Abstract
We describe an efficient inducible system to regulate gene expression in plants based on quorum-sensing components found in Gram-negative bacteria such as Agrobacterium tumefaciens. These bacteria monitor their own population density by utilizing members of the N-acyl homoserine lactone family as inducers and a transcriptional activator as its receptor. In our study, we utilize the components from A. tumefaciens (i.e. 3-oxooctanyl-l-homoserine lactone [OOHL]) synthesized by the TraI protein and its receptor, TraR. When OOHL binds to TraR, it recognizes its specific cis-element, the tra box. We translationally fused the eukaryotic VP16 activation domain to the N terminus of TraR. In the presence of OOHL, the chimeric VP16:TraR transcriptional regulator induces reporter gene expression in moss (Physcomitrella patens), barley (Hordeum vulgare), and carrot (Daucus carota) cells, as well as in transgenic Arabidopsis (Arabidopsis thaliana) seedlings. The inducible system shows a low level of reporter gene expression in the absence of the inducer. Foliar application and a floating-leaf assay in the presence of the inducer shows a 30- and 200-fold induction, respectively. Induction by foliar application of the inducer to whole seedlings is achieved within 8 h. The VP16:TraR activator also shows specificity for binding to its cognate inducer, OOHL. Based on microarray analyses, endogenous gene expression is not significantly affected due to overexpression of the TraR protein or presence of OOHL in either wild-type or lactone-inducible transgenic plants.
Chemically inducible gene expression systems are very useful molecular tools to tightly control temporal and spatial gene expression in cases where constitutive expression of transgenes or RNAi constructs is not desirable or is detrimental to cells. It is also useful to switch on regulator genes in specific tissues to observe whole-genome expression patterns and to construct genetic regulatory networks. Most efforts in developing eukaryotic gene expression systems involve small molecules and their receptor transcriptional regulator either by utilizing inducible systems existing in prokaryotes and/or by developing new and artificial systems based on synthetic transcription factors. The inducer and receptor of these systems that have been adapted for plants include tetracycline and TetR (Weinmann et al., 1994; Blau and Rossi, 1999), ethanol and ALCR (Caddick et al., 1998; Roslan et al., 2001) and its recent modification (Roberts et al., 2005), copper and ACE1 (Mett et al., 1993; McKenzie et al., 1998), steroid hormones dexamethasone and estradiol with glucocorticoid receptor and estrogen receptor, respectively (Bruce et al., 2000; Zuo et al., 2000; Samalova et al., 2005), and insecticide ecdysone and EcR (Koo et al., 2004). Each of these systems has its advantages and disadvantages and successful application depends on a particular experimental system (for review, see Schauder and Bassler, 2001; Padidam, 2003). We show in this article that when the component of the quorum-sensing system found in Agrobacterium tumefaciens is modified, it can serve as an inducible system in a variety of plant cells with many desirable characteristics.
Bacteria such as A. tumefaciens regulate sets of gene expression in response to the density of the population by utilizing small signaling molecules (N-acyl homoserine lactone [HSL]), i.e. quorum sensing. Diverse physiological processes are regulated by quorum-sensing mechanisms, such as bioluminescence in marine bacterium Vibrio fischeri and conjugal transfer of the Ti plasmid in A. tumefaciens, allowing bacteria to better adapt to the environment. The most widely known quorum-sensing system in Gram-negative bacteria is composed of the inducer N-acyl-hexanoyl-l-homoserine lactone synthesized by LuxI from V. fischeri and its receptor/transcriptional regulator LuxR (Fuqua et al., 1994). In A. tumefaciens, the inducer is 3-oxooctanyl-l-homoserine lactone (OOHL) synthesized by TraI, and the receptor/regulator is TraR (Hwang et al., 1994). Crystal structure studies show that TraR contains a helix-turn-helix motif (Zhu and Winans, 1999; Zhang et al., 2002). The C-terminal region of the TraR protein is for DNA binding and transcriptional activation, whereas the N-terminal region is involved in dimerization and proteolysis signaling (Chai and Winans, 2005). A recent study also indicates that a stable homodimer forms only when TraR binds to a single molecule of OOHL in a 1:1 ratio (Zhang et al., 2002). The homodimer TraR-OOHL complex activates an 18-bp cis-acting recognition sequence (i.e. the tra box) in the promoters of at least three different genes, traA, traI, and traR. Both traI and traR are located in the Ti plasmid, and both genes are positively regulated by the TraR-OOHL complex in A. tumefaciens (Fuqua and Winans, 1996). Each member of the HSL family shows specificity to its receptor. The specificity of the inducer is determined by its lactone ring and the number of carbon fatty acid chains (Fuqua and Winans, 1996; Zhu et al., 1998).
TraR has been extensively studied in bacterial systems as described above, as well as in mammalian cells (Neddermann et al., 2003). Here we describe a lactone-inducible system using TraR and OOHL that can induce reporter gene expression in bryophytes (moss [Physcomitrella patens]), dicots (carrot [Daucus carota] and Arabidopsis [Arabidopsis thaliana]), and monocots (barley [Hordeum vulgare]).
RESULTS
OOHL Induces Transient β-Glucuronidase Expression in a Variety of Plant Cells
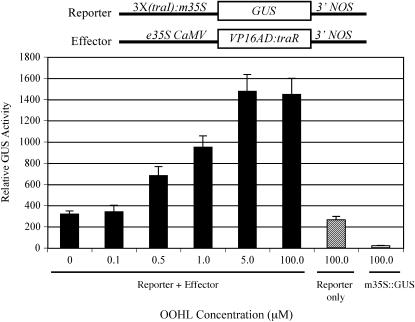
In mammalian cells, a eukaryotic activation domain was required for TraR to be functional in stimulating transcription (Neddermann et al., 2003). In this study, the effector construct was composed of the TraR activator C-terminally fused to the eukaryotic activation domain VP16 AD (VP16:TraR) and linked to the cauliflower mosaic virus (CaMV) 35S promoter. The reporter construct was composed of three copies of the OOHL-responsive traI box [3×(traI)] from the promoter of traI, joined to the minimal (−46 bp) CaMV 35S (m35S) promoter to drive the β-glucuronidase (GUS) reporter. Significant expression of GUS over the basal activity was observed in carrot protoplasts with as little as 0.5 μm OOHL and reached its maximal activity (6-fold) at 5 μm OOHL. However, the basal activity of the 3×(traI)∷GUS reporter in the absence of the inducer OOHL was about 5-fold higher than that of the minimal promoter alone (Fig. 1).
Figure 1.
GUS expression in carrot protoplasts in response to OOHL. Protoplasts cotransfected with the indicated reporter and effector constructs were incubated with OOHL for 36 h. The minimal (−46 bp) 35S driving the GUS reporter (m35S∷GUS) was used as a control. Error bar represents sd from the mean of three samples.
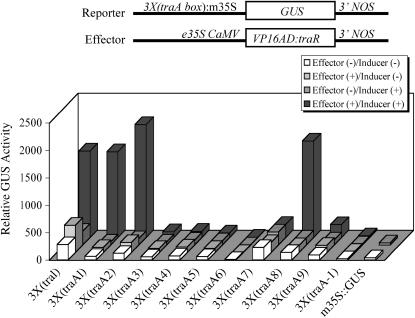
To further reduce the background activity of the 3×(traI)∷GUS reporter, we selected the perfect palindromic binding site from the promoter of traA and systematically changed every pair of nucleotide bases in the traA box. Reporter constructs containing three copies of the corresponding mutant binding site (traA1–traA9) were tested in transient assay along with the original traI box and a single base-pair deletion (traA-1) construct (Fig. 2). Three out of 10 constructs (traA1, 2, and 8) retained the ability to be strongly activated (>11-fold) by the effector plus the OOHL inducer and had significantly reduced background activity when compared with the unmodified traI (Fig. 2). Based upon these results, we chose four copies of the traA8 box [4×(traA8)] joined to the m35S (or Amy64 for barley) linked to either GUS or luciferase (LUC) as the optimal reporter construct in all further experiments.
Figure 2.
Effect of traA box mutagenesis on GUS expression in carrot protoplasts. Protoplasts transfected with the mutated traA box joined to m35S∷GUS (see “Materials and Methods”) were cotransfected with or without effector in the absence or presence of 25 μm OOHL for 36 h. The 3×(traI) indicates three copies of the original traI box reporter construct used in Figure 1. m35S∷GUS was used as a control. Values represent the mean of three samples.
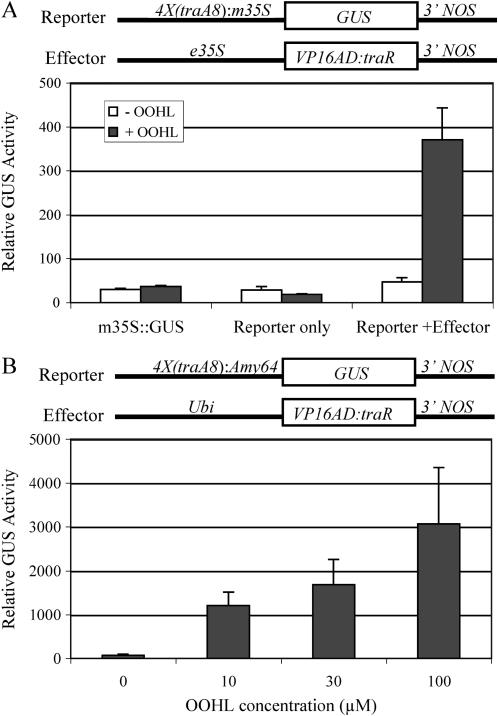
We tested the 4×(traA8) reporter using transient assays in moss protoplasts and barley aleurone cells. We observed a 7-fold induction of 4×(traA8)∷GUS reporter expression in moss protoplasts treated with 100 μm OOHL for 24 h (Fig. 3A). The reporter and effector constructs, modified for use in monocot plants, were cobombarded into barley aleurone cells and treated with various concentrations of OOHL inducers for 24 h, resulting in an over 25-fold increase in activity with 100 μm OOHL (Fig. 3B).
Figure 3.
GUS expression in moss protoplasts and barley aleurone cells in response to OOHL. A, Moss protoplasts were cotransfected with reporter and/or effector constructs and incubated in the absence or presence of 100 μm OOHL for 24 h. m35S∷GUS was used as a control. GUS activity was normalized by LUC activity (Actin∷GFP:LUC). Error bar represents se from the mean of seven samples. B, Barley aleurone cells were cotransfected with the reporter and effector constructs and incubated with different OOHL concentrations. GUS activity was normalized by LUC activity (Ubi-LUC). Error bar represents se from the mean of four samples.
OOHL Induces LUC Expression in Arabidopsis Transgenic Plants
A binary vector of the lactone-inducible system was used for Arabidopsis transformation. The vector contained CaMV 35S∷vp16:traR coupled to the 4×(traA8) box with the m35S promoter controlling the LUC gene (Fig. 4). Single-copy, single-insertion lines were selected on hygromycin and confirmed by Southern analysis (data not shown). Overexpression of the VP16:TraR (CaMV 35S∷vp16:traR) showed normal phenotypes at all developmental stages and over several generations.
Figure 4.
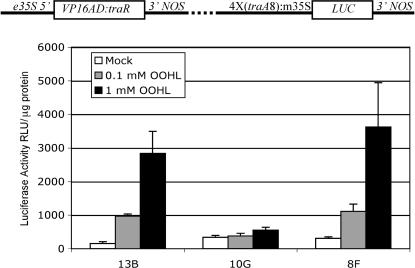
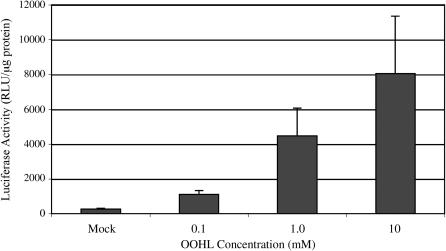
LUC expression in independent transgenic Arabidopsis lines in response to OOHL. The inducible LUC construct used in transgenic Arabidopsis was constructed in a vector as shown in the top. Homozygous 12-d-old seedlings from three independent T3 lines (13B, 10G, and 8F) were assayed for LUC activity after 24 h following a single foliar spray with or without 0.1 or 1.0 mm OOHL. For mock treatment, 0.1% DMSO was used alone. The background LUC activity of 12-d-old wild-type seedlings was 24 ± 4 (se) RLU/μg protein. Error bar represents se from the mean of three samples. RLU, Relative light units.
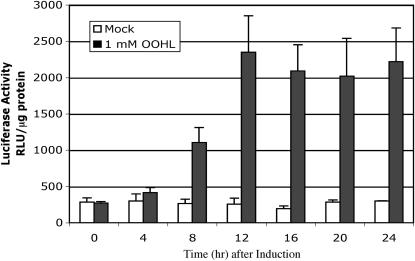
The seedlings of individual T3 homozygous lines were treated with a single foliar spray of OOHL and showed variable levels of inducible LUC activity (4- to 11-fold) after 24 h (Fig. 4). The basal LUC expression of the individual lines without inducer showed 6- to 14-fold higher LUC activity compared to wild-type untransformed plants. However, we have new lactone-inducible plants with a slightly different construct showing only about 2-fold higher basal LUC activity than wild-type seedlings, which indicates the background activity is due to the particular construct (data not shown). The inducer-dependent LUC expression in line 8F (see Fig. 4) was linear from 0.1 to 10 mm OOHL (P < 0.05), and showed 16- and 30-fold induction at 1 and 10 mm, respectively (Fig. 5). Significant LUC activity was first detected at 8 h after seedlings were sprayed once with 1 mm OOHL, with maximal induction (16-fold) attained after 12 h (Fig. 6). After removal of the inducer at 24 h by washing, LUC activity decreased to control levels after an additional 24 h (data not shown).
Figure 5.
LUC expression in transgenic Arabidopsis seedlings in response to OOHL. Two-week-old seedlings were assayed after 24 h following a single foliar spray with different concentrations of OOHL. For mock treatment, 1% methanol was used alone. Error bar represents se from mean of six samples for mock and 1 mm OOHL and three samples for 0.1 and 10 mm OOHL treatment. Activity in all OOHL-treated tissue was significantly different from the mock treatment (P < 0.05). RLU, Relative light units.
Figure 6.
Time course of LUC expression in transgenic Arabidopsis seedlings in response to OOHL. Twelve-day-old seedlings were assayed after 24 h following a single foliar spray with or without 1 mm OOHL. For mock treatment, 0.1% methanol was used. Error bar represents se from the mean of six and three samples for the OOHL and mock treatments. After 8 h, all activity measurements were significantly different from the control (P < 0.05). RLU, Relative light units.
VP16:TraR Activator Shows Specificity to OOHL
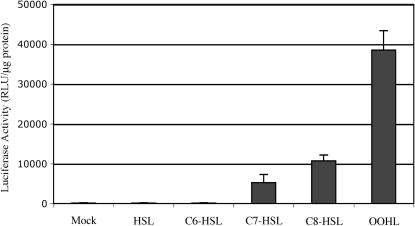
All lactone inducers found in Gram-negative bacteria contain a HSL moiety and a fatty acid acyl group, the latter showing variation in length, saturation chains, and oxidation states (Zhu et al., 1998). To investigate the affinity of TraR to its cognate OOHL, four different commercially available lactone molecules were tested. Leaves taken from 3-week-old seedlings were floated in small volumes of 1 mm of each inducer solution. After 24 h, the leaves were assayed for LUC activity (Fig. 7). HSL and C6-HSL were inactive, while C7-HSL and C8-HSL, which structurally resemble OOHL, induced LUC expression by 30- and 60-fold, respectively, which was statistically significant (P < 0.05). However, neither was as active as the cognate OOHL in the response assay that exhibited a 210-fold increase of LUC activity to the mock treatment.
Figure 7.
Specificity of TraR toward members of the HSL family. Leaves detached from 3-week-old seedlings were floated on 1.0 mm of the indicated HSL solution for 24 h. A 1% solution of DMSO was used as the mock treatment. Error bar represents se from the mean of three samples. RLU, Relative light units.
Endogenous Gene Expression Is Not Affected by OOHL Treatment
We examined the transcription profile of wild-type seedlings treated with the inducer as well as transgenic seedlings containing the lactone-inducible system with and without the inducer. The differential expression of about 20,000 genes was determined using Agilent Arabidopsis DNA microarrays and a two-color (Cy3 and Cy5) labeling and hybridization method. Under the criteria of a 2-fold-change cutoff and a P value <0.05, five genes were differentially expressed when wild-type plants were treated with the inducer. Transgenic seedlings containing all components of the inducible system showed only three genes differentially expressed when compared to wild type and five genes when treated with the inducer. There were no common genes among the total of 13 genes that were differentially expressed by the insertion event and the inducer treatment in both wild-type and transgenic seedlings (Supplemental Table I). In addition, the lists of genes with a 1.5-fold cutoff are available in Supplemental Tables II to IV (compare with “Materials and Methods”).
To address the issue of multiple testing and determine whether the small number of genes changed were simply due to random events, we performed a false discovery rate (FDR; type-I error rate) analysis (Benjamini and Hochberg, 1995). None of the 13 genes met the FDR < 0.05. Therefore, we concluded that the lactone or any components of the inducible system had no effect on the expression of endogenous genes.
DISCUSSION
We documented the use of the quorum-sensing components from A. tumefaciens as a gene-switch system for use in a variety of plants, including bryophytes (moss), dicots (carrot and Arabidopsis), and monocots (barley). The inducible gene expression system was composed of the TraR receptor/transcription factor and the lactone inducer OOHL. Reporter gene expression was induced by OOHL in transient assays as well as by foliar application to transgenic Arabidopsis plants. Induction by OOHL was observed as early as 8 h after spraying Arabidopsis leaves with the inducer. We also showed strong inducer activity (>200-fold) between TraR and its cognate inducer OOHL in a detached-leaf assay using these same transgenic Arabidopsis plants.
Low toxicity and specificity of the inducer to plant cells are also important in inducible systems to avoid nonspecific induction by environmental stress or other types of cues. In this study, overexpression of the chimeric VP16:TraR activator did not cause any developmental abnormality in stable transgenic Arabidopsis plants. Some chemically inducible systems have an effect on endogenous gene expression by the inducer or transcriptional regulator (Padidam, 2003). The lactone-inducible system described here has no effect on the endogenous gene expression profile in Arabidopsis. Microarray analyses confirmed that overexpression of the TraR protein and the OOHL inducer treatment had no effect on expression of endogenous genes. Similar analysis was reported in yeast (Saccharomyces cerevisiae) to address the effect of the tet-regulatable promoter system on endogenous gene expression (Wishart et al., 2005). Furthermore, the OOHL concentration (1 mm) used in this study did not cause any visible toxicity to plant tissues. We also showed strong inducer activity (>200-fold) between TraR and its cognate inducer OOHL in a detached-leaf assay using these same transgenic Arabidopsis plants. In agreement with bacterial studies (Zhu et al., 1998; Luo et al., 2003), we showed that HSL alone and the short carbon-chain representative of the homoserine family (C6-HSL) were unable to activate this system in the detached-leaf assay. Also, the noncognate C7-HSL and C8-HSL inducers showed 3- to 6-fold lower reporter activity than the cognate OOHL. This could be due to the overexpression of TraR (35S∷vp16:traR) in Arabidopsis, resulting in nonspecific binding to these noncognate inducers, which was also observed in a bacterial study (Zhu et al., 1998).
It also appears that one can control the level of expression using this inducible system because there is a wide dynamic range of inducer activity with no detectable toxicity of the inducer. Furthermore, foliar application resulted in local expression followed by a rapid decline in reporter gene activity when the inducer was removed. This characteristic could be used to shorten the switch-off response time by spraying. Another strategy to control the levels of expression with this system is to take advantage of the weakly bound noncognate inducers to TraR. It has been reported that induction by the noncognate inducer exhibited a more rapid loss of activity when removed (Zhu et al., 1998; Luo et al., 2003). This characteristic could be used to induce lower expression levels of specific genes and/or to quicken the switch-off response in postwashing (i.e. a short burst of activity followed by a fast switch-off response could be achieved by applying the noncognate inducer).
Our study of the TraR- and OOHL-based quorum-sensing system from A. tumefaciens reports its use in plants. The lactone-inducible system has a quick response, adjustable switch-off response, inducer specificity, a rapid foliar uptake of the inducer, and no effect on endogenous gene expression. An important characteristic of this system is the tight specificity between the inducer and receptor. There are many quorum-sensing systems found in Gram-negative bacteria showing inducer/receptor specificity in response to induction and/or repression abilities (e.g. a repression [OFF] switch by EsaR from Pantoea stewartii [Minogue et al., 2002]). Our work establishes the background for application of lactone-inducible systems from bacteria to control gene expression in a wide variety of plants.
MATERIALS AND METHODS
TraR Effector Construct for Transient Assays
TraR was isolated by PCR from Agrobacterium tumefaciens strain A348 genomic DNA using Pwo DNA polymerase and the following primers: traR25′, 5′-CATATGCAGCACTGGCTGG and traR13′, 5′-GTCGACCTCAGATGAGTTTCCG. The isolated TraR gene differed slightly from the sequence previously published (Fuqua et al., 1994). Two GC→CG substitutions affecting codons 173, 174, and 212 resulted in C173W, V174L, and R212A changes in the predicted amino acid sequence of the TraR protein. It is not clear whether these differences resulted from sequencing/PCR errors or reflect natural strain polymorphisms. The open reading frame of traR was cloned into a plant expression cassette under the control of the CaMV 35S promoter with a duplicated enhancer region (e35S) followed by a tobacco mosaic virus coat protein translation enhancer. The effector construct encoded a fusion protein containing an HA tag from Influenza hemaglutinin at the N terminus (MGYPYDVPDYAH, actual epitope sequence underlined) followed by a VP16 activation domain (amino acids 413–490) from Herpes simplex and the entire TraR polypeptide. Transcriptional termination and polyadenylation signals were from the 3′ region of the nopaline synthase gene (3′-NOS). Hence the effector construct was stated as e35S∷vp16AD:traR. PCR and cloning fidelity of all constructs were confirmed by DNA sequencing.
TraR Reporter Constructs for Transient Assays
Double-stranded oligonucleotides containing traI and traA elements from the traI and traA gene promoters were created by annealing the following primers: traI1-t-5′-AACTTAACGTGCAGATCTGCACATAACA and traI-b-5′-GTTTGTTATGTGCAGATCTGCACGTTAA; and traA-t-5′-AACTTAATGTGCAGATCTGCACATAACA and traA-b-5′-GTTTGTTATGTGCAGATCTGCACATTAA (sequences of the tra box underlined). Multimers were created by 5′-end phosphorylation using T4 DNA polynucleotide kinase and sticky-end ligation using T4 DNA ligase followed by blunting the ends using Klenow fragment of DNA polymerase I. Each of the tra box elements was linked by a 10-bp spacer sequence. After gel purification, the multimer tra boxes were joined to the CaMV m35S promoter to drive the GUS reporter gene [3×(traA or I):m35S∷GUS].
Mutagenesis of the traA Box
The perfect palindrome of the traA box sequence is 5′-ATGTGCAGATCTGCACAT-3′. The nucleotides were mutated by oligonucleotide synthesis, and each of the mutated traA boxes is shown below. The mutated bases are indicated in lower case: traA1, 5′-gTGTGCAGATCTGCACAc-3′; traA2, 5′-AgGTGCAGATCTGCACcT-3′; traA3, 5′-ATtTGCAGATCTGCAaAT-3′; traA4, 5′-ATGaGCAGATCTGCtCAT-3′; traA5, 5′-ATGTcCAGATCTGgACAT-3′; traA6, 5′-ATGTGaAGATCTtCACAT-3′; traA7, 5′-ATGTGCgGATCcGCACAT-3′; traA8, 5′-ATGTGCAaATtTGCACAT-3′; traA9, 5′-ATGTGCAGtaCTGCACAT-3′; and traA-1, 5′-ATGTGCAGA-CTGCACAT-3′ (“-” indicates deletion of a nucleotide). The flanking sequences outside of the mutant versions of the traA element were not changed for consistency.
Transfection into Carrot Protoplasts
Protoplasts were made from mid-log phase carrot (Daucus carota) cells growing in cell suspension culture as described previously (Ulmasov et al., 1995). After 4 d of subculturing, cells were harvested, treated with digestive enzymes (Driselase; Sigma), and transfected using the polyethylene glycol (PEG)-Ca2+ procedure as described previously (Ulmasov et al., 1995). Transfections were performed in triplicate for both with and without OOHL treatments and at least two independent experiments were performed with each construct. The effector plasmid (e35S∷vp16AD:traR) was cotransfected with appropriate reporter plasmids [3×(traI or traA box):m35S∷GUS] at a 1:1 ratio. After incubation in Murashige and Skoog medium (PhytoTechnology Laboratories) for 36 h in the dark, cells were harvested and assayed for GUS activity.
Transfection into Moss Protoplasts
Moss (Physcomitrella patens) strain Gransden was used as the wild type. The strain was maintained as described previously (Bezanilla et al., 2003). Protonemal tissue was homogenized with a homogenizer (PowerGen 125; Fisher Science) and inoculated onto cellophane overlaid on 0.7% agar plates of PpNH4 media as described previously (Bezanilla et al., 2003) and incubated in a 16/8-h photoperiod at 90 μmol m−2 s−1 at 25°C. DNA delivery into protoplasts was performed as described previously (Schaefer et al., 1991). Protoplasts were isolated and resuspended at 1.5 × 106 protoplasts/mL. Small-scale transient transformations were carried out in 1.5-mL tubes with a total of 3 μg of DNA from the appropriate constructs, 50 μL of protoplasts, and 50 μL of 33% (w/v) PEG. The 3 μg of DNA consisted of 1 μg of actin promoter-driving green fluorescence protein fused to LUC (Actin∷GFP:LUC) as an internal control, 1 μg of each of the reporter [4×(traA8):m35S∷GUS] and effector (e35S∷vp16AD:traR) constructs, or empty pBlueScript vector as balancer plasmid. The transformation reactions were incubated overnight in the dark following a heat shock for 3 min at 45°C. The following day, 0.1 mm OOHL or 0.1% (v/v) dimethyl sulfoxide (DMSO) was added to the transformations. After 24 h of treatment, the protoplasts were harvested at 600g in a swinging bucket rotor and the supernatant was removed. Protein was extracted by a series of freezing in liquid nitrogen and thawing at 37°C with vigorous agitation by vortexing. GUS and LUC activities were measured as described (Lanahan et al., 1992). GUS activity was normalized by the LUC activity and represented as relative GUS activity with se [(GUS/LUC) + se].
Transfection into Barley Protoplasts
Barley (Hordeum vulgare cv Himalaya) seeds from the 1998 harvest at Washington State University (Pullman, WA) were used in all experiments. DNA delivery into barley aleurone cells was performed as described previously (Shen et al., 1993). The reporter construct used above was modified for use in monocot plants by replacing the m35S promoter with the barley α-amylase minimal (−64 bp) promoter, pRZ260 [4×(traA8):Amy64∷GUS]. In addition, the maize (Zea mays) Ubiqutin1 (Ubi) promoter and intron sequence (1,986 bp) were replaced with the e35S promoter in the effector construct, yielding pRZ261 (Ubi∷vp16AD:traR). Maize Ubi promoter-driving LUC (Ubi∷LUC) was used as a bombardment control construct as described previously (Bruce et al., 1989). A total of 2 μg of each reporter construct, effector construct, and bombardment control construct were used to prepare tungsten particles for three bombardments. The bombarded seeds were treated with OOHL for 24 h at room temperature. Protein was extracted from four bombarded barley seeds in extraction buffer (Lanahan et al., 1992) and cell debris was centrifuged down. GUS and LUC activities were measured as described (Lanahan et al., 1992). GUS activity was normalized by the LUC activity and presented as relative GUS activity (GUS/LUC) + se.
Arabidopsis Constructs
The effector construct (e35S∷vp16AD:traR) was cloned into the EcoRI site of pCAMBIA1380 (CAMBIA) plant binary vector, which contained the e35S∷hygromycin∷35S polyA cassette, yielding the pTR80 vector. In the reporter construct, the OOHL-responsive promoter (four copies of traA8 box with m35S promoter) was used to drive the LUC gene and the 3′-NOS polyA was used as a terminator [4×(traA8):m35S∷LUC]. The reporter cassette was cloned into between EcoRI and SpeI sites of the pTR80 vector in cis-orientation to the effector component, yielding the pTRLuc80 vector. The pTRLuc80 vector was then transformed into A. tumefaciens strain ABI for plant transformation.
Arabidopsis Plant Transformation and LUC Assays
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was transformed using the floral-dip method (Clough and Bent, 1998). T1 transgenic Arabidopsis plants were selected on Murashige and Skoog media containing 250 mg/L carbenicillin and 20 mg/L hygromycin. Hygromycin-resistant T1 seedlings were transferred to soil in the greenhouse and seeds of the individual lines were collected by self-fertilization. Seeds from T2 lines were germinated on Murashige and Skoog media with 20 mg/L hygromycin and single-copy insertion lines were selected based on a Mendelian segregation ratio. T3 homozygous seedlings obtained by self-fertilization were grown on Murashige and Skoog + 3% Suc media with hygromycin in a 16/8-h photoperiod at 100 μmol m−2 s−1 at 25°C. About 30 to 50 seeds were sown in a plate and the seeds were germinated in a 25°C incubator after cold treatment at 4°C for 2 to 3 d. Then 12 of the 10-d-old seedlings were carefully transferred to each fresh new Murashige and Skoog + 3% Suc plate and grown for an additional 2 d before being treated with the OOHL inducer at varying times and concentrations. Whole plants or leaf material was frozen in liquid nitrogen, homogenized with a plastic pestle in 200 μL of grinding buffer used in the moss and barley assays, and centrifuged down at 14,000 rpm at 4°C for 10 min. One hundred microliters of the supernatant were used in the LUC assay using 1 mm luciferin (catalog no. L–8220; BIOSYNTH) in a luminescence analyzer (Monolight 2010; Analytical Luminescence Laboratory). The LUC activity was measured as described (Lanahan et al., 1992). Protein concentration was determined by Bradford assay (Bradford, 1976). The statistical analysis of the experiments was performed by t test using a two-tailed distribution with equal variance (α = 0.05).
HSLs Used in This Study
The TraR cognate inducer OHHL was custom ordered from IsoSciences, LLC. The other lactones were purchased from Sigma-Aldrich: HSL, l-HSL hydrochloride; C6-HSL, N-hexanoyl-DL-HSL; C7-HSL, N-heptanoyl-DL-HSL; and C8-HSL, N-octanoyl-HSL. The working concentrations of lactone solutions were prepared in 0.5× Murashige and Skoog solution.
Microarray Experiments and Analysis
Twelve-day-old Arabidopsis seedlings of wild-type and pTRLuc80 line 8F (inducible line) grown in Murashige and Skoog + 3% Suc media, as described above, were treated with either 3 mL of 1 mm OOHL or 0.1% DMSO by foliar application for 24 h. Total RNA was extracted from three individual seedlings per treatment using the Agilent plant RNA isolation mini kit (Agilent Technologies) following the manufacturer's recommendation. The total RNA from three individual seedlings from each treatment was then pooled together. We purchased Arabidopsis 2 (V2) microarrays (product no. G4130B) based on a 60-mer oligonucleotide DNA microarray for two-color gene expression analysis (Cy3 and Cy5 labeling) from Agilent Technologies. The 22K microarray format contains about 20,000 genes/transcripts per microarray. We used an Agilent-certified microarray service lab (MOgene, LC) to perform the following microarray experiment. The quality of the RNA samples was determined on an Agilent 2100 bioanalyzer using an RNA 6000 LabChip kit. The cRNA was amplified from 0.5 μg of the total RNA using Agilent low RNA input fluorescent linear amplification kit (product no. 5184–3523), and 2 μg of cRNA from each sample labeled with Cy3 and Cy5 using ULS aRNA fluorescent labeling kit (product no. EA–006; Kreatech Biotechnology) was mixed and hybridized for each microarray. A total of 12 microarrays were analyzed with dye swaps in two biological samples. The sample pairings are shown below: (1) wild type versus inducible line; (2) wild type + mock treatment versus wild type + OOHL inducer; and (3) lactone-inducible line + mock treatment versus lactone-inducible line + OOHL inducer.
The hybridization, washing, and scanning were conducted according to the manufacturer's recommendation at MOgene, LC. The fluorescent intensities of each feature were extracted by Agilent feature extraction software. The raw intensity data then were log (base e = 2.718) transformed for normalization before ANOVA (mixed model) analysis. Data points for genes (or probes) were discarded if the raw intensities of both Cy3 and Cy5 channels were below 150 (typical background intensity is 40) and the signal-to-background ratio below 2. Log ratios among different samples and the P values were calculated using the mixed model (Wolfinger et al., 2001). FDR was calculated using Benjamini and Hochberg's procedure (Benjamini and Hochberg, 1995) based on the P values. A gene was identified as a significant change in gene expression based on the 2-fold cutoff with P values <0.05 and a FDR < 0.05.
Supplemental Table I contains all the differentially expressed genes from each of the three sample comparisons. Supplemental Table II contains a list of differentially expressed genes from sample pairing 1, with a 1.5-fold cutoff and FDR values. Supplemental Tables III and IV contain a gene list with the above criteria from sample pairings 2 and 3, respectively.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers L08596 (TraR), L17024 (TraI), and AF242881 (TraA).
Supplementary Material
Acknowledgments
We thank the Quatrano laboratory and the Ulmasov group for helpful discussions regarding this work, as well as Paul Klueh and Aihong Pan for technical assistance. We also thank Lesley Tomlin for her assistance in preparing this manuscript.
This work was supported by the Monsanto/Washington University Plant Science Agreement.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ralph S. Quatrano (rsq@wustl.edu).
The online version of this article contains Web-only data.
References
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc (Ser B) 57: 289–300 [Google Scholar]
- Bezanilla M, Pan A, Quatrano RS (2003) RNA interference in the moss Physcomitrella patens. Plant Physiol 133: 470–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Rossi FMV (1999) Tet B or not tet B: advances in tetracycline-inducible gene expression. Proc Natl Acad Sci USA 96: 797–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bruce W, Folkerts O, Garnaat C, Crasta O, Roth B, Bowen B (2000) Expression profiling of the maize flavonoid pathway genes controlled by estradiol-inducible transcription factors CRC and P. Plant Cell 12: 65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce WB, Christensen AH, Klein T, Fromm M, Quail PH (1989) Photoregulation of a phytochrome gene promoter from oat transferred into rice by particle bombardment. Proc Natl Acad Sci USA 86: 9692–9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddick MX, Greenland AJ, Jepson I, Krause KP, Qu N, Riddell KV, Salter MG, Schuch W, Sonnewald U, Tomsett AB (1998) An ethanol inducible gene switch for plants: manipulation of carbon metabolism. Nat Biotechnol 16: 177–180 [DOI] [PubMed] [Google Scholar]
- Chai Y, Winans SC (2005) Amino-terminal protein fusions to the TraR quorum-sensing transcription factor enhance protein stability and autoinducer-independent activity. J Bacteriol 187: 1219–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Fuqua C, Winans SC (1996) Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol 178: 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the luxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176: 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Li P, Zhang L, Piper KR, Cook DM, Tate ME, Farrand SK (1994) TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA 91: 4639–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JC, Asurmendi S, Bick J, Woodford-Thomas T, Beachy RN (2004) Ecdysone agonist-inducible expression of a coat protein gene from tobacco mosaic virus confers viral resistance in transgenic Arabidopsis. Plant J 37: 439–448 [DOI] [PubMed] [Google Scholar]
- Lanahan MB, Ho TH, Rogers JC (1992) A gibberellin response complex in cereal α-amylase gene promoters. Plant Cell 4: 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z-Q, Su S, Farrand SK (2003) In situ activation of the quorum-sensing transcription factor TraR by cognate and noncognate acyl-homoserine lactone ligands: kinetics and consequences. J Bacteriol 185: 5665–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie MJ, Mett V, Reynolds PHS, Jameson PE (1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol 116: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett VL, Lochhead LP, Reynolds PHS (1993) Copper-controllable gene expression system for whole plants. Proc Natl Acad Sci USA 90: 4567–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue TD, Wehland-von Trebra M, Bernhard F, von Bodman SB (2002) The autoregulatory role of EsaR, a quorum-sensing regulator in Pantoea stewartii ssp. stewartii: evidence for a repressor function. Mol Microbiol 44: 1625–1635 [DOI] [PubMed] [Google Scholar]
- Neddermann P, Gargioli C, Muraglia E, Sambucini S, Bonelli F, Francesco RD, Cortese R (2003) A novel, inducible, eukaryotic gene expression system based on the quorum-sensing transcription factor TraR. EMBO Rep 4: 159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padidam M (2003) Chemically regulated gene expression in plants. Curr Opin Plant Biol 6: 169–177 [DOI] [PubMed] [Google Scholar]
- Roberts GR, Garoosi GA, Koroleva O, Ito M, Laufs P, Leader DJ, Caddick MX, Doonan JH, Tomsett AB (2005) The alc-GR system. A modified alc gene switch designed for use in plant tissue culture. Plant Physiol 138: 1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslan HA, Salter MG, Wood CD, White MRH, Croft KP, Robson F, Coupland G, Doonan J, Laufs P, Tomsett AB, et al (2001) Characterization of the ethanol-inducible alc gene-expression system in Arabidopsis thaliana. Plant J 28: 225–235 [DOI] [PubMed] [Google Scholar]
- Samalova M, Brzobohaty B, Moore I (2005) pOp6/LhGR: a stringently regulated and highly responsive dexamethasone-inducible gene expression system for tobacco. Plant J 41: 919–935 [DOI] [PubMed] [Google Scholar]
- Schaefer D, Zrÿd J-P, Knight C (1991) Stable transformation of the moss Physcomitrella patens. Mol Gen Genet 266: 418–424 [DOI] [PubMed] [Google Scholar]
- Schauder S, Bassler BL (2001) The languages of bacteria. Genes Dev 15: 1468–1480 [DOI] [PubMed] [Google Scholar]
- Shen Q, Uknes SJ, Ho TH (1993) Hormone response complex in a novel abscisic acid and cycloheximide-inducible barley gene. J Biol Chem 268: 23652–23660 [PubMed] [Google Scholar]
- Ulmasov T, Liu ZB, Hagen G, Guilfoyle TJ (1995) Composite structure of auxin response elements. Plant Cell 7: 1611–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann P, Gossen M, Hillen W, Bujard H, Gatz C (1994) A chimeric transactivator allows tetracycline-responsive gene expression in whole plants. Plant J 5: 559–569 [DOI] [PubMed] [Google Scholar]
- Wishart J, Hayes A, Wardleworth L, Zhang N, Oliver S (2005) Doxycycline, the drug used to control the tet-regulatable promoter system, has no effect on global gene expression in Saccharomyces cerevisiae. Yeast 22: 565–569 [DOI] [PubMed] [Google Scholar]
- Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS (2001) Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol 8: 625–637 [DOI] [PubMed] [Google Scholar]
- Zhang R-g, Pappas T, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A (2002) Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417: 971–974 [DOI] [PubMed] [Google Scholar]
- Zhu J, Beaber JW, More MI, Fuqua C, Eberhard A, Winans SC (1998) Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol 180: 5398–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Winans SC (1999) Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci USA 96: 4832–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu Q-W, Chua N-H (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.