Abstract
To isolate a gene for stimulating avermectin production, a genomic library of Streptomyces avermitilis ATCC 31267 was constructed in Streptomyces lividans TK21 as the host strain. An 8.0-kb DNA fragment that significantly stimulated actinorhodin and undecylprodigiosin production was isolated. When wild-type S. avermitilis was transformed with the cloned fragment, avermectin production increased approximately 3.5-fold. The introduction of this fragment into high-producer (ATCC 31780) and semi-industrial (L-9) strains also resulted in an increase of avermectin production by more than 2.0- and 1.4-fold, respectively. Subclones were studied to locate the minimal region involved in stimulation of pigmented-antibiotic and avermectin production. An analysis of the nucleotide sequence of the entire DNA fragment identified eight complete and one incomplete open reading frame. All but one of the deduced proteins exhibited strong homology (68 to 84% identity) to the hypothetical proteins of Streptomyces coelicolor A3(2). The orfX gene product showed no significant similarity to any other protein in the databases, and an analysis of its sequence suggested that it was a putative membrane protein. Although the nature of the stimulatory effect is still unclear, the disruption of orfX revealed that this gene was intrinsically involved in the stimulation of avermectin production in S. avermitilis.
Recent studies on various Streptomyces species have shown that many regulatory factors are involved in creating a complex network, which then influences the morphological differentiation and production of secondary metabolites. Such regulatory cascades are consistent with the need of Streptomyces spp. to interact with a variety of environmental changes (3). If the regulatory mechanism of antibiotic biosynthesis can be wholly understood and tightly controlled, this could be a starting point for replacing mutagenesis by UV irradiation or mutagen treatment and the consecutive screening of high-producer strains for improving the productivity of antibiotic biosynthesis. Unfortunately, however, the molecular processes regulating the events leading to differentiation and simultaneous antibiotic biosynthesis are still poorly understood, despite the discovery of numerous useful insights into Streptomyces genetics.
For example, most of the genetic factors regulating avermectin production have not been clearly elucidated, even though avermectin and the avermectin-producing species Streptomyces avermitilis are considered industrially valuable. Avermectin is known to be an excellent anthelminthic agent and highly active against a broad spectrum of nematode and arthropod parasites (6, 15). As a result of its superior activity and widespread market acceptance, the avermectin market exploded during the 1980s and reached U.S. $1 billion at the end of the 1990s.
Recently, the nucleotide sequence of the avermectin biosynthetic gene cluster and the entire genome of S. avermitilis were completely determined (10, 19). The sequencing analysis showed that the avermectin gene cluster spans a distance of 82 kb and has a putative pathway-specific regulatory gene, aveR (10). The aveR product has sequence similarity with RapH in the rapamycin biosynthetic gene cluster and ORF6 in the gene 111 cluster of Streptomyces hygroscopicus. However, despite the completion of the S. avermitilis genome sequencing and the continued accumulation of molecular biological information on this strain, most of the genetic factors regulating avermectin production remain unknown.
Accordingly, the present study describes the cloning, sequencing, and analysis of a putative regulatory fragment of S. avermitilis that causes the overproduction of actinorhodin and undecylprodigiosin in Streptomyces lividans TK21, which only produces a basal level of the pigmented antibiotics under normal conditions. It is also shown that this fragment has a stimulatory effect on avermectin production in various S. avermitilis strains. A sequence analysis of the DNA fragment revealed eight complete and one incomplete open reading frame (ORF) containing a putative regulatory gene. The disruption of this putative regulatory gene severely reduced avermectin production.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Table 1 lists the bacterial strains and plasmids used along with a summary of their characteristics. Three strains of S. avermitilis were used: the wild-type strain ATCC 31267, a high-avermectin-producing mutant, ATCC 31780, and a genetically unstable semi-industrial strain, L-9 (9). S. lividans TK21 was used as both the cloning host and the host for the preparation of all Streptomyces plasmids. Streptomyces plasmids pIJ702, pIJ487 (12), pIJ61 (25), and pIJ941 (14) were used to investigate the copy number effect on antibiotic production in both S. lividans and S. avermitilis. Escherichia coli strains DH5α and JM109 were used for general cloning (21). Plasmids pUC18 and pUC19 were used for subcloning and sequencing (21).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Reference(s) |
|---|---|---|
| Strains | ||
| S. avermitilis ATCC31267 | Wild-type strain (avg avermectin production, 40-60 mg liter−1) | 9 |
| S. avermitilis ATCC31780 | High-producing strain derived from ATCC 31267 (avg avermectin production, 160-200 mg liter−1) | 9 |
| S. avermitilis L-9 | Semi-industrial strain derived from ATCC 31267 (avg avermectin production, 400-600 mg liter−1) | 9 |
| S. lividans TK21 | Cloning host (str-6 SLP2− SLP3−) | 16, 26 |
| E. coli DH5α, JM109 | General cloning hosts | 21 |
| Plasmids | ||
| pIJ702 | Streptomyces cloning vector with mel gene (100-300 copies) | 12, 13 |
| pIJ487 | Streptomyces cloning vector (100-300 copies) | 13 |
| pIJ61 | Streptomyces cloning vector (4-5 copies) | 13, 25 |
| pIJ941 | Streptomyces cloning vector (1-2 copies) | 13, 14 |
| pUC18, pUC19 | E. coli cloning and sequencing vectors | 21 |
| pHP45Ωaac | Source of aacC4 gene | 5 |
| pHP45Ωaac′ | Site-directed mutated aacC4 gene (aac′) without XhoI site | This study |
| pPRA2 | pIJ702 containing 8.0-kb S2 fragment at BglII site | This study |
| pPRA4 | pIJ702 containing 3.6-kb SacI-PstI fragment (S4) of S1 | This study |
| pNS1 | pIJ487 containing 0.7-kb BamHI fragment (NS1) of S1 | This study |
| pNS2 | pIJ487 containing 3.3-kb SacI fragment (NS2) of S1 | This study |
| pNS3 | pIJ487 containing 1.8 kb SacI fragment (NS3) of S1 | This study |
| pNS4 | pIJ487 containing 3.2-kb SacI-BamHI fragment (NS4) of S1 | This study |
| pUC-S18 | pUC19 containing 1.8-kb SacI fragment (NS3) of S1 | This study |
| pUC-S33 | pUC19 containing 3.3-kb SacI fragment (NS2) of S1 | This study |
| pUC-S36 | pUC19 containing 3.6-kb SacI-PstI fragment (NS4) of S1 | This study |
| pMS4 | pIJ61 containing 2.7-kb BamHI-PstI fragment of S1 | This study |
| pLS4 | pIJ941 containing 3.6-kb EcoRI-PstI fragment of pUC-S36 | This study |
| pS2S | pUC19 containing 1.8-kb SphI fragment of S4 | This study |
| pUC19-tsr | pUC19 containing tsr gene on BclI fragment at BamHI site | This study |
| pS2S-tsr | pUC-S36 containing tsr gene isolated from pUC19-tsr | This study |
| pS2S-tsr-aac | pS2S-tsr containing PCR-amplified aac′ gene at XhoI site of orfX | This study |
Media and culture conditions.
S. lividans was grown on R2YE agar plates or in a YEME liquid medium (13). Wild-type S. avermitilis and its high-producing mutants were maintained on Bennet's agar or a YMG agar medium as a sporulation medium (2). To prepare vegetative inocula, spores or mycelia from a plate culture of S. avermitilis on YMG agar were added to 25 ml of liquid SM medium composed of 30 g of glucose, 5 g of soybean meal, 30 g of yeast extract, 5 g of peptone, and 3 g of malt extract per liter. The culture was incubated for 1 to 2 days at 30°C until the production of a melanin-like dark brown pigment started. The avermectin production cultures were inoculated with 1.25 ml of SM culture broth in a modified MF medium composed of 70 g of sucrose, 14 g of soybean meal, 2.5 g of yeast extract, 0.1 g of NaCl, 1 g of CaCO3, 0.5 g of K2HPO4, and 10 g of MOPS (morpholinepropanesulfonic acid) per liter, and 50 glass beads (0.5-mm diameter) were added to each flask. The fermentations were performed in 300-ml baffled flasks on a rotary shaker (225 rpm) at 28°C.
When selecting S. avermitilis transformants, thiostrepton was used at a concentration of 10 μg ml−1 in agar medium and 1 μg ml−1 in liquid cultures. When selecting S. lividans transformants, thiostrepton was used at a concentration of 50 μg ml−1 in agar medium and 5 μg ml−1 in liquid cultures. When selecting gentamicin-resistant E. coli or Streptomyces strains, 20 μg of gentamicin per ml was used for both plate and liquid cultures.
Genomic library construction and DNA manipulations.
Genomic DNA from S. avermitilis ATCC 31267 was isolated with the method described by Kieser et al. (13), and partial digestion of the isolated chromosomal DNA with Sau3AI was optimized. Enriched fractions of 7.0-kb to 10.0-kb fragments were extracted from the agarose gels, and the collected DNA was concentrated by ethanol precipitation. The DNA was ligated into BglII-digested pIJ702, and the ligate was used directly to transform S. lividans TK21 protoplasts. Standard protocols were used for the transformation of S. lividans, plasmid isolation, and colony selection (13). About 7,000 transformants were obtained and analyzed. The isolation of plasmid DNA from E. coli was performed with a Promega Wizard Plus DNA purification kit. The restriction enzymes were obtained from New England Biolabs, Promega, or Boehringer Mannheim, and the digestions were performed according to the manufacturers’ instructions.
Antibiotic production assays.
For the detection of actinorhodin, the absorbance of the culture broth was measured at 590 nm with a spectrophotometer after adjusting the supernatants to pH 12.0 with 1 M NaOH. Undecylprodigiosin was extracted with methanol from the mycelium acidified with 1 M HCl, and the absorption was measured at 530 nm (24). To analyze avermectin, a portion of the culture broth (2 ml) was extracted with 2 ml of methanol by shaking vigorously (300 rpm) for 30 min in a shaking incubator. After removing the methanol, the residue was reextracted with an appropriate volume (0.2 to 2 ml) of dichloromethane. The quantities of the major avermectin components were determined by high-pressure liquid chromatography (HPLC) with a Waters C18 column (3.9 by 150 mm) with methanol-water (85:15, vol/vol) as the mobile phase (7, 18, 22). The elution times of the eight major components, monitored at an absorbance of 246 nm, were 4.65 (B2b), 5.43 (B2a), 6.15 (A2b), 7.54 (A2a), 8.90 (B1b), 11.26 (B1a), 12.36 (A1b), and 16.20 (A1a) min, in the order of elution. An authentic sample of avermectin was kindly provided by LG Biotech and used as the internal standard.
DNA sequencing and nucleotide sequence analysis.
The DNA sequence was read at least two times with the primers indicated in Table 2 with a Perkin Elmer Amplitaq dye terminator sequencing system with double-stranded DNA templates run on an Applied Biosystems 377 automated sequencer. The sequencing reactions were initially performed from vector sequences with universal forward and reverse primers, followed by specific primers generated from the initial sequence data (Table 2). However, one region (bp 6200 to 7500) of the cloned fragment produced unreadable signals when used in the so-called primer-walking method. A high G+C content (average, 74%) can cause such a problem, but the reason was not clearly elucidated. For the sequencing of this region, nested deletions with exonuclease III were generated with an Erase-a-Base kit from Promega. Sequence homology searches were performed with the National Center for Biotechnology Information worldwide BLAST server. The nucleotide sequences were analyzed with PC GENE and FramePlot (11) software.
TABLE 2.
Primers used in sequencing and PCR
| Primer | Nucleotide sequence (5′ to 3′) | Position |
|---|---|---|
| Forward | ||
| S18F1 | ACCTCCACTTCCGGTCGCTGA | 128-148 |
| S18F2 | ATCCATCTCATTCTGGGCGTC | 380-400 |
| S33F1 | TCGTGAGCAGCAGGTTGAGC | 2024-2043 |
| S33F2 | AAACCATCGGCGTTGACGGT | 2455-2474 |
| S33F3 | TCCCACACGTCGCCGATCAA | 2805-2824 |
| S33F4 | ACGCCTGGTACGCGGCGTTC | 3229-3248 |
| S36F1 | CCGGCAATGACTCGTCCCAC | 5239-5258 |
| S36F2 | ACGGGCGTGCGGTGTCGAT | 5536-5554 |
| S36F3 | TCTACACGGACCGGGTCATC | 5871-5890 |
| S36F4 | CCGGGCACTTCGAGCACGTC | 6192-6211 |
| Reverse | ||
| S18R1 | AAGTTCCGCAAGGACGGCGTA | 876-856 |
| S18R2 | AACGGACGGCGTTCCTCGCC | 1220-1201 |
| S18R3 | GGCAGTGAGCTCTTCGAGAA | 1596-1577 |
| S33R1 | GACCTGGCGCACATCGGCAA | 3751-3732 |
| S33R2 | AATCCGCTGTGGATCGAGGC | 4131-4112 |
| S33R3 | TCGACCCGGCAGTACCTGTG | 4548-4529 |
| S33R4 | TACGCCGCATTGCGGACCGT | 4992-4973 |
| S36R1 | TCCGCAAGCCGGCCGACGCGAA | 7456-7435 |
| S36R2 | AAGCTGGGTCTGATCGTCGA | 7817-7798 |
| PCR | ||
| Aac1 | AAGCTCGAGAAGGCCCGATCCTT GGAGCCC | pHP45Ω45aac′ |
| Aac2 | AAGCTCGAGCTCTTGAGTTAAGC CGCGCCG | pHP45Ω45aac′ |
| S36F5 | AAGCCTTACCGTTTCACCCC | 6827-6846 |
| S36R2 | AAGCTGGGTCTGATCGTCGA | 7817-7798 |
Insertional inactivation.
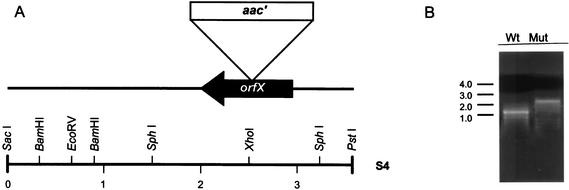
The construction of an S. avermitilis ATCC 31267 mutant by gene disruption (Fig. 5) was accomplished by inserting a gentamicin resistance cassette into the cloned orfX gene in vitro and replacing the chromosomal copy. First, a 1.8-kb SphI fragment of S4 containing orfX was subcloned into pUC19 to create plasmid pS2S. A 1,080-bp PstI-EcoRI fragment containing the thiostrepton resistance gene (tsr) from a pUC19 derivative plasmid, pUC19-tsr, was inserted between the PstI and EcoRI sites in plasmid pS2S to construct plasmid pS2S-tsr. To introduce a gentamicin resistance cassette into a unique XhoI site in orfX, a 1,230-bp gentamicin resistance gene (aac′) was amplified by PCR with plasmid pHP45aac′ as the template. This plasmid was generated from pHP45aac (5) by site-directed mutagenesis with a QuikChange kit from Stratagene, based on replacing an internal XhoI site of CTCGAG with CTGGAG. The resulting plasmid, pS2S-tsr-aac, was used for the transformation of S. avermitilis ATCC 31267.
FIG. 5.
Schematic presentation of construction of orfX mutant. (A) Restriction map and mutagenesis of orfX by insertion of gentamicin resistance gene (aac′). (B) Confirmation of insertion of aac′ gene into orfX by PCR analysis with internal primers S36F5 and S36R2. Wt, wild-type strain; Mut, orfX mutant. Sizes are shown in kilobases.
The recombinant clones were produced by undergoing homologous recombination with the chromosomal copy of orfX and then selected for resistance to gentamicin and sensitivity to thiostrepton. The selected recombinants were subsequently grown without gentamicin on a modified YM agar plate. The gentamicin-resistant colonies containing orfX interrupted by a gentamicin resistance gene were inoculated into medium containing gentamicin at a final concentration of 20 μg ml−1. Confirmation of the final null mutant was achieved by PCR analysis with internal primers S36F5 and S36R2 as described in standard protocols.
RESULTS
Cloning of large DNA fragment activating pigment production and selection of pigment-overproducing colonies.
To identify the regulatory determinant biosynthetically activating quite different antibiotic production in S. lividans and S. avermitilis, a genomic library of S. avermitilis was constructed and transformed into the heterologous host S. lividans TK21. This strain includes all the gene sets required for the biosynthesis of actinorhodin and undecylprodigiosin, yet its pigment production is very low compared to that of its close relative Streptomyces coelicolor under normal conditions (16). In previous reports, S. lividans TK21 has been proved to be an ideal host for this purpose (16, 20, 26).
The genomic library of the pigment-overproducing S. lividans TK21 was first screened by direct color observation. As a result, many colonies (containing possible stimulatory elements) were selected, and their DNA fragments were successfully isolated. The transformants were roughly divided into five groups according to their phenotypic characteristics: blue-pigmented colonies with well-developed aerial mycelia (group I), pale blue- or red-pigmented colonies with well-developed aerial mycelia (group II), nonpigmented or brown-pigmented colonies with well-developed aerial mycelia (group III), nonpigmented or brown-pigmented colonies with poorly developed aerial mycelia (group IV), and blue-pigmented colonies with poorly developed aerial mycelia (group V).
Among these five groups, only the members of group I, with normal differentiation and increased pigment production (90 colonies out of 7,000 transformants), were considered for further analysis. As such, these transformants imply the existence of a class of regulators that act specifically on antibiotic production but not on differentiation. In addition, they also suggest that sporulation can be processed independently of antibiotic biosynthesis through a parallel genetic pathway (1). To verify that the overproductions were based on the unique ability of each individual colony and rule out the effect of signal molecules secreted by other colonies, each colony was restreaked on agar plates and inoculated into liquid medium. As a result, only four transformants exhibited steady pigment production, but the reason for this phenomenon is still unclear. Finally, a DNA analysis of the four dark blue colonies revealed that they all contained the same 8.0-kb DNA fragment, which was designated S2.
Subcloning and quantitative analysis of pigment production.
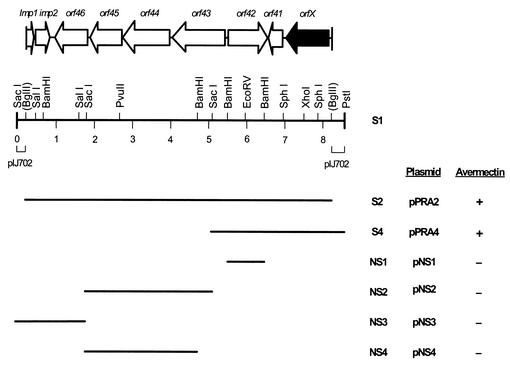
A more detailed restriction map of the S2 fragment was constructed, and the effect of subclones on actinorhodin and undecylprodigiosin production was analyzed (Fig. 1). As shown in Fig. 1, five subclones were generated from the S1 fragment, which contained S2 and two small parts of pIJ702. Among the subclones, only pPRA4, generated from the ligation of the S4 fragment (Table 1) with pIJ702, yielded dark blue colonies on R2YE plates after transformation into S. lividans TK21. The NS1, NS2, NS3, and NS4 fragments with the high-copy plasmid pIJ487 were unable to stimulate pigmented-antibiotic production on the same medium. The absence of pPRA2 or pPRA4 resulted in poor pigment production, yet these plasmids caused a substantial increase in actinorhodin and undecylprodigiosin production when they were reintroduced into S. lividans by retransformation.
FIG. 1.
Restriction map, putative ORFs, and subcloned fragments of 8.0-kb DNA fragment (S2) of S. avermitilis activating both pigmented-antibiotic production in S. lividans and avermectin production in S. avermitilis. The + and − symbols indicate the presence and absence of avermectin production stimulation, respectively, during the growth of the wild-type S. avermitilis strain in MF medium.
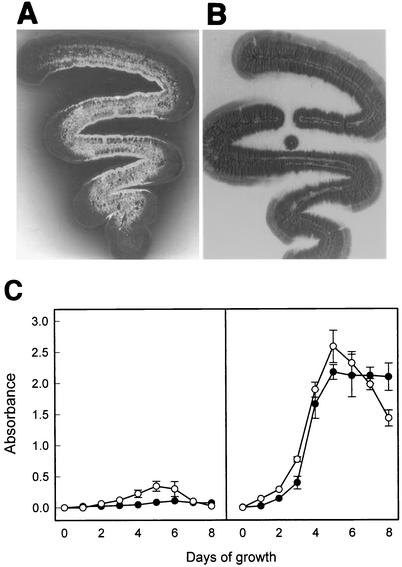
For quantitative analysis of pigment production induced by the S2 fragment, the transformants of S. lividans TK21 carrying pPRA2 (pIJ702 containing the S2 fragment) were cultured in liquid YEME medium. The production of actinorhodin and undecylprodigiosin was followed by spectrophotometric determination (13). After 2.5 to 3.5 days of growth at 30°C, the production increased exponentially (Fig. 2). When the transformants were cured of pPRA2, the amounts of the two pigments produced were equal to those produced by plasmid-free S. lividans. The retransformation of the cured strain with pPRA2 induced a change in the color of the culture broth from yellow to purple or dark blue. Originally, transformants of S. lividans TK21 carrying pIJ702 were used as the control strain. However, untransformed S. lividans was finally used because melanin production confused the analysis of the pigment production.
FIG. 2.
Effect of 8.0-kb Sau3AI DNA fragment (S2) on pigment production in S. lividans TK21 on 5-day-old R2YE plates (upper panels) and in liquid YEME medium (lower panels). (A) TK21 carrying no plasmid; (B) TK21 carrying pPRA2. The dark gray diffusion into the agar medium indicates the blue pigment. (C) Production of actinorhodin (•) and undecylprodigiosin (○) by strain TK21 carrying no plasmid (left side) or carrying plasmid pPRA2 (right side). The A530 and A590 values for undecylprodigiosin and actinorhodin were analyzed and plotted. Each plot represents the mean values of duplicate experiments. Bars indicate standard error.
Effect of copy number of orfX-containing fragments on pigment production.
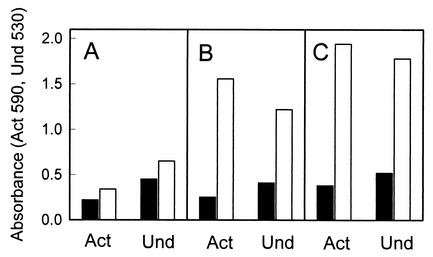
The stimulation of actinorhodin and undecylprodigiosin production caused by the cloned 3.6-kb S4 fragment exhibited a copy number dependency in S. lividans TK21. When the effect of a low copy number was investigated with pIJ941 (14) derived as pLS4 (Table 1) in the S. lividans strain, no dark blue pigment was detected on the agar plates. The absorbance of the pigmented antibiotics visibly increased in the culture broth of S. lividans carrying pLS4 (Fig. 3A). However, a moderate or high number of extrachromosomal copies of an essential region containing orf41 and orfX, in pMS4 (Fig. 3B) and pPRA4 (Fig. 3C), respectively, significantly increased pigment production in the same host.
FIG. 3.
Effect of copy number of orfX-containing fragments on pigment production in S. lividans TK21 cultured in liquid YEME medium for 5 days. (A) ▪, pIJ941; □, pLS4 (pIJ941 containing 3.6-kb EcoRI-PstI fragment of S1). (B) ▪, pIJ61; □, pMS4 (pIJ61 containing 2.7-kb BamHI-PstI fragment of S1). (C) ▪, pIJ702; □, pPRA4 (pIJ702 containing 3.6-kb SacI-PstI fragment of S2). Act, actinorhodin; Und, undecylprodigiosin.
Enhancement of avermectin production by introduction of cloned S2 fragment into wild-type S. avermitilis and its high-producing mutant strains.
Based on the copy number effect on pigment production, it would appear that multiple fragment copies can substantially increase avermectin production in S. avermitilis. As expected, the high-copy-number plasmid pPRA4 increased overall avermectin production the most among the three S. avermitilis strains. The eight major avermectin components were quantified in cultures of the wild type and its high-producer mutants as well as their transformants carrying the high-copy-number plasmid pPRA4 (Fig. 4). Plasmid pMS4 was found to be unsuitable for S. avermitilis because the SLP1.2 origin seemed to prevent autonomous replication in this Streptomyces species, as in S. coelicolor (13). In the case of pLS4, plasmid replication was normally processed in S. avermitilis, yet no detectable increase in avermectin production was observed (data not shown).
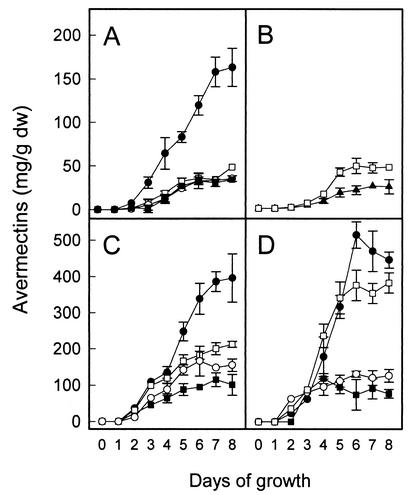
FIG. 4.
Overall avermectin production in wild-type S. avermitilis (A and B), high-producer strain ATCC 31780 (C), and semi-industrial strain L-9 (D) cultured in modified MF medium for 8 days. Symbols: □, no plasmid; ▴, orfX disrupted; •, pPRA4 introduced by electroporation of intact cells; ○, pPRA4 introduced by polyethylene glycol-mediated transformation; ▪, pIJ702 introduced by polyethylene glycol-mediated transformation. Thiostrepton addition had no effect on the growth of the transformants, and each plot represents the mean values of three independent experiments. Bars indicate standard error.
Accordingly, these data prompted an investigation of the effect of the 3.6-kb SacI-PstI region of S1 in the high-copy-number plasmid pPRA4. Figure 4 shows that avermectin was initially detected after 2 days of growth; thereafter, the level of avermectin increased steadily during the exponential phase and subsequent stationary phase. S. avermitilis carrying pPRA4 exhibited a profound increase in avermectin production, while the level of avermectin production in the S. avermitilis transformants carrying only pIJ702 remained unchanged. When the transformants lost plasmid pPRA4, avermectin production returned to the original level produced by the plasmid-free cells. However, retransformation of the cured strain with pPRA4 restored increased antibiotic production. In the case of the high-producing mutant strains ATCC 31780 and L-9, polyethylene glycol-mediated transformation with pPRA4 severely reduced overall avermectin production (Fig. 4C and D). These reductions were found to be related to the removal and regeneration of the cell wall, which are essential for the polyethylene glycol-mediated transformation procedure (9). Therefore, to prevent this unnecessary decrease, the previously developed electrotransformation procedure (9) was applied to these high-producing strains with satisfactory results (Fig. 4C and D).
Analysis of nucleotide sequences.
To sequence the 8.0-kb S2 fragment, three subclones were constructed, and the determination of the entire nucleotide sequence was completed (EMBL accession number AF440828). A coding region analysis of the 7,996-bp sequence with the FramePlot (11) and PC Gene programs revealed the presence of eight complete and one incomplete ORF, which were named (from left to right) imp1, imp2, orf46, orf45, orf44, orf43, orf42, orf41, and orfX (Fig. 1). imp1, imp2, and orf42 were transcribed from right to left, whereas orf46, orf45, orf44, orf43, orf41, and orfX were divergently transcribed. These genes exhibit the characteristics of typical Streptomyces genes because they contain an overall G+C content of 72.0 mol% and 90.1% G+C in the third-letter position.
The putative ORFs and their features deduced from the nucleotide sequences are summarized in Table 3. A similarity search highlighted a significant similarity between the incomplete IMP1 and complete IMP2 proteins and the putative integral membrane proteins IMP1 and IMP2 of S. coelicolor, respectively. The sequences of the ORF46, ORF45, ORF44, ORF43, ORF42, and ORF41 proteins showed high levels of similarity with the hypothetical proteins of S. coelicolor. The similarity or identity levels observed were within a range of 71 to 84% for the entire amino acid sequences. Unfortunately, the S. coelicolor proteins homologous to ORF46, ORF45, ORF44, ORF43, ORF42, and ORF41 from S. avermitilis are hypothetical gene products, and thus their functions are unknown. The orfX protein exhibited no significant similarity with any of the proteins in the SWISSPROT and EMBL databases. A low level of similarity (35% identity) was found between the orfX protein and the putative membrane protein SC66T3.18c (EMBL database accession number AL079348) of S. coelicolor.
TABLE 3.
Relevant features deduced from DNA sequences
| ORF | Homologous protein (% similarity) | Initiation-stop codons | No. of amino acids | Predicted function |
|---|---|---|---|---|
| imp1 | S. coelicolor SC10H5.02c (71) | −148 TGA | 49 | Integral membrane protein |
| imp2 | S. coelicolor SC10H5.03c (68) | ATG182-628TGA | 149 | Integral membrane protein |
| orf46 | S. coelicolor SCM1.46 (78) | GTG1764-763TGA | 333 | Unknown |
| orf45 | S. coelicolor SCM1.45 (78) | ATG2588-1761TGA | 275 | Unknown |
| orf44 | S. coelicolor SCM1.44 (82) | ATG3910-2588TGA | 441 | Unknown |
| orf43 | S. coelicolor SCM1.43 (74) | GTG5205-3907TGA | 433 | Unknown |
| orf42 | S. coelicolor SCM1.42c (72) | GTG5466-6341TGA | 291 | Unknown |
| orf41 | S. coelicolor SCM1.41 (84) | GTG6750-6295TGA | 151 | Unknown |
| orfX | S. coelicolor SC66T3.18c (35) | ATG7823-6840TGA | 327 | Putative membrane protein |
Accordingly, compared to the arrangement of the S. coelicolor chromosome, the current results suggest that a chromosomal rearrangement of S. avermitilis may have occurred as a result of internal genetic instability. So far, no homology has been found in the ongoing but still incomplete S. avermitilis genome sequencing (10, 19). However, since a database search is insufficient to elucidate the whole function of the large DNA fragment, as many of the functions of the Streptomyces proteins are still in question, further research, including disruption of each ORF, protein isolation, characterization, and comparison of the effects between orfX and aver, is needed to provide a more accurate answer to why multiple copies of the S2 fragment and orfX-containing fragments caused an increase in antibiotic production, as described previously.
Distribution of orfX sequence among avermectin-high-producing mutant strains.
To identify the distribution of the orfX sequence among high-producing S. avermitilis strains, a PCR analysis with the internal primers S36F5 and S36R2 (Table 2) was performed. A 973-bp orfX region of the S4 fragment was used as the target. The amplified bands were found in the wild-type strain ATCC 31267 as well as in the high avermectin producers ATCC 31780 and L-9 (data not shown). These results, together with the results of the gene disruption experiment, strongly suggest that either the entire sequence or a considerably conserved sequence of orfX exists in the high producers and that the orfX product appears to play an essential role in the production and regulation of avermectin in both the normal strain and the high producers.
Construction of orfX disruptant.
To investigate the possible role of the orfX gene product in S. avermitilis, nucleotide sequencing was performed and an orfX disruptant was generated by insertional inactivation, as described in Materials and Methods. The finally constructed vector, pS2S-tsr-aac, contained the orfX gene interrupted at a unique XhoI site by the aac′ gene oriented in the direction of the transcription of orfX. pS2S-tsr-aac was used to transform protoplasts of S. avermitilis ATCC 31267 for the disruption of orfX in its chromosome (Fig. 5). The orfX-disrupted S. avermitilis strain ATCC 31267 grew and sporulated normally on modified YM agar medium. However, the amount of avermectin produced by the disruptant was less than that produced by the wild-type S. avermitilis ATCC 31267 both on agar medium and in liquid culture (Fig. 4B). As such, the disruption of the orfX gene resulted in a significant yet incomplete loss of avermectin production, and the negative effect of the disruption on avermectin production was similar to other examples of positive regulatory gene disruption (8, 16, 17, 23).
As expected, retransformation of the orfX-disrupted mutant with pPRA4 restored its avermectin production ability, implying that the mutation was compensated for by the introduction of this plasmid. However, because pPRA4 carrying both orfX and orf41 was used to complement the null mutant, the gentamicin resistance cassette could have polar effects on orf41, and thus the avermectin restoration could be the result of the introduction of orfX or a combination of orfX and orf41. The former and latter results indicate that the orfX gene is required for the stimulation of avermectin production, while the other gene(s) in the S2 fragment appears to have more of an assisting role in avermectin production.
DISCUSSION
The primary goal of the present study was to enhance avermectin production through cloning and manipulating a stimulatory genetic element instead of the traditional methods for strain development, for example, random mutagenesis and genome recombination by protoplast fusion. Although S. avermitilis is considered an industrially important Streptomyces strain, comprehensive studies on the essential molecular factors influencing avermectin productivity are still incomplete. To clone a stimulatory gene positively affecting avermectin production, a genomic DNA library of S. avermitilis was constructed and an 8.0-kb DNA fragment which stimulated actinorhodin and undecylprodigiosin production in S. lividans TK21 was successfully isolated. It was particularly interesting that the same DNA fragment also increased avermectin biosynthesis in various S. avermitilis strains, including the high-producing mutant strain ATCC 31780 and a semi-industrial strain L-9.
To verify the enhancing effect caused by this large DNA fragment, DNA sequencing and molecular biological analyses were performed. The subcloning of fragments into various vectors with different copy numbers, gene disruption (insertional inactivation) of a determinant region, and comparison of the sequences with known sequences in the databases demonstrated that a DNA region containing orfX and orf41 was essential for the stimulatory effect and that orfX may be a membrane-bound putative regulatory gene. A comparison of the ORFs in the 8.0-kb S. avermitilis DNA with the chromosome sequence of S. coelicolor A3(2) revealed that homologous counterparts to the ORFs were scattered all over the S. coelicolor genome (SCM1, SC66T3, and SC10H5), suggesting the possibility that this distribution was caused by severe genetic rearrangement in S. avermitilis. As such, it will be interesting to investigate the relationship between aveR (a putative pathway-specific regulatory gene for avermectin production) (10) and the cloned genes, especially orfX.
The current examples of yield enhancement based on the isolation and manipulation of a stimulatory factor in a relatively high-producing strain and a semi-industrial strain are indeed encouraging and meaningful to the microbial and biotechnological field as this approach can be applied directly to numerous industrial strains.
Acknowledgments
This work was funded by the Brain Korea 21 program, supported by the Korean Ministry of Education and partially aided by the Hungarian-Korean Intergovernmental Science and Technological Cooperation project (KOR-2/99) supported by the Hungarian Ministry of Education.
REFERENCES
- 1.Adamidis, T., and W. Champness. 1992. Genetic analysis of absB, a Streptomyces coelicolor locus involved in global antibiotic regulation. J. Bacteriol. 174:4622-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas, R. M. 1993. Handbook of microbiological media, 1st ed., p. 1006-1007. CRC Press, Boca Raton, Fla.
- 3.Bibb, M. J. 1996. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142:1335-1344. [DOI] [PubMed] [Google Scholar]
- 4.Bibb, M. J., P. R. Findlay, and M. W. Johnson. 1984. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene 30:157-166. [DOI] [PubMed] [Google Scholar]
- 5.Blondelet-Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the Ω interposon for use in E. coli and Streptomyces. Gene 190:315-317. [DOI] [PubMed] [Google Scholar]
- 6.Croft, S. L. 1997. The current status of antiparasite chemotherapy. Parasitology 114:3-15. [PubMed] [Google Scholar]
- 7.Curdova, E., J. Zima, V. Jechova, and Z. Vanek. 1989. The effect of inorganic phosphate on the production of avermectin in Streptomyces avermitilis. J. Basic Microb. 29:341-346. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Bernardo, J., A. F. Brana, C. Mendez, and J. A. Salas. 2000. Insertional inactivation of mtrX and mtrY genes from the mithramycin gene cluster affects production and growth of the producer organism Streptomyces argillaceus. FEMS Microbiol. Lett. 186:61-65. [DOI] [PubMed] [Google Scholar]
- 9.Hwang, Y. S., J. Y. Lee, E. S. Kim, and C. Y. Choi. 2001. Optimization of transformation procedures in avermectin high-producing Streptomyces avermitilis. Biotechnol. Lett. 23:457-462. [Google Scholar]
- 10.Ikeda, H., T. Nonomiya, M. Usami, T. Ohta, and S. Omura. 1999. Organization of the biosynthetic gene cluster for the polyketide anthelminthic macrolide avermectin in Streptomyces avermitilis. Proc. Natl. Acad. Sci. USA 96:9509-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa, J., and K. Hotta. 1999. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G+C content. FEMS Microbiol. Lett. 174:251-253. [DOI] [PubMed] [Google Scholar]
- 12.Katz, E., C. J. Thompson, and D. A. Hopwood. 1983. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J. Gen. Microbiol. 129:2703-2714. [DOI] [PubMed] [Google Scholar]
- 13.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom
- 14.Lydiate, D. J., F. Malpartida, and D. A. Hopwood. 1985. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene 35:223-235. [DOI] [PubMed] [Google Scholar]
- 15.Martin, R. J., A. P. Robertson, and H. Bjorn. 1997. Target sites of anthelminthics. Parasitology 114:111-124. [PubMed] [Google Scholar]
- 16.Martínez-Costa, O. H., A. J. Martín-Triana, E. Martínez, M. A. Fernádez-Moreno, and F. Malpartida. 1999. An additional regulatory gene for actinorhodin production in Streptomyces lividans involves a LysR-type transcriptional regulator. J. Bacteriol. 181:4353-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto, A., S. K. Hong, H. Ishizuka, S. Horinouchi, and T. Beppu. 1994. Phosphorylation of the AfsR protein involved in secondary metabolism in Streptomyces species by a eukaryotic-type protein kinase. Gene 146:47-56. [DOI] [PubMed] [Google Scholar]
- 18.Omura, S., H. Ikeda, and H. Tanaka. 1991. Selective production of specific components of avermectins in Streptomyces avermitilis. J. Antibiot. 44:560-563. [DOI] [PubMed] [Google Scholar]
- 19.Omura, S., H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, and M. Hattori. 2001. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 98:12215-12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero, N. M., V. Parro, and R. P. Mellado. 1994. Expression of a heterologous gene activating actinorhodin biosynthesis in Streptomyces lividans and Streptomyces coelicolor. FEMS Microbiol. Lett. 116:301-306. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 22.Schulman, M. D., D. Valentino, M. Nallin, and L. Kaplan. 1986. Avermectin B2 O-methyltransferase activity in Streptomyces avermitilis mutants that produce increased amounts of the avermectins. Antimicrob. Agents Chemother. 29:620-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekurova, O., H. Sletta, T. E. Ellingsen, S. Valla, and S. Zotchev. 1999. Molecular cloning and analysis of a pleiotropic regulatory gene locus from the nystatin producer Streptomyces noursei ATCC 11455. FEMS Microbiol. Lett. 177:297-304. [DOI] [PubMed] [Google Scholar]
- 24.Strauch, E., E. Takano, H. A. Baylis, and M. J. Bibb. 1991. The stringent response in Streptomyces coelicolor A3(2). Mol. Microbiol. 5:289-298. [DOI] [PubMed] [Google Scholar]
- 25.Thompson, C. J., T. Kieser, J. M. Ward, and D. A. Hopwood. 1982. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene 20:51-62. [DOI] [PubMed] [Google Scholar]
- 26.Vogtli, M., P. C. Chang, and S. N. Cohen. 1994. afsR2: a previously undetected gene encoding a 63-amino-acid protein that stimulates antibiotic production in Streptomyces lividans. Mol. Microbiol. 14:643-653. [DOI] [PubMed] [Google Scholar]
- 27.Wright, F., and M. J. Bibb. 1992. Codon usage in the G+C-rich Streptomyces genome. Gene 113:55-65. [DOI] [PubMed] [Google Scholar]