Abstract
The Arabidopsis (Arabidopsis thaliana) root epidermal bulger1-1 (reb1-1) mutant (allelic to root hair defective1 [rhd1]) is characterized by a reduced root elongation rate and by bulging of trichoblast cells. The REB1/RHD1 gene belongs to a family of UDP-d-Glucose 4-epimerases involved in the synthesis of d-Galactose (Gal). Our previous study showed that certain arabinogalactan protein epitopes were not expressed in bulging trichoblasts of the mutant. In this study, using a combination of microscopical and biochemical methods, we have investigated the occurrence and the structure of three major Gal-containing polysaccharides, namely, xyloglucan (XyG), rhamnogalacturonan (RG)-I, and RG-II in the mutant root cell walls. Our immunocytochemical data show that swollen trichoblasts were not stained with the monoclonal antibody CCRC-M1 specific for α-l-Fucp-(1→2)-β-d-Galp side chains of XyG, whereas they were stained with anti-XyG antibodies specific for XyG backbone. In addition, analysis of a hemicellulosic fraction from roots demonstrates the presence of two structurally different XyGs in reb1-1. One is structurally similar to wild-type XyG and the other is devoid of fuco-galactosylated side chains and has the characteristic of being insoluble. Similar to anti-XyG antibodies, anti-bupleuran 2IIC, a polyclonal antibody specific for galactosyl epitopes associated with pectins, stained all root epidermal cells of both wild type and reb1-1. Similarly, anti-RG-II antibodies also stained swollen trichoblasts in the mutant. In addition, structural analysis of pectic polymers revealed no change in the galactosylation of RG-I and RG-II isolated from reb1-1 root cells. These findings demonstrate that the reb1-1 mutation affects XyG structure, but not that of pectic polysaccharides, thus lending support to the hypothesis that biosynthesis of Gal as well as galactosylation of complex polysaccharides is regulated at the polymer level.
The plant cell wall plays a vital role in growth and development as well as in mediating interactions with the environment and other organisms. It is a dynamic and complex structure comprising cellulose microfibrils and a xyloglucan (XyG) network embedded within a matrix of polysaccharides and proteins (i.e. glycoproteins and proteoglycans). Four major types of noncellulosic polysaccharides are found in the primary walls of plant cells (in taxa outside the graminae), namely, the neutral hemicellulosic polysaccharide XyG, and three pectic polysaccharides, homogalacturonan (HG), rhamnogalacturonan (RG)-I, and RG-II (Carpita and Gibeaut, 1993).
XyG consists of a β-d-(1→4)-glucan backbone to which are attached side chains containing either xylosyl, galactosyl-xylosyl, or fucosyl-galactosyl-xylosyl residues. In dicotyledonous and nongrass monocotyledonous plants, XyG is the principal polysaccharide that cross-links the cellulose microfibrils. XyG is able to bind cellulose tightly because its β-d-(1→4)-glucan cellulose-like backbone can form many hydrogen bonds with the microfibrils, whereas the side chains give rise to regions where microfibril binding is interrupted and thus a single XyG molecule can interconnect separated cellulose microfibrils. This XyG-cellulose network forms a major load-bearing structure that contributes to the structural integrity of the wall and the control of cell expansion (Cosgrove, 1999).
In Arabidopsis (Arabidopsis thaliana) cell walls, generally six structurally distinct oligomers are released from XyG upon treatment with endo-β-(1→4) endoglucanase. According to the nomenclature introduced by Fry et al. (1993), these fragments are XXXG, XLFG, XLLG, XLXG, XXLG, and XXFG (according to Lerouxel et al., 2002; Madson et al., 2003; Peña et al., 2004). It has been suggested that the terminal Fuc present in XyG side chains is required for the stabilization of a planar conformation that facilitates binding to cellulose microfibrils (Levy et al., 1991). However, recent studies on mur2 and mur3 mutants have indicated that galactosylation rather than fucosylation of XyG is essential for maintaining the tensile strength of the cell wall during growth (Vanzin et al., 2002; Peña et al., 2004).
The pectic matrix is structurally complex and heterogeneous. HG domains consist of α-d-(1→4)-GalUA (GalA) residues, which can be methyl esterified, acetylated, and/or substituted with Xyl (Willats et al., 2001). Deesterified blocks of HG can be cross-linked by calcium, resulting in the formation of a gel that is believed to be essential for cell adhesion (Jarvis, 1984). Such an association is also important in controlling wall porosity (Carpita and Gibeaut, 1993). RG-I domains contain repeats of the disaccharide (→4-α-d-GalA-(1→2)-α-l-Rha-1→) in which rhamnosyl residues can carry oligosaccharide side chains consisting predominantly of β-d-(1→4)-galactosyl- and/or α-l-(1→5)-arabinosyl-linked residues (McNeil et al., 1982). Side chains of RG-I are believed to decrease the ability of pectic molecules to cross-link and form a stable gel network and are thereby able to influence the mechanical properties of the cell wall (Hwang and Kokini, 1991). In addition, the structure and tissue distribution of arabinan- or galactan-rich side chains of RG-I have been shown to be regulated during cell growth and differentiation of many species (for review, see Willats et al., 2001).
RG-II is the most structurally complex pectic polysaccharide discovered so far in plants (Ridley et al., 2001). It occurs in the cell walls of all higher plants as a dimer (dRG-II-B) that is cross-linked by a borate diester (Matoh et al., 1993; Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O'Neill et al., 1996). The backbone of RG-II is composed of a HG-like structure containing at least eight α-d-(1→4)-GalA-linked residues to which four structurally different oligosaccharide chains, denoted A, B, C, and D, are attached. The C and D side chains are attached to C-3 of the backbone, whereas A and B are attached to C-2 of the backbone (O'Neill et al., 2004). The C disaccharide contains Rha and 2-keto-3-deoxy-d-manno-octulosonic acid (Kdo), whereas the D disaccharide contains 2-keto-3-deoxy-d-lyxo-heptulosaric acid (Dha) and Ara (O'Neill et al., 2004). The A and B oligosaccharide chains are both composed of eight to 10 monosaccharides and are attached by a β-d-apiose residue to O-2 of the backbone. A d-galactosyl residue (d-Gal) occurs on the B chain.
The structure of RG-II has been shown to be conserved throughout the primary cell walls of vascular plants (Ridley et al., 2001; Matsunaga et al., 2004). RG-II plays an important role in the structural organization of the cell wall and the control of cell growth. Cross-linking of RG-II via a borate ester and the formation of dimers in muro contribute significantly to the control of cell wall porosity (Fleischer et al., 1999) and tensile strength (Ryden et al., 2003). For instance, abnormally swollen cell walls have been shown to result from boron deficiency and decrease in RG-II dimer formation (Matoh, 1997; Ishii et al., 2001). In addition, it has been shown that plants carrying mutations that affect the boron-mediated cross-linking of RG-II have reduced intercellular attachment (Iwai et al., 2002) and growth (O'Neill et al., 2001).
In the past 10 years, screening and analysis of Arabidopsis mutants have become a widely used approach to unravel the mechanisms of cell wall biosynthesis and function. For instance, the identification and analysis of cellulose-deficient Arabidopsis mutants, such as rsw (root swelling) and irx (irregular xylem), have led to the finding that multiple cellulose synthase proteins are required for cellulose synthesis in the primary and secondary cell walls (Fagard et al., 2000; Peng et al., 2000; Taylor et al., 2000, 2003). The characterization of other mutants, such as mur1, 2, 3, and 4 (Reiter et al., 1997), with deficiencies in specific sugars of the noncellulosic fraction of the cell wall, is currently providing significant knowledge of the enzymatic machinery involved in complex polysaccharide biosynthesis. In particular, the mur mutants have allowed molecular cloning and characterization of genes encoding glycosyltransferases active in the synthesis of XyG as well as genes encoding sugar-synthesizing enzymes (Burget et al., 2003; Madson et al., 2003; Mølhøj et al., 2004).
The root epidermal bulger1-1 (reb1-1) mutant of Arabidopsis, which is allelic to root hair defective1 (rhd1) is characterized by a reduced elongation rate of the primary root and bulging of root trichoblast cells (Baskin et al., 1992; Andème-Onzighi et al., 2002). Bulging of trichoblasts has been observed mostly in the elongation and differentiation zones of the root. The REB1/RHD1 gene has been cloned and shown to encode for a UDP-d-Glc 4-epimerase (UGE4) involved in the synthesis of d-Gal (Seifert et al., 2002). In our previous study using immunocytochemistry, it was shown that arabinogalactan proteins (AGPs) recognized by LM2 or JIM14 antibodies are not expressed in bulging trichoblasts of the mutant, although they are present in other cell types (Andème-Onzighi et al., 2002). Similarly, Seifert et al. (2002) have shown that the CCRC-M1-recognized epitope of XyG is absent from the epidermal and cortical cells of rhd1. Thus, it has been postulated that the UDP-d-Gal transfer to wall polymers can be regulated at the tissue and the cellular level (Seifert et al., 2002).
To expand upon these findings and gain further insight into the role of Gal-containing cell wall polysaccharides, we have investigated the localization and structure of RG-I and RG-II in addition to XyG in the mutant root cells by combining immunocytochemical and biochemical techniques. Our data show that swollen trichoblasts in the root elongation zone do express a galactosylated epitope associated with pectic polysaccharides recognized by anti-bupleuran 2IIC antibodies. In contrast, they do not express the α-l-Fucp-(1→2)-β-d-Galp side chain recognized by the monoclonal antibody (mAb), CCRC-M1, as previously described (Seifert et al., 2002). Structural analysis of XyG isolated from reb1-1 mutant roots demonstrates the presence of two structurally different types. One has a normal wild-type structure and the other a XyG that is devoid of α-l-Fucp-(1→2)-β-d-Galp and β-d-Galp side chains and insoluble. In contrast, no change in the structure of the pectic polysaccharides RG-I and RG-II isolated from reb1-1 root cells is found. Based on these findings and those of Andème-Onzighi et al. (2002), we postulate that UGE4 might be part of a protein complex involved in the galactosylation of XyG and AGPs, but not in that of pectic polysaccharides in trichoblast cells.
RESULTS
Immunofluorescence Localization of XyG in Root Cells
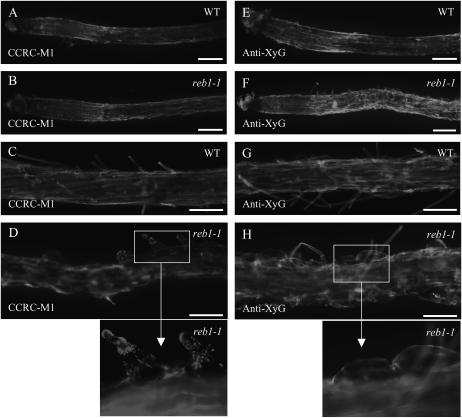
To investigate the occurrence of XyG epitopes in the outer epidermal cell wall of roots, we used a whole-mount labeling technique, which is a rapid and reliable method for mapping polysaccharide epitopes (Willats et al., 2001; Vicré et al., 2005). We studied the distribution of epitopes recognized by the mAb, CCRC-M1, which is specific for α-l-Fucp-(1→2)-β-d-Galp side chains (Puhlmann et al., 1994) and those recognized by a polyclonal anti-XyG antiserum, which is specific for the β-d-(1→4)-glucan backbone (Lynch and Staehelin, 1992). The CCRC-M1-recognized epitope is detected in all cells of the wild type except in a small region around the quiescent center (Fig. 1, A and C). Root hairs are also labeled with the CCRC-M1 antibody (Fig. 1C). In the reb1-1 mutant, an overall heterogeneous staining of the root is observed with some regions more stained than others (Fig. 1B). A close examination of swollen trichoblasts in elongation and differentiation zones shows either a patchy staining of these cells (Fig. 1D) or no staining at all (data not shown). The anti-XyG-recognized epitope is also detected in root cells of both the wild type and reb1-1 mutant (Fig. 1, E and F). The intensity of the staining, however, appears much higher in the elongation zone of the reb1-1 mutant (Fig. 1F). A close examination of swollen trichoblasts shows a uniform staining of their cell walls (Fig. 1H). Root hairs are also labeled (Fig. 1, G and H).
Figure 1.
Immersion immunofluorescence staining of XyG in wild-type (A, C, E, and G) and reb1-1 (B, D, F, and H) roots. A to D, Anti-XyG antiserum. E to H, mAb CCRC-M1 antibody. Bars = 140 μm (A, B, E, and F) and 100 μm (C, G, D, and H).
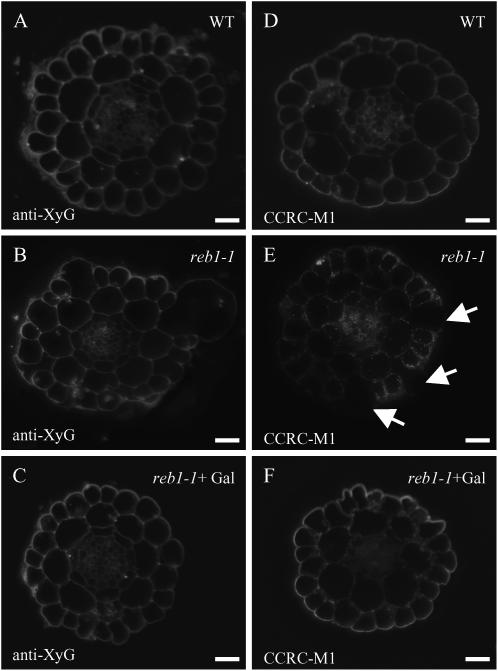
To confirm and extend these observations, we immunolabeled resin-embedded cross sections of wild-type and reb1-1 roots using the same antibodies. We focused on the examination of the elongation zone where trichoblasts were shown to bulge (Andème-Onzighi et al., 2002). The anti-XyG antiserum stains all cell types equally in both the wild type and the reb1-1 mutant (Fig. 2, A and B). The mAb CCRC-M1 labels all cell types in the wild type, although with different intensities (Fig. 2D). The antibody stains epidermal cells (i.e. trichoblasts and atrichoblasts) more strongly than other tissues. In contrast, in reb1-1 mutant roots, the mAb CCRC-M1 does not stain swollen trichoblasts, although it does stain atrichoblasts and nonswollen trichoblasts (Fig. 2E; see also Seifert et al., 2002). Interestingly, CCRC-M1 staining of trichoblasts is restored in mutant roots grown in the presence of 10 mm d-Gal (Fig. 2F). In this connection we also found that the addition of 10 mm d-Gal to the culture medium of reb1-1 causes a complete recovery of the wild-type root length and morphology in addition to restoring AGP content (data not shown).
Figure 2.
Fluorescence micrographs of cross sections in the root elongation zone showing staining with anti-XyG antiserum (A–C) and the mAb CCRC-M1 (D–F) for wild type (A and D), reb1-1 (B and E), and reb1-1 grown in the presence 10 mm d-Gal (C and F). Bars = 20 μm (A and D) and 25 μm (B, C, E, and F). Arrows indicate trichoblasts.
Taken together, these findings indicate that the structure of fuco-galactosylated side chains of XyG, recognized by CCRC-M1, might be modified in reb1-1 trichoblasts.
Immunolocalization of the Pectic Polysaccharides RG-I and RG-II
We next examined distribution of epitopes associated with RG-I and RG-II using immunofluorescence microscopy and three antibodies. These are: (1) the polyclonal anti-bupleuran 2IIC, which recognizes an epitope consisting of β-d-galactosyl residues carrying glucuronic or 4-O-methylglucuronic acids found in RG-I (Sakurai et al., 1998; Andème-Onzighi et al., 2000); (2) the mAb, LM5, which is specific for β-d-(1→4)-galactan side chains of RG-I (Jones et al., 1997); and (3) a polyclonal anti-RG-II, which binds to an unknown epitope associated with the borate-RG-II complex (Matoh et al., 1998). Similar to XyG localization, we focused on the elongation zone of both wild-type and mutant roots and examined cross sections of resin-embedded roots.
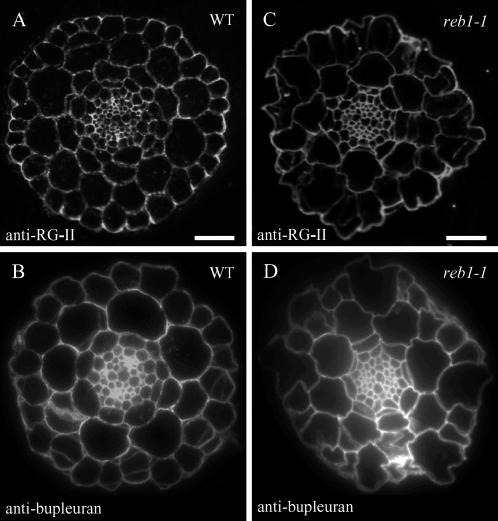
The anti-RG-II antiserum stains all cell types in wild-type and mutant roots (Fig. 3, A and C). Epidermal cells appear to be strongly labeled as compared to other cell types. Similarly, the anti-bupleuran 2IIC antiserum stains all cell types equally, including trichoblasts, in both wild-type and mutant roots (Fig. 3, B and D). In contrast, the mAb LM5 labels epidermal cells in neither wild-type nor reb1-1 mutants, whereas it labels other cell types, including the cortex, endodermis, and pericycle (data not shown). Evidently, LM5 is not a suitable probe to assess the occurrence of pectic Gal in epidermal cells of the elongation zone in Arabidopsis roots (see also Seifert et al., 2004).
Figure 3.
Fluorescence micrographs of cross sections in the root elongation zone showing staining with anti-pectin antibodies for wild type (A and B) and reb1-1 (C and D). A and C, Anti-RG-II antiserum. B and D, Anti-bupleuran 2IIC antiserum. Bar = 30 μm.
Based on all these observations, we conclude that galactosylated pectins occur in the swollen trichoblasts of the mutant.
Chemical Analyses of Gal-Containing Cell Wall Polysaccharides in reb1-1 Root Cells
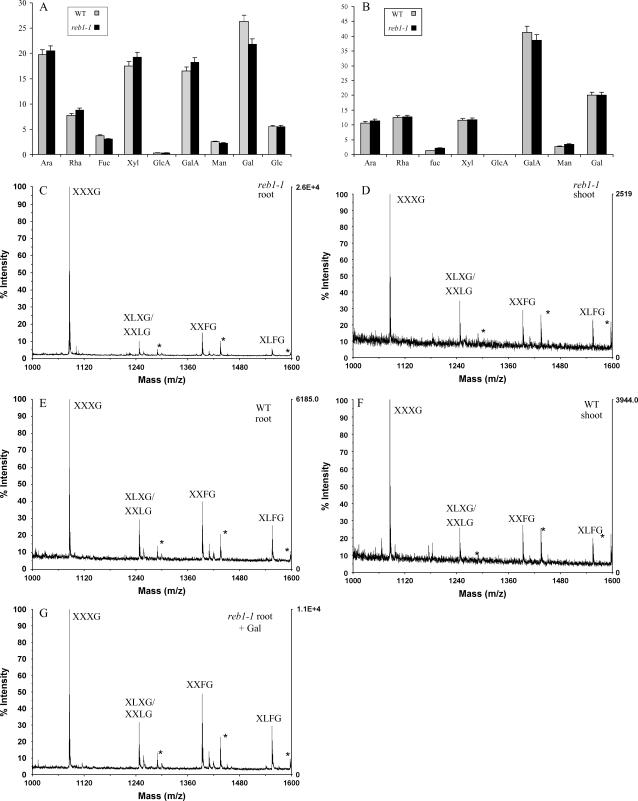
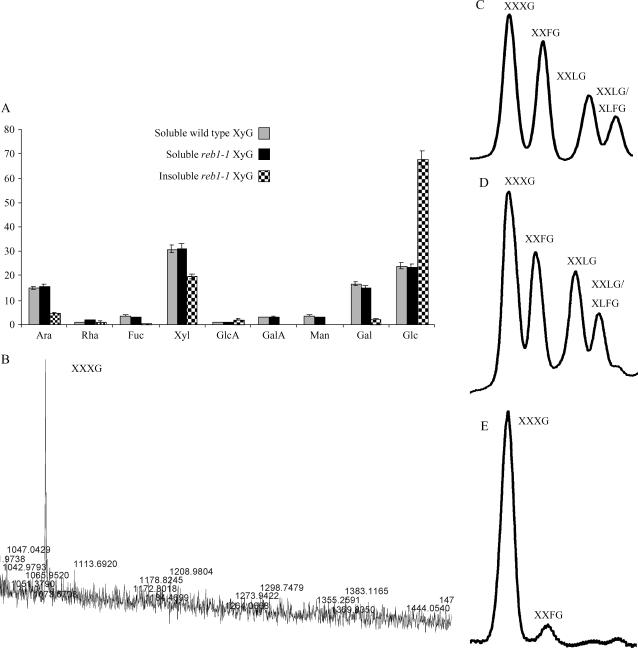
First, we determined the monosaccharide composition of cell walls isolated from roots and shoots using gas chromatography (GC; Fig. 4). As previously reported (Seifert et al., 2002), reb1-1 root cell walls contain less Gal than the wild type (Fig. 4A), whereas there is no significant difference in monosaccharide composition between the wild-type and reb1-1 shoot cell walls (Fig. 4B). Second, we investigated the structure of XyG, RG-I, and RG-II polysaccharides (see below).
Figure 4.
Monosaccharide composition and analysis of XyG structure released from the cell wall. A and B, Monosaccharide composition of crude cell wall material extracted from reb1-1 and wild-type roots (A) and shoots (B). C to F, MALDI-TOF MS spectra of XyG oligosaccharides released from the wild-type and reb1-1 cell wall isolated from root and shoot tissues. G, MALDI-TOF MS spectrum of XyG oligosaccharides released from the cell wall isolated from the root of reb1-1 grown in the presence 10 mm d-Gal. Asterisks (*) indicate ions related to acetylated fragments.
Analysis of XyG Structure
To determine XyG structure, we used the enzymatic oligosaccharide fingerprinting strategy (Lerouxel et al., 2002; Ray et al., 2004) to determine the XyG structure. reb1-1 and wild-type crude cell wall material from roots and shoots were treated separately with an endo-β-d-(1→4)-glucanase and the resulting fragments characterized using matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). The ions corresponding to the fragments XXFG, XXLG/XLXG, and XLFG decrease dramatically in reb1-1 mutant roots as compared to wild-type roots (Fig. 4, C and E; Table I). In contrast, no significant difference in the structure of XyG is found between wild-type and reb1-1 shoots (Fig. 4, D and F). These results show that the reb1-1 mutation specifically affects XyG structure in the root, but not in the shoot, consistent with the known alteration of morphology in the mutant specifically in the root. Interestingly, a complete recovery of a wild-type XyG structure occurs when reb1-1 grows in the presence of 10 mm d-Gal (Fig. 4G).
Table I.
Comparison of relative abundance (percentage) of XyG oligosaccharides obtained by HPAEC-PAD from root cell walls of wild type, reb1-1, and reb1-1 grown in the presence of 10 mmd-Gal
HPAEC-PAD signals representing the XyG fragments were integrated. Data represent the mean and sd of three independent experiments. HPAEC-PAD profiles are presented as supplemental data (Supplemental Fig. 1).
| XyG Fragments | XXXG | XXFG | XLXG | XXLG/XLFG |
|---|---|---|---|---|
| Wild-type roots | 42.5 ± 3 | 29.1 ± 1 | 17.2 ± 3 | 11.2 ± 3 |
| reb1-1 roots | 77.3 ± 3 | 12.6 ± 3 | 5.5 ± 1 | 4.6 ± 2 |
| reb1-1 roots + Gal | 44.0 ± 3 | 29.4 ± 1 | 16.6 ± 1 | 10.0 ± 3 |
To further assess XyG structure, we analyzed hemicellulosic fractions isolated from reb1-1 and wild-type root cell wall material using 4 m KOH. It is worth noting that the 4 m KOH extract obtained from reb1-1, unlike from the wild type, contained two hemicellulosic subfractions: one soluble and the other insoluble. As shown in Figure 5, A, C, and D, soluble hemicellulosic fractions isolated from both wild-type and reb1-1 roots share identical chemical features. Consistent with the previously reported structure of Arabidopsis XyG (Zablackis et al., 1995), the monosaccharide composition includes Fuc, Xyl, Gal, and Glc residues (Fig. 5A). Both high-performance anion-exchange chromatography-pulsed amperometric detection (HPAEC-PAD) profiles (Fig. 5, C and D) and MALDI-TOF mass spectra (data not shown) of fragments generated by an endoglucanase treatment of soluble XyG fractions show the presence of XXXG, XXFG, XLXG, and XLFG as major oligosaccharides, with similar relative intensities in both wild-type and reb1-1 preparations. As a consequence, we conclude that both wild-type and reb1-1 soluble hemicelluloses consist of a XXXG-type XyG bearing α-d-Xylp (X), β-d-Galp-(1→2)-α-d-Xylp (L), or α-l-Fucp-(1→2)-β-d-Galp-(1→2)-α-d-Xylp (F) side chains in similar proportions.
Figure 5.
Monosaccharide composition and analysis of XyG structure released from 4 m KOH hemicellulosic fractions. A, Monosaccharide composition of soluble and insoluble hemicellulosic fractions isolated from the wild type and reb1-1. B, MALDI-TOF MS spectra of XyG oligosaccharides released from reb1-1-insoluble hemicellulosic fraction. C and E, HPAEC-PAD profiles of XyG oligosaccharides released from soluble hemicellulosic fractions of wild type (C) and reb1-1 (D) and from insoluble hemicellulosic fraction of reb1-1 (E).
The insoluble XyG fraction from reb1-1, which accounts for approximately 3% of the total XyG, strongly differs from soluble fractions. Sugar composition shows that the insoluble material consists mainly of Xyl and Glc in a 2.5:4 ratio (Fig. 5A). The HPAEC-PAD profile and the MALDI-TOF mass spectrum of the endoglucanase-generated fragments demonstrate that this XyG fraction is composed nearly exclusively of XXXG subunits (Fig. 5, B and E). The XXFG and XLFG fragments do not exceed 5% of the oligosaccharide population. As a consequence, we conclude that, in contrast to the wild type, reb1-1 roots contain two types of XyG: a major species identical to the wild type and a minor species almost completely devoid of fucosylated and galactosylated side chains. Considering that trichoblast cells are not immunolabeled with the mAb CCRC-M1, specific for Fuc-containing XyG side chains, we propose that the XyG structure in reb1-1 is cell type-specific with a wild-type structure in atrichoblasts and other inner tissues and a nongalactosylated (and thus a nonfucosylated) XyG in trichoblast cells.
Analysis of RG-I and RG-II
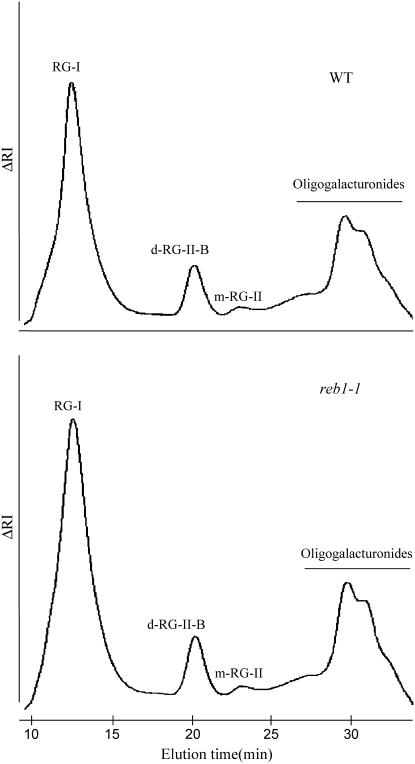
Pectic polysaccharides were extracted from alcohol-insoluble residue (AIR) following treatment by endopolygalacturonase (EPG) as previously described (Ishii et al., 2001). The purified EPG-soluble fraction was then separated by size-exclusion chromatography (SEC) gel permeation into RG-I and RG-II polysaccharide fractions. In both chromatograms of wild-type and reb1-1 root cell walls, RG-I is the main polysaccharide present, whereas RG-II occurs as a minor polysaccharide, mostly under its dimer form (dRG-II; Fig. 6). In addition, the ratio between RG-I and RG-II polysaccharides as well as dimerization levels of RG-II are similar in both wild type and reb1-1 (Fig. 6).
Figure 6.
SEC-refractive index profiles of the material released following EPG treatment of wild-type and reb1-1 root cell walls.
The monosaccharide composition of RG-I and d-RG-II isolated from root cell walls is similar between the reb1-1 mutant and the wild type (Tables II and III). In addition, no differences were found in a liquid chromatography-MS analysis of the B chains of RG-II released by mild acid treatment (data not shown). Together, these results indicate that the reb1-1 mutation has no effect on the structure of the two major Gal-containing pectic polysaccharides of the cell wall, RG-I and RG-II.
Table II.
Composition of neutral sugars (mol %) of RG-I isolated from roots of wild type and reb1-1
nd, Not detectable.
| Glycosyl Residues
|
RG-I
|
|
|---|---|---|
| Wild Type | reb1-1 | |
| Ara | 34 | 31 |
| Rha | 10 | 12 |
| Xyl | 8 | 9 |
| Fuc | 8 | 8 |
| Gal | 40 | 40 |
| Api | nd | nd |
| 2-O-Me-Fuc | nd | nd |
| 2-O-Me-Xyl | nd | nd |
Table III.
Monosaccharide composition (mol %) of RG-II isolated from roots of wild type and reb1-1
| Glycosyl Residues
|
RG-II
|
|
|---|---|---|
| Wild Type | reb1-1 | |
| Ara | 18 | 16 |
| Rha | 9 | 9 |
| Xyl | 3 | 3 |
| Fuc | 3 | 3 |
| Gal | 18 | 19 |
| Api | 5 | 5 |
| 2-O-Me-Fuc | 2 | 3 |
| 2-O-Me-Xyl | 3 | 3 |
| GalA | 31 | 32 |
| GlcA | 3 | 2 |
| Kdo | 1 | 1 |
| Dha | 2 | 2 |
| Aceric A | 2 | 2 |
DISCUSSION
The reb1-1 plant is deficient in one of the five Glc epimerases that synthesize d-Gal and is known to produce altered AGPs (Andème-Onzighi et al., 2002; Seifert et al., 2002). The goal of this study was to determine the effect of the reb1-1 mutation on the structure and cellular localization of three major Gal-containing cell wall polymers, XyG, RG-I, and RG-II. Our principal findings are: (1) reb1-1 mutant root cells make two types of XyG that are structurally different: a typical, wild-type polymer and a polymer that is insoluble and devoid of Gal and Fuc residues; (2) swollen trichoblasts do express galactosylated epitopes associated with pectins, but not fuco-galactosylated epitopes associated with XyG; and (3) the structure of the pectic polysaccharides RG-I and RG-II is unchanged. Thus, the reb1-1 mutation appears to affect only a certain type of cell wall polymer (i.e. XyG and AGP). These findings suggest that UGE4 might be involved specifically in the galactosylation of XyG and AGPs, but not in that of pectic polysaccharides, and link galactosylation of XyG, in addition to AGPs, to cell expansion and morphogenesis.
Galactosylation of XyG Is Possibly Required for Cell Expansion
Using an enzymatic fingerprinting strategy, we show that reb1-1 root cells produce an abnormal XyG in addition to the normal one. The abnormal XyG lacks α-l-Fucp-(1→2)-β-d-Galp substitutions and has the feature of being water insoluble. Additionally, and as shown previously (Seifert et al., 2002), immunolabeling shows that swollen trichoblasts are not stained with CCRC-M1. Therefore, we conclude that the synthesis of the abnormal XyG may occur predominantly, if not exclusively, in swollen trichoblasts, although we cannot exclude a change in XyG structure in some other tissues in the mutant. XyG is known to tether cellulose microfibrils in the primary wall, resulting in an extensive cellulose-XyG network that acts as the major tension-bearing structure (Hayashi, 1989; Carpita and Gibeaut, 1993). In muro reorganization of XyG, cross-links are believed to be a key factor in controlling wall strength and extensibility and, therefore, the directionality of cell expansion (Fry, 1995; Cosgrove, 2001).
The reb1-1 mutant can now be added to the list of Arabidopsis mutants with altered XyG structure, even though this occurs only in a specific set of root cells. Two other mutants that make altered XyG are mur2 and mur3 (Vanzin et al., 2002; Madson et al., 2003). MUR2 and MUR3 genes encode for, respectively, fucosyl- and galactosyl-transferases that are specific for XyG (Vanzin et al., 2002; Madson et al., 2003). Mutation of the MUR2 gene leads to the absence of XyG fucosylation (Vanzin et al., 2002), whereas mutation of MUR3 leads to the absence of Fuc-Gal from the first Xyl residue and an increase in galactosylation at the middle Xyl in leaves, but not in etiolated hypocotyls (Madson et al., 2003). The only visible phenotype detected is in hypocotyls of dark-grown mur3 seedlings, which exhibit a marked swelling of epidermal and cortical cells at the base along with a significant loss of tensile strength (<40% that of wild type). The XyG of these cells, unlike that in leaves, has no increased galactosylation of the middle Xyl residues. Our results (see above) indicate that the swollen trichoblasts lack galactosylated XyG as well. These observations on reb1-1 roots and mur3-etiolated hypocotyls point to a possible link between the galactosylation of XyG and the control of cell expansion. Although tensile strength was not measured in reb1-1, it is reasonable to think that the lack of galactosylation of XyG, in addition to that of AGPs, induces a reduction in the mechanical strength of the cell wall in trichoblasts as it has been found for epidermal cells of mur3-etiolated hypocotyls. However, unlike XyG, AGPs have been shown to be required for anisotropic cell expansion (Willats and Knox, 1996). Therefore, the fact that bulging of trichoblasts in reb1-1 is much more pronounced than swelling of the mur3-etiolated hypocotyl cells may be due more to the structural alteration of AGPs than to that of XyG.
Changes in the mechanical properties of the cell wall during turgor-driven expansion is dependent on several loosening enzymes, including XyG-endotransglycosidase and expansins (Fry, 1995; Cosgrove, 2001), whose action could be modulated by the structure of the substrate (Nishitani and Tominaga, 1992; Catalá et al., 2001). Thus, the altered structure of XyG in reb1-1 may well influence the activity of wall-loosening enzymes, which in turn would contribute to a decrease in tensile strength of trichoblasts. The abnormal XyG in reb1-1 trichoblasts is ungalactosylated and is water insoluble, two features that have been shown to reduce strongly the accessibility of the XyG-endotransglycosidase to its substrate in the mur3 hypocotyl (Peña et al., 2004). Thus, besides AGP, galactosylation of XyG could contribute indirectly to the regulation of anisotropic cell expansion and growth.
UGE4 Is Confined to a Protein Complex Specifically Involved in the Galactosylation of XyG and AGPs: A Hypothesis
Cell wall matrix polysaccharides and proteoglycans are synthesized in the Golgi apparatus and transported via secretory vesicles to the cell surface (Driouich et al., 1993). This requires the action of a set of Golgi glycosyltransferases, in addition to nucleotide sugar transporters and nucleotide sugar interconversion enzymes (Keegstra and Raikhel, 2001; Seifert, 2004). It has been postulated that these partners could interact physically to form complexes within Golgi membranes to coordinate sugar supply and polymer synthesis (Seifert, 2004). The REB1/RHD1 gene encodes one of five UDP-d-Glc 4-epimerase isoforms, UGE4, which is responsible for the conversion of UDP-d-Glc into UDP-d-Gal (Seifert et al., 2002). The observation that the reb1-1 mutation affects the galactosylation of AGPs (Andème-Onzighi et al., 2002) and XyG (this study; Seifert et al., 2002) specifically in trichoblasts indicates that the supply of UDP-d-Gal for the biosynthesis of these polymers is regulated at the cell-type level. Thus, it is postulated that, in reb1-1, ungalactosylated XyG and AGPs are associated with trichoblasts, but not with atrichoblasts. This could be explained by a possible compensation for the lack of UGE4 in atrichoblasts by the expression of a functional UGE1 gene, an isoform shown to be strongly expressed in all root cells of the mutant, including the epidermis (Seifert et al., 2004), although it is not clear why such compensation might occur in atrichoblasts, but not in trichoblasts. An explanation is that the UGE1 enzyme in reb1-1 trichoblasts could be specifically involved in channeling Gal to other polymers, such as pectins, rather than XyG and AGPs. Factors in support of this include (1) the observation that Gal-containing pectic epitopes revealed by the anti-bupleuran 2IIC antibodies are present in reb1-1 trichoblasts and (2) the finding that Gal content of isolated RG-I is unchanged. Additionally, our chemical analysis of RG-II molecules demonstrates that neither Gal content nor dimerization is affected by the reb1-1 mutation. Therefore, we hypothesize that UGE4 might be specifically involved in channeling of UDP-d-Gal to XyG and molecules of the arabinogalactan-II type (e.g. AGPs), whereas another isoform, possibly UGE1, would be responsible for providing Gal to pectic polysaccharides.
This hypothesis implies the occurrence of different biosynthetic complexes containing different UGE isoforms in addition to specific galactosyltransferases and UDP-d-Gal transporters. From our hypothesis, it follows that UGE4 is confined to complexes specifically involved in galactosylation of XyG and AGP. The UGE1 isoform would be confined to another complex involved in pectin galactosylation, including the addition of galactans to RG-I. Also, it is possible that such complexes might be associated with different Golgi cisternae, which would explain why some sugar epitopes of complex polysaccharides are added in some cisternae, but not in others (Zhang and Staehelin, 1992; Driouich et al., 1993). In mammalian cells, the formation of such complexes has been demonstrated for 2-O-sulfotransferase and uronosyl 5-epimerase, two enzymes involved in glycosaminoglycan biosynthesis (Pinhal et al., 2001).
Our hypothesis of a specific biosynthetic complex involved in XyG galactosylation is supported by transcript-profiling data comparing the expression of UGE4 (At1g64440), MUR2 (At2g03220), and MUR3 (At2g20370) from which it emerges that these genes have identical patterns of transcription (http://jsp.weigelword.org/atgendev/atgen.jsp). Likewise, the three genes are coexpressed in various experiments related to root development, as revealed by the two-gene scatter-plot tool from NASCArray (http://affymetrix.arabidopsis.info/narrays/twogenescatter.pl). The coordinated expression of UGE4 with two other genes involved in XyG synthesis is consistent with our hypothesis of a complex of the polymer- and sugar-synthesizing enzymes. In contrast, using the same tools, we found that the other UGE genes (UGE1: At1g1278; UGE2: At4g23920; and UGE3: At1g63180) have distinct expression profiles compared with MUR2 and MUR3. Clearly, this hypothesis could be confirmed or refuted by detailed work combining electron microscopy and immunocytochemistry of the Golgi polysaccharide biosynthetic complexes along with assays of protein-protein interaction among the putative components.
MATERIALS AND METHODS
Plant Growth Conditions
Two lines of Arabidopsis (Arabidopsis thaliana) L. (Heynh.) were used: the wild-type Columbia and the reb1-1 mutant (Baskin et al., 1992). Growth conditions were identical to those described by Andème-Onzighi et al. (2002). For phenotype recovery experiments, the culture medium of the reb1 -1 mutant was supplemented with 10 mm d-Gal.
Cell Wall Extraction, Isolation of Hemicellulosic Fractions, and Digestion with Endoglucanase
Frozen root material (5 g) was heated at 70°C for 15 min in 70% (v/v) ethanol to inactivate enzymes. The roots were ground in a potter homogenizer, and the homogenate was washed twice with hot 70% (v/v) ethanol and once with water. The remaining AIR pellet was then freeze dried. The lyophilized AIR was used for the determination of monosaccharide composition by using GC and for the extraction of hemicellulosic wall polymers. To extract a hemicellulosic fraction, AIR was treated with boiled ammonium oxalate at 0.5% (2 × 1 h) followed by incubation in 4 m KOH overnight at room temperature as described by Ray et al. (2004). Two fractions were distinctly obtained from each sample (wild type, reb1-1, and reb1-1 + 10 mm Gal): One is a soluble fraction (4 m KOH-soluble hemicellulosic fraction) and one is an insoluble fraction (4 m KOH-insoluble hemicellulosic fraction). It is worth noting that, unlike in the wild type and reb1-1 + 10 mm Gal, only the biochemical analysis of the 4 m KOH-insoluble hemicellulosic fraction from reb1-1 roots revealed the presence of XyG. The amount of the insoluble XyG in reb1-1 roots was determined to be 3% of the total root XyG by quantification of monosaccharides from GC of the soluble and insoluble fractions.
XyG oligomers were generated either from AIR extract or from 4 m KOH hemicellulosic fractions after enzymatic digestion with endo-β-d-(1→4)-glucanase (EC 3.2.1.4; catalog no. E–CELTR; Megazyme) as described previously (Lerouxel et al., 2002; Ray et al., 2004).
Extraction and Analysis of RG-I and RG-II
Extraction of pectic material and further purification of RG-I and RG-II were performed as described by Ishii et al. (2001). Briefly, 3 g of frozen roots were suspended in aqueous 80% (v/v) heated ethanol and centrifuged at 2,500 rpm for 5 min. The insoluble residue was washed with 80% to 95% to 100% (v/v) ethanol, chloroform:methanol (1:1, v/v), acetone, and then air dried. The AIR was treated for 4 h at 4°C with 0.1 n NaOH to saponify the methyl and acetyl esters. The suspensions were adjusted to pH 5 with 10% (v/v) glacial acetic acid and then treated for 16 h at 30°C with a homogeneous preparation of EPG from Aspergillus niger (2.5 units; Megazyme). The suspensions were centrifuged and the insoluble residues washed with water. The EPG-soluble fractions were dialyzed (1-kD cutoff dialysis tubing) against deionized water and freeze dried. RG-I and RG-II polysaccharides were purified from the EPG-solubilized material by elution from SEC on a Sephadex G-75 (2.5 × 90 cm) and then a Superdex Prep 75 (Amersham-Pharmacia Biotech; 1.6 × 38 cm) column. SEC-refractive index was performed with a Shimadzu LC-10A system with a refractive index detector (model RID-10A; Shimadzu) connected to a Sephadex G-75 column or a Superdex-75 HR 10/30 column eluted at 0.6 mL min−1 with 50 mm ammonium formate, pH 5.3, as previously described (Ishii and Matsunaga, 1996). The presence of mRG-II and dRG-II-B in the EPG digests was determined by comparing their retention times with those of the authentic mRG-II and dRG-II-B from sugar beet (Beta vulgaris) and red wine.
Monosaccharide Composition Analysis
Monosaccharide composition of crude cell wall (AIR) and hemicelulosic fractions was determined as previously described by Ray et al. (2004). Neutral glycosyl composition of RG-I was determined by GC of their alditol acetate derivatives (York et al., 1986). Combined neutral and acidic glycosyl composition of RG-II was determined by GC of their trimethylsilyl methyl ester methyl glycoside derivatives (York et al., 1986).
HPAEC-PAD
Endoglucanase-generated XyG fragments were analyzed by HPAEC (DX 500 system; Dionex) equipped with a CarboPac PA-1 column and a GP 50 gradient pump. XyG fragments were separated using a gradient from 100 mm NaOH (solvent A) to 1 m NaOAc in 100 mm NaOH (solvent B) at 1 mL min−1 using the following conditions: 0 min, 100% A; 5 min, 95% A; and 30 min, 92% A. Assignments of peaks to XyG fragments were carried out according to published data (Vincken et al., 1996) and by comparison of their retention times with major XyG oligosaccharides prepared by digestion of XyG from tamarind with endoglucanase (see Lerouxel et al., 2002).
MALDI-TOF MS Analysis
MALDI-TOF mass spectra of the XyG fragments solubilized by endoglucanase were acquired on a Voyager DE-Pro MALDI-TOF instrument (Applied Biosystems) equipped with a 337-nm nitrogen laser. Mass spectra were performed in the reflector-delayed extraction mode using 2,5-dihydroxybenzoic acid (Sigma-Aldrich) as matrix. The matrix, freshly dissolved at 5 mg mL−1 in 30%:70% acetonitrile/0.1% trifluoroacetic acid, was mixed with the solubilized oligosaccharides in a ratio of 1:1 (v/v). These spectra were recorded in a positive mode, using an acceleration voltage of 20,000 V with a delay time of 100 ns. They were smoothed once and externally calibrated using commercially available mixtures of peptides and proteins (Applied Biosystems). In this study, the MALDI-TOF mass spectra of XyG oligosaccharides were calibrated using des-Arg-1-bradykinin (904.4681 D), angiotensin I (1296.6853), Glu-1-fibrinopeptide B (1570.6774 D), ACTH clip 18 to 39 (2465.1989), and bovine insulin (5730.6087). Laser shots were accumulated for each spectrum to obtain an acceptable signal-to-noise ratio.
Immunofluorescence Microscopy
Fixation, embedding, immunolabeling, and microscopy were performed as previously described (Andème-Onzighi et al., 2002). The antibodies were used at 1:10 for CCRC-M1 and anti-XyG (originally designated as anti-XG; see Moore and Staehelin, 1988), at 1:5 for anti-RG-II and LM5, and at 1:200 for anti-bupleuran 2IIC.
Immersion immunofluorescence staining of XyG epitopes was done on roots after a brief fixation with 4% paraformaldehyde according to the procedure of Willats et al. (2001).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number At1g64440 for the REB1/RHD1 gene.
Supplementary Material
Acknowledgments
We wish to give special thanks to T. Baskin (University of Massachusetts) for his valuable comments and helpful suggestions on the manuscript as well as for the provision of the reb1-1 seeds. We also acknowledge M. Hahn (University of Georgia), A. Staehelin (University of Colorado), and H. Yamada (Kitasato Institute at Tokyo) for gifts of antibodies. We are grateful to O. Lerouxel, M. Seveno, and C. Rihouey (University of Rouen) for help and advice throughout this work, as well as A. Faik (University of Ohio) for critical reading of the first version of the manuscript.
This work was supported by the Centre National de la Recherche Scientifique and the University of Rouen (to A.D.) and by the “PROBRAIN” (to T. I.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Azeddine Driouich (azeddine.driouich@univ-rouen.fr).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.074997.
References
- Andème-Onzighi C, Lhuissier F, Vicré M, Yamada H, Driouich A (2000) A (1→3,6)-β-d-galactosyl epitope containing uronic acids associated with bioactive pectins occurs in discrete cell wall domains in hypocotyl and root tissues of flax seedlings. Histochem Cell Biol 113: 61–70 [DOI] [PubMed] [Google Scholar]
- Andème-Onzighi C, Sivaguru M, Judy-March J, Baskin TI, Driouich A (2002) The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan-proteins and the organisation of cortical microtubules. Planta 215: 949–958 [DOI] [PubMed] [Google Scholar]
- Baskin TI, Betzner AS, Hoggart R, Cork A, Williamson RE (1992) Root morphology mutants in Arabidopsis thaliana. Aust J Plant Physiol 19: 427–437 [Google Scholar]
- Burget EG, Verma R, Molhoj M, Reiter WD (2003) The biosynthesis of l-arabinose in plants: molecular cloning and characterization of a Golgi-localized UDP-d-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis. Plant Cell 15: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cells in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Catalá C, Rose JKC, York WS, Albersheim P, Darvill AG, Bennett AB (2001) Characterization of a tomato xyloglucan endotransglycosylase gene that is down-regulated by auxin in etiolated hypocotyls. Plant Physiol 127: 1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ (1999) Enzymes and others agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50: 391–417 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2001) Wall structure and wall loosening: a look backwards and forwards. Plant Physiol 125: 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A, Faye L, Staehelin LA (1993) The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci 18: 210–214 [DOI] [PubMed] [Google Scholar]
- Fagard M, Höfte H, Vernhettes S (2000) Cell wall mutants. Plant Physiol Biochem 38: 15–25 [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R (1999) The boron requirement and cell wall properties of growing and stationary suspension-cultured Chenopodium album L. cells. Plant Physiol 117: 1401–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC (1995) Polysaccharides-modifying enzymes in plant cell wall. Annu Rev Plant Physiol Plant Mol Biol 46: 497–520 [Google Scholar]
- Fry SC, York W, Albersheim P (1993) An unambiguous nomenclature for xyloglucan derived oligosaccharide. Physiol Plant 89: 1–3 [Google Scholar]
- Hayashi T (1989) Xyloglucans in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol 40: 139–168 [Google Scholar]
- Hwang JW, Kokini JL (1991) Structure and rheological function of side branches of carbohydrate polymers. J Texture Stud 22: 123–167 [Google Scholar]
- Ishii T, Matsunaga T (1996) Isolation and characterisation of a boron-rhamnogalacturonan II complex from cell walls of sugar beet pulp. Carbohydr Res 284: 1–9 [Google Scholar]
- Ishii T, Matsunaga T, Hayashi N (2001) Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue. Plant Physiol 126: 1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H, Masaoka N, Ishii T, Satoh S (2002) From the cover: a pectin glucuronyltransferase is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci USA 99: 16319–16324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MC (1984) Structure and properties of pectin gels in plant cell wall. Plant Cell Environ 7: 153–164 [Google Scholar]
- Jones L, Seymour GB, Knox JP (1997) Localization of pectic galactan in tomato cell wall using a monoclonal antibody specific to (1-4)-β-d-galactan. Plant Physiol 113: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K, Raikhel N (2001) Plant glycosyltransferases. Curr Opin Plant Biol 4: 219–224 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110: 1017–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouxel O, Choo TS, Séveno M, Usadel B, Faye L, Lerouge P, Pauly M (2002) Rapid structural phenotyping of plant cell wall mutants by enzymatic oligosaccharide fingerprinting. Plant Physiol 130: 1754–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, York WS, Stuikeprill R, Meyer B, Staehelin LA (1991) Simulations of the static and dynamic molecular conformations of xyloglucan: the role of the fucosylated side-chain in surface-specific side-chain folding. Plant J 1: 195–215 [PubMed] [Google Scholar]
- Lynch MA, Staehelin LA (1992) Domain-specific and cell type-specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J Cell Biol 118: 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson M, Dunand C, Li X, Verma R, Vanzin GF, Caplan J, Shoue DA, Carpita NC, Reiter WD (2003) The MUR3 gene of Arabidopsis encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. Plant Cell 15: 1662–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T (1997) Boron in plant cell walls. Plant Soil 193: 59–70 [Google Scholar]
- Matoh T, Ishigaki K, Ohno K, Azuma J (1993) Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol 34: 639–642 [Google Scholar]
- Matoh T, Takasaki M, Takabe K, Kobayashi M (1998) Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol 39: 483–491 [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, Higuchi M, Darvill AG, Albersheim P, O'Neill MA (2004) Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes and bryophytes: implications for the evolution of vascular plants. Plant Physiol 134: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M, Darvill AG, Albersheim P (1982) Structure of plant cell walls. XII. Identification of seven glycosyl residues attached to O-4 of the 2-4 linked l-rhamnosyl residues of rhamnogalacturonan I. Plant Physiol 70: 1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølhøj M, Verma R, Reiter WD (2004) The biosynthesis of d-galacturonate in plants: functional cloning and characterization of a membrane-anchored UDP-d-glucuronate 4-epimerase from Arabidopsis. Plant Physiol 135: 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PJ, Staehelin LA (1988) Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pretense L.: implication for secretory pathways. Planta 174: 433–445 [DOI] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R (1992) Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem 267: 21058–21064 [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846–849 [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Ishii T, Albersheim P, Darvill AG (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55: 109–139 [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P (1996) Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem 271: 22923–22930 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Ryden P, Madson M, Smith AC, Carpita NC (2004) The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth. Plant Physiol 134: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Hocart CH, Redmond JR, Williamson RE (2000) Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production. Planta 211: 406–414 [DOI] [PubMed] [Google Scholar]
- Pinhal MA, Smith B, Olson S, Aikwa J, Kimata K, Esko JD (2001) Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5-epimerase and 2-O-sulfotransferase interact in vivo. Proc Natl Acad Sci USA 98: 12984–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hahn MG (1994) Generation of monoclonal antibodies against plant cell wall polysaccharides. Plant Physiol 104: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Loutelier-Bourhis C, Condamine E, Driouich A, Lerouge P (2004) Structural investigation of hemicellulosic polysaccharides from Argania spinosa: characterisation of a novel xyloglucan motif. Carbohydr Res 339: 201–208 [DOI] [PubMed] [Google Scholar]
- Reiter WD, Chapple C, Somerville C (1997) Mutant of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J 12: 335–345 [DOI] [PubMed] [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signalling. Phytochemistry 57: 929–967 [DOI] [PubMed] [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter W-D, McCann MC (2003) Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol 132: 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai MH, Kiyohara H, Matsumuto T, Tsumuraya Y, Hashimoto Y, Yamada H (1998) Characterization of antigenic epitopes in anti-ulcer pectic polysaccharides from Bupleurum falcatum L. using several carbohydrates. Carbohydr Res 311: 219–229 [DOI] [PubMed] [Google Scholar]
- Seifert GJ (2004) Nucleotide sugar inter-conversions and cell wall biosynthesis: how to bring the inside to the outside. Curr Opin Plant Biol 3: 277–284 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Barber C, Wells B, Dolan L, Roberts K (2002) Galactose biosynthesis in Arabidopsis: genetic evidence for substrate channeling from UDP-D-galactose into cell wall polymers. Curr Biol 12: 1840–1845 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Barber C, Wells B, Roberts K (2004) Growth regulators and the control of nucleotide sugar flux. Plant Cell 16: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR (2003) Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc Natl Acad Sci USA 100: 1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Laurie S, Turner SR (2000) Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. Plant Cell 12: 2529–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter WD (2002) The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc Natl Acad Sci USA 99: 3340–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicré M, Santaella C, Blanchet S, Gateau A, Driouich A (2005) Root border-like cells of Arabidopsis, microscopical characterization and role in the interaction with rhizobacteria. Plant Physiol 138: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincken JP, Beldman G, Niessen WMA, Voragen AGJ (1996) Degradation of apple fruit xyloglucan by endoglucanase. Carbohydr Polym 29: 75–85 [Google Scholar]
- Willats WGT, Knox JP (1996) A role of arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J 9: 919–925 [DOI] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- York WS, Darvill AG, Stevenson TT, Albersheim P (1986) Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol 118: 3–40 [Google Scholar]
- Zablackis E, Huang J, Muller B, Darvill AG, Albersheim P (1995) Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol 107: 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA (1992) Functional compartmentalization of the Golgi apparatus of plant cells: an immunochemical analysis of high pressure frozen/freeze substituted sycamore suspension-cultured cells. Plant Physiol 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.