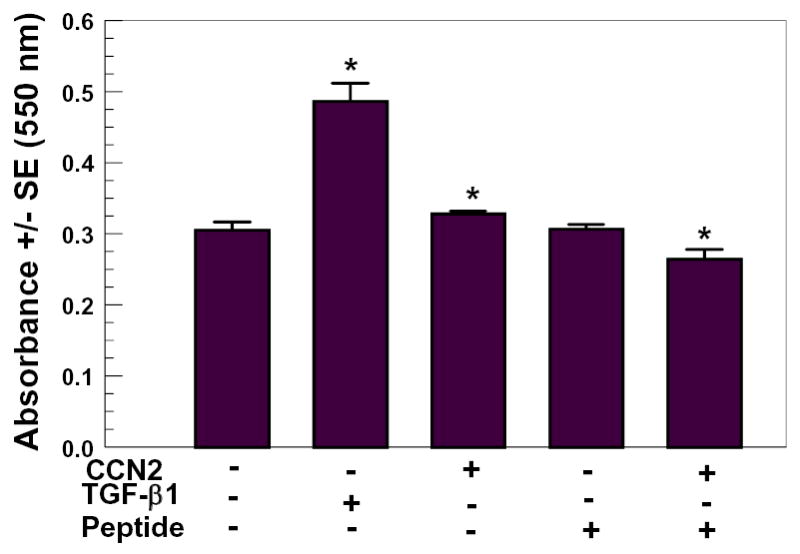
Module 3 CCN2/CTGF peptide inhibits CCN2/CTGF stimulation of collagen deposition by human gingival fibroblasts. CCN2/CTGF (100 ng/ml) in the presence and absence of 50 μM peptide (see Materials and Methods for sequence), or no additions (negative control) or TGF-β1 (10 ng/ml, positive control) were incubated to confluent human gingival fibroblasts. Media containing respective additions were changed twice during the seven day treatment period. Cell layers were then fixed and subjected to the Sirius Red assay for collagen deposition. *, p< 0.05 compared to untreated controls.
DISCUSSION
As a multi-domain matricellular factor, CCN2/CTGF has multiple biological activities and multiple binding partners [Leask and Abraham, 2003]. In the present study we have investigated structure function relationships of CCN2/CTGF with respect to its stimulation of collagen deposition by gingival fibroblasts. Assays were highly reproducible and consistent, although the effect of CCN2/CTGF on collagen deposition was modest and ranged between 5 and 25%. Collagen deposition assays in response to exogenous addition of CCN2/CTGF were performed in the presence of serum to maintain cell viability during the several days of culture required. In addition, this protocol allows for CCN2/CTGF interaction with the effects of other factors present in serum that together elicit increased extracellular matrix production. In our experiments, no synergistic effects were seen on collagen deposition in studies in which both CCN2/CTGF and TGF-β1 were applied together to gingival fibroblasts (data not shown). However, from in vivo studies, it is known that as a matricellular factor, CCN2/CTGF more efficiently elicits fibrosis in combination with other factors, and that CCN2/CTGF alone is a relatively weak fibrogenic factor [Mori et al., 1999].
Inhibition studies done with CCN2/CTGF region specific antibodies, and complementary assays done with N-terminal and C-terminal halves of CCN2/CTGF all indicate that that the C-terminal half of CCN2/CTGF contains sequences required for stimulation of collagen deposition. This region of CCN2/CTGF contains two modules or domains known, respectively as the thrombospondin-like domain (module 3) and the cysteine knot domain (module 4) [Blom et al., 2002; Brigstock, 1999]. Domain 4 binds to heparin sulphate proteoglycans and may serve to enhance binding of domain 3 to binding partners including integrins or low density lipoprotein receptor related protein (LRP) [Gao and Brigstock, 2003; Chen et al., 2000]. Consistent with the importance of module 3 of CCN2/CTGF stimulating collagen deposition, neutralizing antibodies against integrins implicate α6 and β1 subunits each inhibited CCN2/CTGF stimulated collagen deposition by gingival fibroblasts. The integrin α6β1 is a ligand for module 3 of CCN1 and CCN2/CTGF in endothelial cells and skin fibroblasts [Leu et al., 2003]; and we demonstrate that a peptide that contains the CCN2/CTGF binding site for α6β1 inhibits collagen deposition by gingival fibroblasts. These findings support the hypothesis that CCN2/CTGF is likely to stimulate collagen deposition by module 3 interaction with α6β1 integrin.
There is an apparent discrepancy between our studies which shown that the C-terminal half of CCN2/CTGF is required for increased collagen deposition by primary human gingival fibroblasts, and studies in a rat kidney cell line (NRK cells) that show that the N-terminal half of CCN2/CTGF stimulates collagen synthesis [Grotendorst and Duncan, 2005]. It is recognized that collagen synthesis is sometimes uncoupled from functional collagen deposition [Trackman, 2005], hence our decision to assay directly for collagen deposition. In addition, regulation of extracellular matrix genes by CCN2/CTGF may be different in gingival fibroblasts compared to kidney cells, as type I collagen mRNA levels are not increased by CCN2/CTGF in gingival fibroblasts [Hong et al., 1999] but are increased in NRK cells [Frazier et al., 1996]. Thus, assay methodology and tissue or species specificity of CCN2/CTGF activity each likely contributes to the data obtained.
The mechanisms by which CCN2/CTGF/integrin binding could stimulate collagen deposition are not yet known. Increased fibroblast cell adhesion could promote more efficient extracellular processing or assembly of collagen precursors. Alternatively, signaling pathways leading to enhanced production of extracellular enzymes and proteins that control collagen deposition may be regulated [Hong et al., 2004]. As noted, collagen deposition is enhanced in gingival fibroblasts by CCN2/CTGF without increases in collagen mRNA levels, suggesting that this enhancement is caused by posttranslational events [Hong et al., 1999]. Collagen biosynthesis is a complex process that includes intracellular synthesis, modification and assembly of procollagen chains, followed by secretion, processing by procollagen N- and C- proteinases, and finally lysyl oxidase-dependent cross-linking [Prockop and Kivirikko, 1995]. Candidate downstream targets of CCN2/CTGF in this context are diminished degradative proteolysis of collagen precursors, enhanced production or activation of procollagen C-proteinases or N-proteinases, or enhanced production or activation of lysyl oxidase or its relatives (LOXL1-LOXL4) [Csiszar, 2001; Molnar et al., 2003]. We have previously reported that lysyl oxidase activity is enhanced by CCN2/CTGF in these cultures [Hong et al., 1999]. This increased enzyme activity may depend in part on enhanced lysyl oxidase activation, rather than production, as lysyl oxidase mRNA levels were not regulated by CCN2/CTGF [Hong et al., 1999]. With the new knowledge that a CCN2/CTGF peptide can inhibit collagen deposition stimulated by CCN2/CTGF, we now have a new reagent that will help in determining the signal transduction pathways and mechanisms by which CCN2/CTGF stimulates collagen deposition by gingival fibroblasts.
Data include the interesting observation that CCN2/CTGF increases collagen deposition without increasing the growth of these cultures. By contrast TGF-β1 stimulated both growth and collagen deposition. TGF-β1 has been shown previously to stimulate the proliferation of apparently confluent normal human primary dermal fibroblasts [Clark et al., 1997]. The absence of a mitogenic effect of CCN2/CTGF on confluent human fibroblasts distinguishes it from the effects of TGF-β1. The absence of a mitogenic effect and the presence of a modest collagen matrix stimulating effect by CTGF/CCN2 seem likely to contribute to tissue fibrosis by effectively increasing the deposition of a collagenous extracellular matrix over time. This could ultimately result in a tissue containing greater levels of deposited collagen than would occur in the absence of CTGF/CCN2.
Drug induced gingival fibrosis is a condition caused by three classifications of drugs [Trackman and Kantarci, 2004]. Phenytoin, an anti-seizure medication, causes the most fibrotic lesions, and is accompanied by elevated levels of CTGF [Uzel et al., 2001]. To the extent that CTGF contributes to gingival fibrosis and to the extent that these mechanisms apply to other tissues, insights into mechanisms by which CTGF promotes collagen deposition are likely to be of great significance. One can begin to envision the development of anti-fibrotic therapeutic strategies based on inhibition of CCN2/CTGF interactions with functionally important binding partners such as α6β1 integrins.
Figure 3.
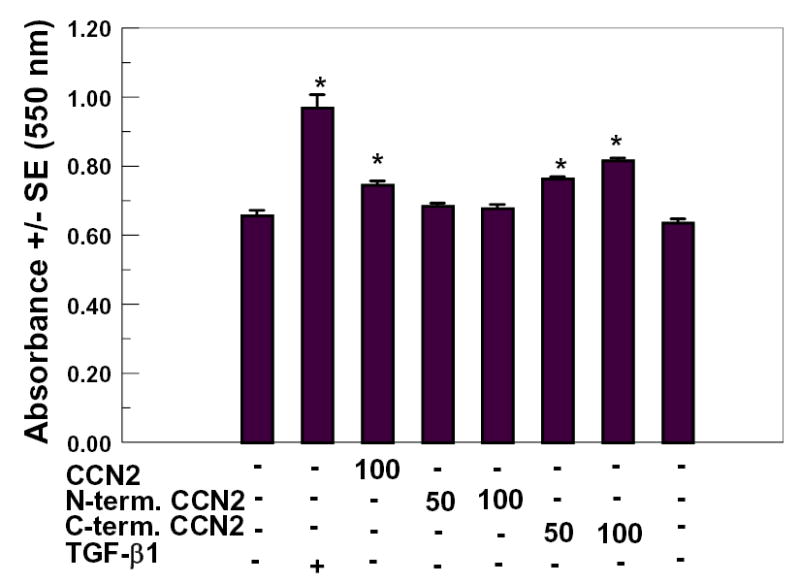
Increased collagen deposition stimulated by truncated forms of CCN2/CTGF in human gingival fibroblast cultures. Confluent human gingival fibroblast cultures were treated for seven days with the indicated concentrations of full length CCN2/CTGF, N-terminal half of CCN2/CTGF, or C-terminal half of CCN2/CTGF or no additions (controls). The Sirius Red assay for collagen deposition was then performed. TGF-β1 (10 ng/ml) was a positive control. *, p < 0.05, compared to untreated controls.
Acknowledgments
Research was supported by the following grants: NIH/NIDCR DE11004 and M01 RR00533. We thank Dr. Michael Davey for performing preliminary studies related to developing the collagen deposition assay.
References
- Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–66. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–82. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Matricellular proteins: an overview. Matrix Biol. 2000;19:555–6. doi: 10.1016/s0945-053x(00)00103-7. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127–8. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272:20275–82. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001;276:10443–52. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin alpha 6beta 1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–61. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170:69–80. doi: 10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Crean JK, Finlay D, Murphy M, Moss C, Godson C, Martin F, Brady HR. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem. 2002;277:44187–94. doi: 10.1074/jbc.M203715200. [DOI] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- Edwards CA, O'Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–7. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–46. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–11. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- French DM, Kaul RJ, D'Souza AL, Crowley CW, Bao M, Frantz GD, Filvaroff EH, Desnoyers L. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165:855–67. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–55. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Riva A, Hodgens K, Eyre DR, Landis WJ. Post-translational control of collagen fibrillogenesis in mineralizing cultures of chick osteoblasts. J Bone Miner Res. 1993;8:1031–43. doi: 10.1002/jbmr.5650080903. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Duncan M. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. Faseb J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Duncan M, Williams S, Klapper H. Structural and functional analysis of CTGF. Molecular Pathology. 2001;54 Abstract # A16. [Google Scholar]
- Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143:1441–50. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Nakaki T, Fujii T. Connective tissue growth factor induces apoptosis via caspase 3 in cultured human aortic smooth muscle cells. Eur J Pharmacol. 2000;392:19–22. doi: 10.1016/s0014-2999(00)00115-1. [DOI] [PubMed] [Google Scholar]
- Hishikawa K, Oemar BS, Tanner FC, Nakaki T, Luscher TF, Fujii T. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem. 1999;274:37461–6. doi: 10.1074/jbc.274.52.37461. [DOI] [PubMed] [Google Scholar]
- Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, Guo Y, Uzel MI, Ma D, Trackman PC. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- Hong HH, Uzel MI, Duan C, Sheff MC, Trackman PC. Regulation of lysyl oxidase, collagen, and connective tissue growth factor by TGF-beta1 and detection in human gingiva. Lab Invest. 1999;79:1655–67. [PubMed] [Google Scholar]
- Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3) J Biol Chem. 1999;274:24321–7. doi: 10.1074/jbc.274.34.24321. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090–6. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Kostenuik PJ, Halloran BP, Morey-Holton ER, Bikle DD. Skeletal unloading inhibits the in vitro proliferation and differentiation of rat osteoprogenitor cells. Am J Physiol. 1997;273:E1133–9. doi: 10.1152/ajpendo.1997.273.6.e1133. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–63. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Liu Y, Chen N, Chen CC, Lam SC, Lau LF. Identification of a novel integrin alpha 6 beta 1 binding site in the angiogenic inducer CCN1 (CYR61) J Biol Chem. 2003;278:33801–8. doi: 10.1074/jbc.M305862200. [DOI] [PubMed] [Google Scholar]
- Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647:220–4. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–9. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name? Mol Genet Metab. 2000;71:276–92. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–19. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- Oemar BS, Luscher TF. Connective tissue growth factor. Friend or foe? Arterioscler Thromb Vasc Biol. 1997;17:1483–9. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- Palaiologou AA, Yukna RA, Moses R, Lallier TE. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J Periodontol. 2001;72:798–807. doi: 10.1902/jop.2001.72.6.798. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Piche JE, Carnes DL, Jr, Graves DT. Initial characterization of cells derived from human periodontia. J Dent Res. 1989;68:761–7. doi: 10.1177/00220345890680050201. [DOI] [PubMed] [Google Scholar]
- Pischon N, Darbois LM, Palamakumbura AH, Kessler E, Trackman PC. Regulation of collagen deposition and lysyl oxidase by tumor necrosis factor-alpha in osteoblasts. J Biol Chem. 2004;279:30060–5. doi: 10.1074/jbc.M404208200. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7:683–92. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–65. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem (Tokyo) 1999;126:137–45. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- Shimo T, Wu C, Billings PC, Piddington R, Rosenbloom J, Pacifici M, Koyama E. Expression, gene regulation, and roles of Fisp12/CTGF in developing tooth germs. Dev Dyn. 2002;224:267–78. doi: 10.1002/dvdy.10109. [DOI] [PubMed] [Google Scholar]
- Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol. 2003;194:256–66. doi: 10.1002/jcp.10206. [DOI] [PubMed] [Google Scholar]
- Trackman PC. Diverse biological functions of extracellular collagen processing enzymes. J Cell Biochem. 2005;96:927–37. doi: 10.1002/jcb.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trackman PC, Kantarci A. Connective tissue metabolism and gingival overgrowth. Crit Rev Oral Biol Med. 2004;15:165–75. doi: 10.1177/154411130401500305. [DOI] [PubMed] [Google Scholar]
- Tullberg-Reinert H, Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric microassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histochem Cell Biol. 1999;112:271–6. doi: 10.1007/s004180050447. [DOI] [PubMed] [Google Scholar]
- Uzel MI, Kantarci A, Hong HH, Uygur C, Sheff MC, Firatli E, Trackman PC. Connective tissue growth factor in phenytoin-induced gingival overgrowth. J Periodontol. 2001;72:921–931. doi: 10.1902/jop.2001.72.7.921. [DOI] [PubMed] [Google Scholar]
- van der Pauw MT, Everts V, Beertsen W. Expression of integrins by human periodontal ligament and gingival fibroblasts and their involvement in fibroblast adhesion to enamel matrix-derived proteins. J Periodontal Res. 2002;37:317–23. doi: 10.1034/j.1600-0765.2002.00349.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–64. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15:1430–40. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]


