Abstract
The apoplast is considered the leaf compartment decisive for manganese (Mn) toxicity and tolerance in cowpea (Vigna unguiculata). Particularly apoplastic peroxidases (PODs) were proposed to be key enzymes in Mn toxicity-induced processes. The presented work focuses on the characterization of the role of hydrogen peroxide (H2O2)-producing (NADH peroxidase) and H2O2-consuming peroxidase (guaiacol POD) in the apoplastic washing fluid (AWF) of leaves for early stages of Mn toxicity and genotypic differences in Mn tolerance of cowpea. Leaf AWF of the Mn-sensitive cultivar (cv) TVu 91 but not of the Mn-tolerant cv 1987 showed an increase of guaiacol-POD and NADH-peroxidase activities at elevated AWF Mn concentrations. two-dimensional resolutions of AWF proteins revealed that cv TVu 91 expressed more and additional proteins at high Mn treatment, whereas Mn-tolerant cv TVu 1987 remained nearly unaffected. In both cultivars, NADH-peroxidase activity and accompanied H2O2 formation rate in vitro were significantly affected by Mn2+, p-coumaric acid, and metabolites occurring in the AWF. The total phenol concentration in the AWF was indicative of advanced stages of Mn toxicity but was rather unrelated to early stages of Mn toxicity and genotypic differences in Mn tolerance. The NADH oxidation by AWF PODs was significantly delayed or enhanced in the presence of the protein-free AWF from cv TVu 1987 or cv TVu 91, respectively. High-performance liquid chromatography analysis of AWF indicates the presence of phenols in cv TVu 1987 not observed in cv TVu 91. We conclude from our studies that the H2O2-producing NADH peroxidase and its modulation by stimulating or inhibiting phenolic compounds in the leaf apoplast play a major role for Mn toxicity and Mn tolerance in cowpea.
Manganese (Mn) excess represents an important factor limiting growth and crop yields particularly on acid and insufficiently drained soils with low redox potential (Foy, 1984; Schlichting and Sparrow, 1988). The oxidation of MnII and phenolic compounds catalyzed by peroxidase (apoplastic peroxidase [POD]) in the leaf apoplast is considered as a key reaction leading to Mn toxicity symptoms and finally leaf injury in cowpea (Vigna unguiculata L. Walp.; Horst, 1988). This assumption is based mainly on two observations: (1) Mn toxicity symptoms (brown spots on leaves) occur in the cell wall and consist of oxidized Mn and oxidized phenolic compounds (Wissemeier and Horst, 1992), (2) Kenten and Mann (1950, 1956) observed a close relationship between POD-catalyzed phenol and Mn oxidation. The oxidation of MnII and phenols is probably mediated by the formation of highly reactive MnIII and phenoxyradicals, which are considered as phytotoxic agents (Horst, 1988; Horst et al., 1999). Particularly the simultaneous enhancement of POD activity and expression of Mn toxicity symptoms (brown spots) suggest a close relationship between PODs and the oxidation of Mn and phenolic compounds (Fecht-Christoffers et al., 2003a, 2003b). However, the activation of PODs by Mn excess in cotton (Gossypium hirsutum) was considered a secondary effect (Sirkar and Amin, 1974), and also from our own work in cowpea the enhanced apoplastic POD activity could be the reason for or the consequence of Mn-induced leaf injury.
Peroxidases are widely distributed in plant tissues with different functions associated with an apparent lack in substrate specificity (Campa, 1991; Mäder, 1992; Hiraga et al., 2001). In the plant apoplast, they were attributed to act as hydrogen peroxide (H2O2)-consuming and phenol-oxidizing enzymes, thus leading to secondary cell wall and lignin formation (Polle et al., 1994; Sato et al., 1995; Christensen et al., 1998; Kärkönen et al., 2002). Peroxidases using NADH as electron donor were also termed NADH oxidases and have been suggested to play an important role in the formation of H2O2 needed for lignification (Elstner and Heupel, 1976; Gross et al., 1977; Halliwell, 1978; Mäder et al., 1980; Mäder and Amberg-Fisher, 1982; Bolwell et al., 1995). Mn and phenolic compounds presumably control the activity of the NADH peroxidase (Elstner and Heupel, 1976; Gross et al., 1977; Halliwell, 1978; Mäder and Füssl, 1982; Stich and Ebermann, 1984; Pedreño et al., 1987). Proteins purified from the apoplastic washing fluid (AWF) of leaves from the Mn-sensitive cowpea cv TVu 91 showed NADH-peroxidase activity, which was strongly enhanced at high leaf Mn concentrations (Fecht-Christoffers et al., 2003a). The stimulation of this H2O2-producing NADH peroxidase in the leaf apoplast of Mn-treated plants might be the cause for the elevated H2O2 formation of washed intact leaf segments from cowpea showing Mn toxicity symptoms (Horst et al., 1999).
Plant species and cultivars within species show considerable differences in Mn tolerance. Several mechanisms of Mn tolerance have been proposed: (1) sequestration of Mn in the apoplast thus preventing Mn oxidation (Maier, 1997; Horst and Maier, 1999), (2) transport of Mn into the vacuoles (Hirschi et al., 2000; Schaaf et al., 2002; Delhaize et al., 2003) and sequestration by organic acids (Maier, 1997; Horst and Maier, 1999), (3) maintaining low cytosolic Mn concentrations by enhanced transport of Mn into the endoplasmic reticulum (Wu et al., 2002), and (4) scavenging the Mn-induced enhanced formation of reactive oxygen species (ROS), phenoxy radicals, and MnIII by an efficient ascorbic acid turnover in the apoplast and cytoplasm (Fecht-Christoffers et al., 2003b; Fecht-Christoffers and Horst, 2005). But so far, none of the factors can satisfactorily explain genotypically enhanced Mn tolerance in cowpea (Horst et al., 1999).
In one of our recent studies, the emphasis was on the characterization of the Mn toxicity-induced alterations in the proteome of the leaf AWF of a Mn-sensitive cultivar. This work focuses on the characterization of genotypic differences in Mn tolerance with emphasis on the role of H2O2-producing (NADH peroxidase) POD and the apoplastic metabolites affecting the resulting H2O2 formation at early stages of Mn toxicity.
RESULTS
Mn Uptake and Formation of Visible Mn Toxicity Symptoms
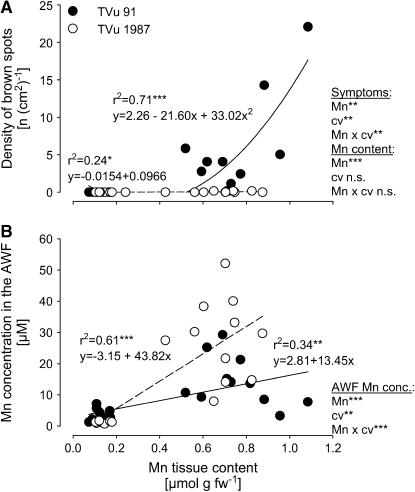
Plants of the Mn-sensitive and Mn-tolerant cowpea cv TVu 91 and cv TVu 1987, respectively, readily took up Mn, thus causing 10-fold higher Mn contents in the leaf tissue than those of control plants. Characteristic Mn toxicity symptoms (brown spots) were expressed on leaves of cv TVu 91, whereas cv TVu 1987 was unaffected (Fig. 1A). In spite of similar Mn tissue contents, cv TVu 1987 showed significantly higher Mn concentration in the AWF than cv TVu 91 (Fig. 1B).
Figure 1.
Relationship between Mn content in the bulk-leaf tissue and the density of brown spots on leaves (A) and the Mn concentration in the AWF (B). Plants of cowpea cv TVu 91 and cv TVu 1987 were treated with 50 μm Mn for 4 d, whereas control plants received 0.2 μm Mn continuously, n = 12. Levels of significance of regression analysis and F test are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively. n.s., Not significant.
Effect of Mn Treatment on the Protein Composition in the AWF
Two-dimensional (2D) resolutions of AWF proteins of control leaves did not show markedly different protein patterns between the two cultivars (Supplemental Fig. 1). However, they indicate an enhanced release of proteins from the symplast into the AWF particularly in the acid (pH 3–4) and neutral (pH 6) range of the polyacrylamide gel in leaves of cv TVu 91 treated with an elevated Mn supply compared to control plants. However, the protein pattern of cv TVu 1987 was hardly affected by the Mn treatment. The 2D resolution from cv TVu 91 showed a high conformity to previously shown results. Therefore, strongly expressed proteins on the gels can be considered identical with proteins identified previously by nanoliquid chromatography-mass spectrometry (MS)/MS (Fecht-Christoffers et al., 2003a), e.g. pathogenesis-related-like proteins, chitinase, peroxidase, thaumatin-like protein, and glucanase.
Effect of Mn Treatment on Activities of Guaiacol POD, NADH Peroxidase, and the Protein Concentration in the AWF
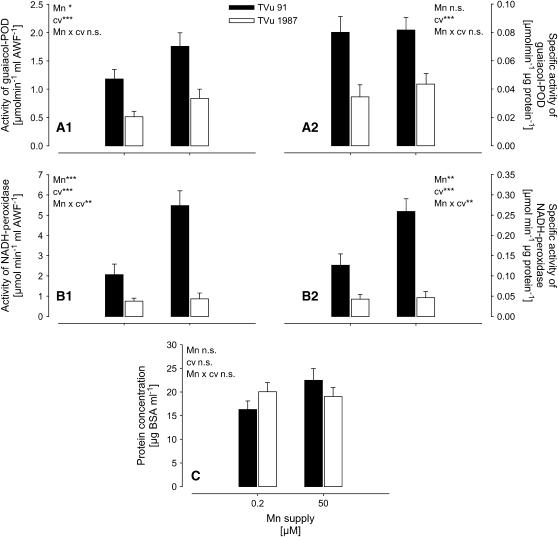
Total (Fig. 2, A1 and B1) and specific (Fig. 2, A2 and B2) activities of guaiacol POD and particularly of NADH peroxidase in the AWF were much higher in Mn-sensitive cv TVu 91 than in Mn-tolerant cv TVu 1987. Mn treatment induced a significant increase of apoplastic NADH peroxidase only in cv TVu 91 (highly significant Mn/cultivar interaction; Fig. 2, B1 and B2). In contrast to the total activity the specific guaiacol-POD activity did not respond to Mn treatment. This was due to enhanced (P = 0.0558, comparison of means for TVu 91; data not shown) protein concentrations of the AWF in cv TVu 91 (Fig. 2C).
Figure 2.
Effect of excess Mn on the total activity (A1) and specific activity (A2) of guaiacol POD, NADH peroxidase (B1 and B2; measurements were done in the presence of 16 mm MnCl2 and 1.6 mm p-coumaric acid), and the protein concentrations in the AWF (C) extracted from leaves of cowpea cv TVu 91 and cv TVu 1987. Plants were treated with 50 μm Mn for 4 d, whereas control plants received 0.2 μm Mn continuously. Values are means of independent replicates ±se, n = 12. Levels of significance of F test are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively. n.s., Not significant.
The Formation of H2O2 by Leaf-AWF NADH Peroxidase in Vitro
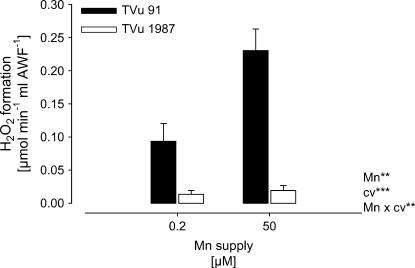
Because NADH peroxidase was proposed to generate H2O2, the H2O2 formation by apoplastic (AWF) NADH peroxidase was measured in vitro. The rate of H2O2 formation was significantly lower in the leaf AWF of cv TVu 1987 than in the AWF of cv TVu 91 (Fig. 3). The H2O2 formation was significantly enhanced by Mn treatment in cv TVu 91, but not in cv TVu 1987.
Figure 3.
In vitro formation of H2O2 by AWF proteins extracted from leaves of cowpea cv TVu 91 and cv TVu 1987 as affected by Mn supply during cultivation of plants. Plants were treated with 50 μm Mn for 4 d, whereas control plants received 0.2 μm Mn continuously. Values are means of independent replicates ±se, n = 12. Levels of significance of F test are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively.
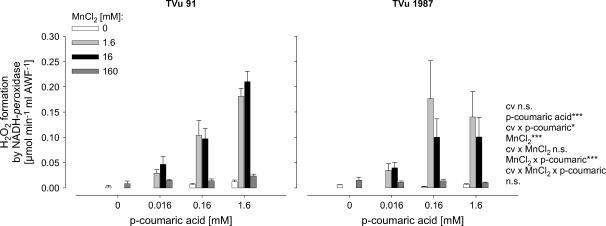
Effect of Mn and p-Coumaric Acid on the Leaf-AWF NADH Peroxidase and H2O2 Formation in Vitro
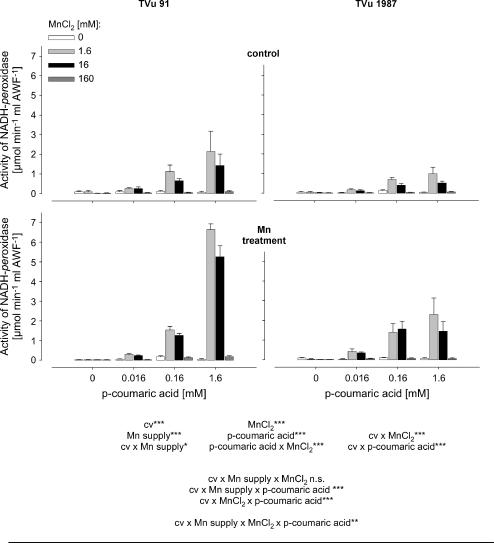
To get a better quantitative understanding of the role of Mn and phenols for the activity of leaf-AWF NADH peroxidase, Mn and p-coumaric acid concentrations were varied in the enzyme test. The NADH-oxidation rate by NADH peroxidase was significantly affected by the concentrations of p-coumaric acid and Mn (Fig. 4). Enzyme activities increased with increasing concentration of p-coumaric acid and increasing Mn concentrations up to 1.6 mm. The application of 16 mm Mn did not further enhance NADH-peroxidase activity significantly, and the highest Mn concentration applied (160 mm) strongly suppressed the NADH-oxidation rate. The AWF from plants treated with 50 μm Mn during cultivation showed generally higher NADH-peroxidase activities than control plants. This was observed in both cultivars. However, NADH-peroxidase activity was significantly lower in the Mn-tolerant cv TVu 1987 than in the Mn-sensitive cv TVu 91, particularly at the high p-coumaric acid concentration (significant cultivar × p-coumaric acid interaction).
Figure 4.
Effect of MnCl2 and p-coumaric acid concentrations on activity of NADH peroxidase in the AWF extracted from leaves of controls and Mn-treated plants (50 μm Mn for 4 d) of cowpea cv TVu 91 and cv TVu 1987. Concentrations of added p-coumaric acid varied from 0 to 1.6 mm and of MnCl2 from 0 to 160 mm. Values are means of independent replicates ±se, n = 4. Levels of significance of F test are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively. n.s., Not significant.
In agreement with the NADH-peroxidase activity, the formation rate of H2O2 in vitro was similarly dependent on the presence of p-coumaric acid and Mn (Fig. 5). The highest H2O2 formation rate was measured in the presence of 1.6 mm p-coumaric acid and 16 mm Mn in cv TVu 91. Although the overall cultivar difference was not significant, the H2O2 formation was significantly lower in the Mn-tolerant cv 1987 at the high p-coumaric acid concentration (significant cultivar × p-coumaric acid interaction) in agreement with the higher NADH-peroxidase activity shown in Figure 4.
Figure 5.
Effect of MnCl2 and p-coumaric acid concentrations on the in vitro formation rate of H2O2 by AWF proteins from Mn-treated plants (50 μm Mn for 4 d) of cowpea cv TVu 91 and cv TVu 1987. Concentrations of added p-coumaric acid varied from 0 to 1.6 mm, and of MnCl2 from 0 to 160 mm. Values are means of independent replicates ±se, n = 4. Levels of significance of F test are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively. n.s., Not significant.
Effect of Apoplastic Water-Soluble Metabolites on the NADH-Peroxidase Activity in the AWF
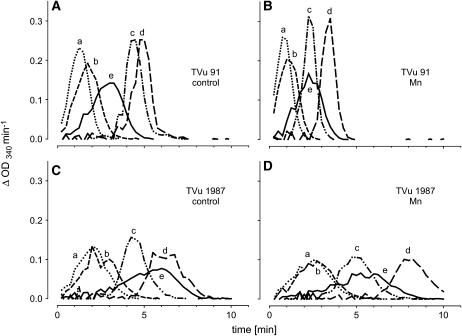
The differences in NADH-peroxidase activity in the AWF between the cultivars could be due to stimulating or inhibiting metabolites in the AWF. To investigate the influence such AWF metabolites have on the NADH-peroxidase activity (measurements were made in the presence of optimal Mn and p-coumaric acid concentrations), the purified apoplastic proteins from controls and Mn treatments of both cultivars were crosswise combined with protein-free AWF (AWF filtrate) or water. AWF filtrate alone and boiled AWF samples did not show any NADH-peroxidase activity (data not shown). The AWF filtrates added to the enzyme assay had differential effects on the kinetics of NADH oxidation (Fig. 6). In the presence of AWF filtrates from cv TVu 91 or water, the NADH oxidation by apoplastic enzymes of cv TVu 91 and cv TVu 1987 (controls and Mn treatments) started shortly after addition of NADH (lines a, b, and e in each plot). AWF filtrates from cv TVu 91 (lines a and b) accelerated the oxidation compared to water (line e). In the presence of AWF filtrates from cv TVu 1987 (lines c and d in each plot), NADH oxidation showed a lag time of 2 to 8 min. The longest lag time was observed in the combination of both proteins and filtrates of cv TVu 1987 treated with high Mn (plot D, line d). The combination of proteins from cv TVu 1987 and water (plots C and D, line e) also produced a lag time.
Figure 6.
Effect of water-soluble apoplastic metabolites (AWF filtrate) or water on the consumption of NADH by purified apoplastic proteins. Oxidation of NADH is displayed as changes in absorption at λ = 340 nm per min. AWF from controls and Mn treatments (50 μm for 4 d) from cowpea cv TVu 91 and cv TVu 1987 were divided into two fractions (purified proteins and AWF filtrate) using centrifugal concentrators with MWCO 5 kD. Protein concentrates (A, TVu 91 control; B, TVu 91 Mn; C, TVu 1987 control; and D, TVu 1987 Mn), and AWF-filtrates (a, TVu 91 control; b, TVu 91 Mn; c, TVu 1987 control; and d, TVu 1987 Mn) or water (e) were combined. For measurement of enzyme activities, 1 μL of protein concentrate and 50 μL AWF filtrate or double demineralized water were mixed with 16 mm MnCl2, 1.6 mm p-coumaric acid, and 0.3 mm NADH.
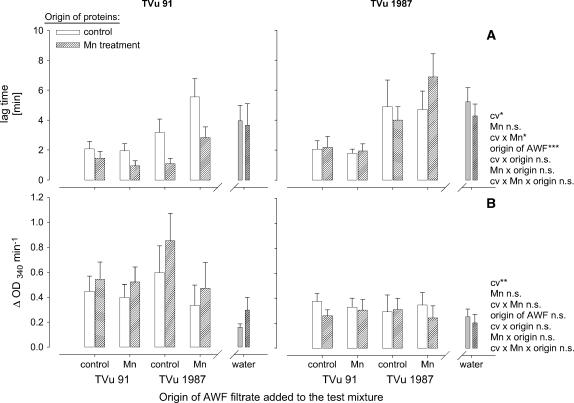
From the kinetics of the NADH oxidation, the duration of the lag time and the slope at maximum NADH-oxidation rate was submitted to statistical analysis (Fig. 7). The lag time (Fig. 7A) was significantly affected by the origin of AWF filtrates added to the test mixture, the origin of the AWF proteins, and the Mn status of the plants: AWF filtrates from leaves of cv TVu 91 reduced the lag time particularly in combination with AWF proteins from the same Mn-treated cultivar. Filtrates from TVu 1987 delayed the NADH oxidation especially of proteins from the same Mn-treated cultivar. No significant effect of the origin of the AWF filtrates on the maximum NADH-oxidation rate existed (Fig. 7B), but AWF proteins from cv TVu 91 generally, but more clearly when Mn treated, showed a significantly higher NADH-oxidation capacity than proteins from cv TVu 1987. This compares well with the NADH-oxidation capacity of the crude AWF shown in Figure 2.
Figure 7.
Effect of the origin of protein-free AWF filtrate and of water on the consumption of NADH by apoplastic protein purified from the AWF extracted from leaves of cowpea cv TVu 91 and cv TVu 1987. Time course of NADH oxidation is characterized by the delay (lag time) of NADH oxidation (A) and the slope of NADH-oxidation rate at maximum (max V; B). AWF from controls and Mn treatments of cowpea plants were divided into two fractions (purified proteins and protein-free AWF filtrate) using centrifugal concentrators with MWCO 4 kD. Protein concentrates, and AWF filtrates or water were combined. For measurement of enzyme activities, 1 μL of protein concentrate and 50 μL AWF filtrate or double demineralized water were mixed with 16 mm MnCl2, 1.6 mm p-coumaric acid, and 0.3 mm NADH. Values are means of independent replicates ±se, n = 6. Levels of significance of F test are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively. n.s., Not significant.
Concentrations and Composition of Phenolic Compounds of the AWF
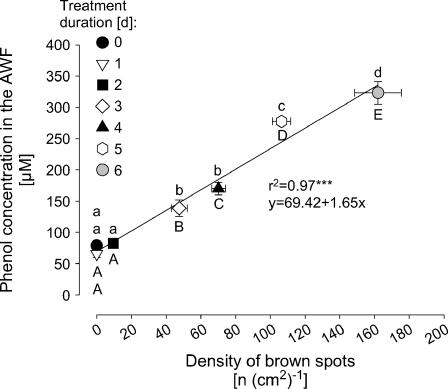
In a kinetic experiment with the Mn-sensitive cv TVu 91 that allowed to vary the severity of Mn toxicity as expressed by the number of brown spots over a wide range [0–160 (cm2)−1], the apoplastic phenol concentration increased with the severity of Mn toxicity (Fig. 8). Only in leaves with clearly expressed Mn toxicity symptoms [>50 spots (cm2)−1] phenol concentrations in the AWF were significantly enhanced. The Mn-tolerant cv TVu 1987 did not show any brown spots under the same experimental conditions (data not shown).
Figure 8.
Relationship between the total phenol concentrations in the leaf AWF and the density of brown spots in cowpea cv TVu 91. Plants were precultured in nutrient solution and Mn concentration was increased to 50 μm for 1, 2, 3, 4, 5, or 6 d, respectively. Control plants received 0.2 μm continuously. Values are means of independent replicates ±se, n = 14. Significant differences between means are indicated by different letters at P < 0.05 (Tukey): uppercase letters for density of brown spots and lowercase letters for phenol concentrations. Levels of significance are shown by *, **, and *** for P < 0.05, 0.01, and 0.001, respectively.
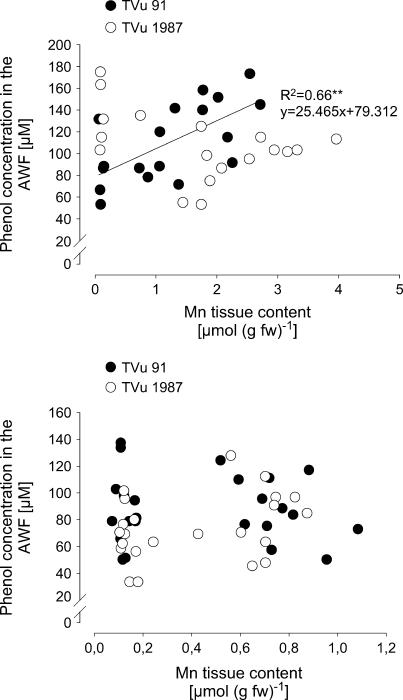
In a further experiment the relationship between the AWF phenol concentrations and a wide range of Mn leaf-tissue contents were studied (Fig. 9A). With increasing Mn contents the phenol concentrations increased in cv TVu 91 but not in cv TVu 1987. To elucidate primary rather than secondary lesions of Mn toxicity the cultivars differing in Mn sensitivity were additionally compared at much lower Mn toxicity stress than in the previous experiment [0–20 brown spots (cm2)−1; Fig. 9B]. Phenol concentrations in the AWF showed a considerable variability. They appeared not to be affected by the Mn tissue content up to 1.2 μmol (g fresh weight)−1, and there was no difference between the cultivars.
Figure 9.
Relationship between Mn tissue contents and total phenol concentrations in the leaf AWF of cowpea cv TVu 91 and cv TVu 1987. Plants were treated with 50 μm Mn for different treatment periods of 0 to 5 d resulting in a wide (top) or a narrow (bottom) range of Mn leaf-tissue contents and Mn toxicity symptoms only in the Mn-sensitive cv TVu 91 [up to 60 or <20 brown spots (cm)−2, respectively]. Phenol concentrations were determined with the Folin-Denis reagent and calculated using standard solutions of p-coumaric acid in the range from 0 to 300 μm.
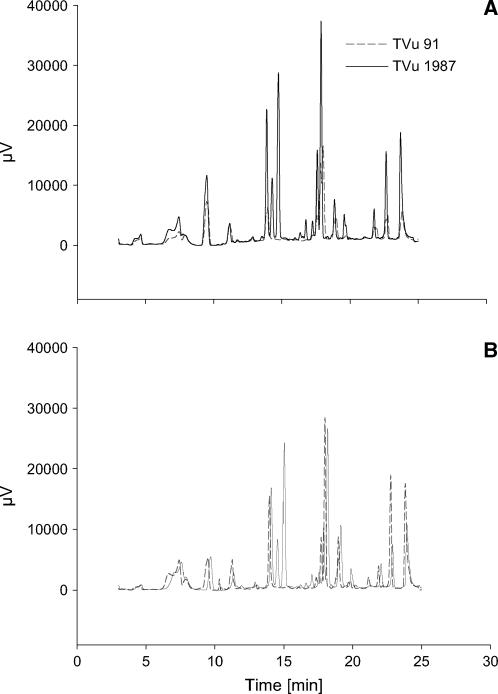
Since quantitative differences between Mn supplies and cultivars could not be measured, the phenols in the leaf AWF were separated using HPLC to study possible qualitative differences. The HPLC chromatograms revealed differences in the pattern of phenols not only between optimum and elevated Mn supply (compare Fig. 10A with Fig. 10B) but also between the cultivars at optimum (Fig. 10A) and elevated (Fig. 10B) supply. The qualitative difference between the cultivars was particularly marked: Compared to the Mn-sensitive cv TVu 91 the Mn-tolerant cv TVu 1987 showed at least four additional peaks independent of the Mn treatment. Since no similarities between absorption spectra of apoplastic and commercial available phenolic compounds existed, the identities of the phenolic compounds in the AWF remain unknown so far.
Figure 10.
HPLC chromatograms of phenols in the AWF from the leaves of controls (A) and Mn treatments (B) of cowpea cv TVu 91 and cv TVu 1987. Plants were treated with 50 μm Mn for 4 d, whereas control plants received 0.2 μm Mn continuously. AWF was acidic hydrolyzed at 80°C and phenols extracted by solid-phase extraction. Results show the chromatograms of three biological repetitions and three measurements combined using the Kontron system 3000 software (Kontron Instruments).
DISCUSSION
Mn Uptake and Development of Mn Toxicity Symptoms
Excess Mn supply during the cultivation of cowpea plants caused the formation of visible small, dark-brown spots on leaves, representing oxidized Mn and phenols (Wissemeier and Horst, 1992). The density of these brown spots is a reliable parameter for the severity of Mn toxicity (Wissemeier and Horst, 1991). In this work, the formation of 5 to 10 brown spots per cm2 leaf area (Fig. 1A) represents an early and moderate stage of Mn toxicity without further distinct Mn toxicity symptoms, such as chlorosis and necrosis. As previously shown (Horst et al., 1999; Fecht-Christoffers et al., 2003b), cowpea cv TVu 91 and cv TVu 1987 show significant differences in the expression of Mn toxicity symptoms at elevated Mn tissue contents. Therefore, the Mn-resistance of cv TVu 1987 is due to the ability to tolerate high Mn concentrations in the leaf tissue. In the Mn-sensitive cv TVu 91 but not in the Mn-tolerant cv TVu 1987, the appearance of Mn toxicity symptoms appears to be related to an increase of the Mn concentration of the AWF (Fig. 1B). The rapid accumulation of Mn in the AWF (Fecht-Christoffers et al., 2003a) indicates a particular role of free apoplastic Mn for the expression of Mn toxicity in spite of its low contribution to the total Mn content of the leaf. The significantly higher Mn concentration in the leaf AWF of Mn-treated plants of Mn-tolerant cv TVu 1987 without inducing Mn toxicity symptoms (Fig. 1B) demands for a particularly efficient Mn-tolerance mechanism in this cultivar.
The Apoplast Proteome: 2D Isoelectric Focusing/SDS-PAGE Resolutions of Proteins in the AWF from Cultivars Differing in Mn Tolerance
In the Mn-sensitive cv TVu 91, development of toxicity symptoms is accompanied by an enhanced release of proteins into the leaf apoplast (Fecht-Christoffers et al., 2003a). The protein concentration in the AWF is dependent on the severity of Mn toxicity symptoms. The only slight increase of the apoplastic protein concentration (Fig. 2C) reflects the mild expression of Mn toxicity in this experiment. The 2D resolutions of water-soluble apoplastic proteins from cv TVu 91 (Supplemental Fig. 1) in this study were carried out with AWF from leaves with a stronger expression of Mn toxicity (density of brown spots higher than 30 per cm2). The 2D resolutions showed high conformity to previously shown results (Fecht-Christoffers et al., 2003a). Therefore, strongly expressed leaf AWF proteins in Mn-treated cv TVu 91 presented in Supplemental Figure 1 were most probably identical with the proteins identified previously by nanoliquid chromatography-MS/MS (Fecht-Christoffers et al., 2003a). Among the most prominently expressed proteins were pathogenesis-related proteins, thaumatin-like proteins, chitinase, peroxidase, and glucanase. The role of most of these pathogenesis-related-like proteins in Mn toxicity is not clear (Fecht-Christoffers et al., 2003a). The 2D resolutions of proteins in the AWF extracted from Mn-tolerant cv TVu 1987 did not show Mn-induced changes of the apoplast proteome (Supplemental Fig. 1). This confirms the assumption that the release of proteins in the Mn-sensitive cultivar reflects Mn sensitivity rather than a Mn-tolerance mechanism (Fecht-Christoffers et al., 2003a). However, it has to be considered that minor changes in the apoplast proteome could not be detected due to a too low amount of proteins loaded on the gel. Therefore, it cannot yet be excluded that apoplastic proteins are involved in the enhanced Mn tolerance of TVu 1987. Also, only water-soluble proteins of the leaf apoplast have been analyzed so far.
PODs in the Leaf Apoplast and Their Role in Mn Toxicity
Particularly basic (cationic) POD isoenzymes, present in young and developing tissues (MacAdam et al., 1992; Polle et al., 1994) are attributed to play a role in the production of H2O2 in the cell wall (Mäder et al., 1980; Mäder and Amberg-Fisher, 1982). Because NADH was shown to act as the electron donor, H2O2-producing POD was also named NADH oxidase. Apoplastic acidic (anionic) PODs were frequently detected in maturing plant tissues (MacAdam et al., 1992; Polle et al., 1994; de Souza and MacAdam, 1998; Klotz et al., 1998) and showed high affinities to H2O2 and monophenolic compounds (Mäder et al., 1977; Mäder et al., 1980). Therefore, they are considered to be involved in secondary cell wall formation and lignification by reduction of H2O2 and oxidation of phenolic compounds (Mäder et al., 1980; Mäder et al., 1986; Lagrimini et al., 1993; Ros Barcelo, 1997). Beside the discrimination of POD isoforms according to their functionality, PODs were also proposed to act as polyfunctional enzymes (Pedreño et al., 1995). Such peroxidases undergo at least two reaction cycles: (1) The peroxidase-oxidase cycle is characterized by the formation of ferrous POD (Fe2+) and the inactive compound III (oxyperoxidase Fe6+) in the presence of NADH under aerobic conditions (Yamazaki and Yokota, 1973; Pedreño et al., 1987). This cycle was proposed to be the source of H2O2 formation (Halliwell, 1978). (2) A two-electron transfer to H2O2, followed by two single-reduction steps back to the ferric (Fe3+) enzyme, characterizes the peroxidatic reaction. This classical peroxidatic cycle represents probably the source of phenol oxidation and polymerization in the cell wall.
In cowpea, the magnitude of the POD activity particularly in the leaf AWF is indicative of the severity of Mn toxicity (Fecht-Christoffers et al., 2003a, 2003b), like other indicators, e.g. the formation of brown spots and of callose (Wissemeier and Horst, 1991; Wissemeier et al., 1992). The AWF of cv TVu 91 contains several POD isoenzymes, which were not fully characterized up to now. Mn toxicity-released PODs are presumably acidic (anionic) POD isoforms (Supplemental Fig. 1; Fecht-Christoffers et al., 2003a). The release of acidic isoenzymes was attributed to a late response to several stresses (Gaspar et al., 1985; Castillo, 1986), catalyzing the typical H2O2-consuming peroxidatic cycle. Therefore, released acidic PODs probably catalyze the formation of brown depositions in the cell wall. Observations of Lagrimini (1991) support this hypothesis, because transgenic plants overexpressing acidic PODs showed more rapid wound-induced browning of tissues than control plants.
In this study, the Mn treatment only slightly affected the total AWF guaiacol-POD activity (Fig. 2A1) in both cultivars, whereas the specific activity was unaffected (Fig. 2A2). This can be related to the mild expression of Mn toxicity in this experiment and confirms the above-made assumption that the guaiacol-POD activity is closely related to the formation of the brown depositions in the leaf apoplast. Guaiacol-POD activity was lower in the Mn-tolerant cv TVu 1987 compared to the Mn-sensitive cv TVu 91. This genotypic difference was even greater regarding the activity of the NADH peroxidase. Both total and specific activities were highly significantly increased by Mn treatment exclusively in the Mn-sensitive cv TVu 91 (Fig. 2, B1 and B2) and remained on a low level in the Mn-tolerant cv TVu 1987. This suggests that NADH-peroxidase activity in the leaf AWF is more closely related to early events of Mn toxicity than guaiacol POD and that the control of the NADH-peroxidase activity is important for genotypic Mn leaf-tissue tolerance.
The Effect of Cofactors on NADH-Peroxidase Activity
The regulatory effect of phenolic compounds and Mn on NADH-peroxidase activity is widely documented (Yamazaki and Piette, 1963; Halliwell, 1978; Mäder et al., 1980; Mäder and Füssl, 1982; Pedreño et al., 1987). MnII was proposed to affect the NADH-peroxidase cycle by reducing reactive oxygen, thus forming MnIII (Yamazaki and Piette, 1963; Halliwell, 1978). Indeed, the beneficial property of MnII as scavenger for ROS is documented (Kawano et al., 2002) and discussed as a superoxide dismutase-equivalent system in lactic acid bacteria (Archibald and Fridovich, 1982a, 1982b). Therefore, the suppression of NADH peroxidase in the presence of 160 mm MnCl2 (Fig. 4) might be caused by a complete scavenging of reactive oxygen radicals produced in the POD cycles. The scavenging of 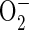 by MnII in vitro is significantly affected by the presence of several buffers and complexing agents. Aqueous Mn2+ proved to be a poor scavenger for ROS, whereas in the presence of orthophosphate, pyrophosphate, and a number of organic acids e.g. lactate, succinate, and malate the ROS scavenging activity of MnII increased (Archibald and Fridovich, 1982a). The nature of the ligand determines the stability of the manganous complex. Apoplastic Mn concentrations up to 500 μm in vivo (calculated from Fig. 1 assuming a 10× dilution of the leaf apoplastic sap in the AWF through infiltration) might stimulate NADH peroxidase more effectively due to the association of Mn to beneficial ligands, e.g. organic acids. Since no significant differences in the occurrence and concentrations of organic acids in the apoplast between cv TVu 91 and cv TVu 1987 have been observed (Maier, 1997), differences in their Mn tolerance are probably not due to such differences.
by MnII in vitro is significantly affected by the presence of several buffers and complexing agents. Aqueous Mn2+ proved to be a poor scavenger for ROS, whereas in the presence of orthophosphate, pyrophosphate, and a number of organic acids e.g. lactate, succinate, and malate the ROS scavenging activity of MnII increased (Archibald and Fridovich, 1982a). The nature of the ligand determines the stability of the manganous complex. Apoplastic Mn concentrations up to 500 μm in vivo (calculated from Fig. 1 assuming a 10× dilution of the leaf apoplastic sap in the AWF through infiltration) might stimulate NADH peroxidase more effectively due to the association of Mn to beneficial ligands, e.g. organic acids. Since no significant differences in the occurrence and concentrations of organic acids in the apoplast between cv TVu 91 and cv TVu 1987 have been observed (Maier, 1997), differences in their Mn tolerance are probably not due to such differences.
The phenolic compounds had inconsistent effects on the functionality of PODs (Lee, 1977; Mäder and Füssl, 1982; Pedreño et al., 1987). Already Kenten and Mann (1950) detected a significant influence of several phenolic compounds on the POD-catalyzed oxidation of manganous pyrophosphate. Mn oxidation was stimulated by monohydroxy phenols but not by dihydroxy and trihydroxy phenols. Phenols have been proposed to affect the peroxidase-catalyzed NADH oxidation via different pathways (Yamazaki and Yokota, 1973; Halliwell, 1978; Mäder and Füssl, 1982; Pedreño et al., 1987). The time course of NADH oxidation showed a characteristic lag time, followed by a high turnover rate of NADH (Yokota and Yamazaki, 1977; Pedreño et al., 1987). The length of the lag time was significantly influenced by the concentrations of enzyme and NADH and was affected by phenols. Pedreño et al. (1987) showed that the addition of p-coumaric acid and coniferyl alcohol caused a lag time, followed by a sudden and rapid NADH oxidation. No distinct lag time was observed in the presence of ferulic acid and guaiacol. The addition of sinapinic acid alone or together with ferulic acid inhibited NADH oxidation completely. Interestingly, the oxidation of NADH by purified proteins from the AWF showed a similar characteristic initial lag time followed by a rapid oxidation of NADH (Fig. 6). Particularly the length of the initial lag time was affected by the origin of the AWF filtrate (Figs. 6 and 7). The AWF filtate from cv TVu 91 (Mn sensitive) shortened the lag time and the AWF filtrate from cv TVu 1987 delayed NADH oxidation (Fig. 6). Since no NADH oxidation was observed by AWF filtrates alone, particularly the interaction between enzymes and apoplastic promoters (cv TVu 91) or inhibitors (cv TVu 1987) governs the oxidation of NADH in the leaf apoplast. A similar effect of phenolic compounds on the time courses of POD-catalyzed indole-3-acetic acid oxidation (Gelinas and Postlethwait, 1969; Lee, 1977) support the close relationship between phenolic compounds and the functionality of POD. Gelinas and Postlethwait (1969) reported a logarithmic increase in lag period by the addition of linearly increasing concentrations of boiled plant extracts from maize (Zea mays) plants, containing presumably ferulic acid. The similar effects of phenols and AWF filtrates on the functionality of NADH peroxidase suggest that the phenol composition and concentration in the apoplast play a crucial role in Mn toxicity and particular Mn tolerance. The lower NADH-peroxidase activity in cv TVu 1987 at elevated levels of guaiacol-POD activity (Fig. 2) may be caused by the inhibitory effect of specific phenols on NADH oxidation present in the AWF of cv TVu 1987.
Relationship between NADH Peroxidase and H2O2 Formation and Its Role in Mn Toxicity
The presented results support the assumed linkage between NADH-peroxidase activity and H2O2 formation. The formation rate of H2O2 in vitro was significantly increased by Mn treatment only in the Mn-sensitive cv TVu 91 (Fig. 3). The addition of the cofactors Mn and p-coumaric acid influenced both NADH-peroxidase activities (Fig. 4) and H2O2 formation rates (Fig. 5) in both cultivars, but stronger in TVu 91.
The development of Mn toxicity was attributed to an increased formation of H2O2 in the apoplast (Horst et al., 1999). Due to the close relationship of POD and NADH-peroxidase activity and the formation of H2O2 in vivo, a H2O2-producing and -consuming POD system in the apoplast appears to be very important for the development and avoidance of Mn toxicity in the apoplast. However, a role of further H2O2-consuming enzymes in vivo such as catalase cannot be excluded and should be studied additionally in the future.
Effect of Mn Treatment on Phenol Concentration and Composition in the AWF and Its Role in Mn Toxicity and Tolerance
In relation to the presumably significant effect of phenolic compounds on the functionality of PODs, phenol concentration and phenol composition in the AWF of cowpea leaves were investigated. In the Mn-sensitive cowpea cv TVu 91, total phenol concentrations of the leaf tissue (water and NaOH extracts; Maier, 1997) and the AWF increased with increasing severity of Mn toxicity caused by higher Mn concentration in the leaf tissue (Fig. 9A) and visualized by the enhanced formation of brown spots (Fig. 8). This is also reflected by higher and additional peaks in the HPLC chromatogram of the AWF phenols of Mn-treated cv TVu 91 (Fig. 10). In the Mn-tolerant cowpea cv TVu 1987 total phenol concentrations in the AWF did not increase with increasing Mn leaf-tissue contents (Figs. 9A and 10). At Mn tissue concentrations leading to mild Mn toxicity [0–20 (cm2)−1], in both cultivars no relationship could be found between total AWF phenol concentrations and leaf Mn contents (Fig. 9B). Therefore, in cowpea a positive correlation between Mn tolerance and the quantity of total phenols in the leaf tissue and the AWF could not be found. Such a correlation was proposed by Aoba (1986) to explain differences in Mn tolerance of several tree species. Detoxification of other metals through sequestration by phenolic compounds has been suggested (Heim et al., 2001; Kidd et al., 2001; Lavid et al., 2001). Our results rather suggest that the release of phenolic compounds into the apoplast reflects an advanced stress response to excess Mn rather than a tolerance mechanism. The stimulating effect of Mn on Phe ammonia lyase activity (Engelsma, 1972; Burnell, 1988) and on phenol contents in the plant tissue (Langheinrich et al., 1992) support the hypothesis that Mn excess induces the release of phenolic compounds into the leaf apoplast (Wissemeier and Horst, 1992).
Not the quantity of total phenols but rather the composition of the phenols in the leaf apoplast appears to be important for the difference in Mn tolerance of the cowpea cv TVu 91 and cv TVu 1987. Such qualitative differences are indicated by the HPLC chromatograms shown in Figure 10A. Moreover, the cross combining of AWF proteins with AWF filtrates (Figs. 6 and 7) suggests that the metabolome of the apoplast is equally or even more important for the expression of Mn tolerance than the proteome. Therefore, an in-depth analysis of the metabolites present in the AWF is necessary. As already mentioned and shown above, particularly phenolic compounds affect the functionality of PODs. Genotypic differences in phenol compositions of the AWF might be important for the formation of H2O2 and, therefore, the oxidation of MnII and phenolic compounds (see discussion above).
We conclude from our results that PODs in the leaf AWF of cowpea are capable of producing and consuming H2O2 in the apoplast. For the H2O2-producing NADH oxidase cycle, the interaction between PODs and phenolic compounds in the leaf apoplast is crucial for the development and avoidance of Mn toxicity. The further characterization of the phenol and protein composition of the apoplast is expected to give in-depth insights into the regulation of H2O2 production and consumption in the leaf apoplast and its importance for the expression of Mn toxicity and Mn tolerance in the leaves of cowpea.
MATERIALS AND METHODS
Plant Material and Cultivation
Plants of cowpea (Vigna unguiculata L. Walp) cv TVu 91 and cv TVu 1987 differing in Mn leaf-tissue tolerance (Horst et al., 1999) were grown hydroponically in a growth chamber under controlled environmental conditions at 30°C/25°C day/night temperature, 75% ± 5% relative humidity, and a photon flux density of 270 μmol m−1 s−1 photosynthetic active radiation at midplant height during a 16-h photoperiod. After germination in 1 mm CaSO4, one seedling per cultivar was transferred to a constantly aerated nutrient solution in a 5-L plastic pot. The concentration of the basic nutrient solution was (in micrograms): Ca(NO3)2 1,000, K2SO4 375, KH2PO4 100, MgSO4 325, FeEDDHA 20, NaCl 10, H3BO3 8, MnSO4 0.2, CuSO4 0.2, ZnSO4 0.2, and Na2MoO4 0.05.
After preculture for at least 14 d, the MnSO4 concentration in the nutrient solution was increased to 50 or 100 μm Mn for 1 to 6 d. Control plants received 0.2 μm Mn continuously. Plants were treated until distinct visible symptoms were detectable on leaves of the sensitive cultivar. All plants were harvested at the same day.
The nutrient solution was changed two to three times a week to provide optimum mineral nutrition.
Quantification of Mn Toxicity Symptoms
For the quantification of Mn toxicity symptoms, leaf discs (1.54 cm2) were cut out at the base, middle, and tip of the trifoliate leaf and incubated in ethanol for at least 3 d. Numbers of brown spots per leaf disc were counted. Density of brown spots per square centimeter was calculated.
Extraction of AWF
AWF was extracted by a vacuum infiltration/centrifugation technique. Therefore, leaflets of second trifoliate leaves were cut from plants, weighted, and infiltrated with demineralized water. The pressure was reduced to 35 hPa (1 min) by using a water jet pump followed by slow relaxation for 2 min. Leaflets were removed from the water and dry blotted. Infiltrated leaflets were weighted again and AWF was recovered by centrifugation at 1,324g for 5 min at room temperature. There were no differences in the yield of AWF between cultivars and Mn treatments. According to the activity of malate dehydogenase in the AWF, the contamination of the AWF by cytosolic proteins was less than 1%.
Determination of Protein Concentration in the AWF
Measurements were carried out according to Bradford (1976).
Protein Precipitation and 2-D Isoelectric Focusing/SDS-PAGE
For the extraction of proteins from the AWF, the AWF of the second-oldest trifoliate leaves of all plants in each variant were pooled and equal volumes were used for the precipitation of the proteins by phenol and acetate/methanol (Fecht-Christoffers et al., 2003a).
For separation of proteins by their pI, the IPGphor system (Amersham Pharmacia Biotech) was used. Proteins were resuspended in demineralized water and supplemented with a rehydration solution (8 m urea, 2% [w/v] CHAPS, 0.5% [v/v] carrier ampholyte mixture [IPG buffer; Amersham Pharmacia], 0.28% [w/v] dithiothreitol, and a trace of bromphenol blue). Focusing was carried out using Immobiline DryStrip gels (18 cm) with a nonlinear pH gradient (pH 3–10) under conditions outlined by Werhahn and Braun (2002). Afterward, the Immobiline DryStrip gels were incubated with equilibration solution (50 mm Tris-Cl [pH 8.8], 6 m urea, 30% [v/v] glycerin, 2% [w/v] SDS, and bromphenol blue) supplemented with 1% (w/v) dithiothreitol and 2.5% (w/v) iodoacetamide each for 15 min, respectively. DryStrip gels were placed horizontally on a Tricine SDS-PAGE. The second-dimension electrophoresis was carried out according to Schägger and von Jagow (1987).
Staining
The 2-D gels were stained with colloidal Coomassie Blue (Neuhoff et al., 1985, 1990). Gels were fixed in 40% (v/v) MeOH/10% (v/v) HAc for at least 2 h and incubated in 80% (v/v) staining solution and 20% (v/v) MeOH for at least 3 h. The staining solution contained 98% (v/v) solution A (2% [w/v] phosphoric acid and 10% [w/v] ammonium sulfate) and 2% (v/v) solution B (5% [w/v] Coomassie Blue).
Determination of Guaiacol-POD Activity
For the measurement of H2O2-consuming POD activity in the AWF, the oxidation of the artificial substrate guaiacol was determined spectrophotometrically at λ = 470 nm (UVIKON 943, BioTek Instruments GmbH). Samples were mixed with guaiacol solution (20 mm guaiacol in 10 mm Na2HPO4 buffer [pH 6]) and 0.03% (w/w) H2O2. For calculation of enzyme activities the molar extinction coefficient 26.6 mm−1 cm−1 was used.
Determination of NADH Peroxidase
For the measurement of NADH-peroxidase activity in the AWF, AWF samples were combined with 0.3 mm NADH, 1.6 mm p-coumaric acid, and 16 mm MnCl2. All components were dissolved in 100 mm sodium acetate buffer, pH 5. The activity was measured at λ = 340 nm using a microplate reader (μQuant, BioTek Instruments GmbH). For calculation of enzyme activities, the extinction coefficient of 4.23 mm−1 was used.
Determination of the H2O2 Formation by Apoplastic NADH Peroxidase (in Vitro)
The detection of H2O2 was based on the peroxidase-catalyzed oxidation of guaiacol. AWF was used for the measurement of NADH-peroxidase activity (as described above). After at least 1 min detection of NADH oxidation (measurements were done in the presence of 16 mm MnCl2, 1.6 mm p-coumaric acid, and 0.3 mm NADH), 3 mm guaiacol (in 10 mm sodium phosphate buffer, pH 6) were added to the enzyme assay. Changes in absorption were monitored at λ = 470 nm for 2 min. For the calculation of the H2O2 formation rate, the extinction coefficient 0.00378 μm−1 was used.
The Effect of Varied Mn and p-Coumaric Acid Concentrations on NADH-Peroxidase Activity and Related H2O2 Formation (in Vitro)
NADH peroxidase and H2O2 formation were determined as described above. However, the concentration of added p-coumaric acid was varied from 0 to 1.6 mm and of MnCl2 from 0 to 160 mm. Extinction coefficients were calculated at each p-coumaric acid concentration: for 0, 0.016, 0.16, and 1.6 mm p-coumaric acid ɛ = 4.94, 4.68, 4.60, and 4.23 mm−1, respectively.
Separation of Proteins from the AWF and Measurement of the Effect of AWF Filtrate on NADH-Peroxidase Activity
To verify the effect of apoplastic water-soluble nonenzyme compounds of the apoplast on the activity of NADH peroxidase, proteins of the AWF were separated from the AWF by using mini centrifuge filter with a molecular weight cutoff (MWCO) at 4 kD (Nalgene Nunc International). Sample volumes of 500 μL were centrifuged at 6,000g, 4°C, for 1 to 2 h. AWF filtrates were removed and protein concentrates were washed with double demineralized water at 4°C. Purified proteins were recovered by centrifugation at 3,000g, 4°C, and 10 min.
Just before detection of enzyme activities, proteins and AWF filtrate of controls and Mn-treated plants from TVu 91 and TVu 1987 were combined. For measurement of enzyme activities, 1 μL of protein concentrate and 50 μL AWF filtrate or double demineralized water were mixed with 16 mm MnCl2, 1.6 mm p-coumaric acid, and 0.3 mm NADH.
Measurement of Total Phenol Concentration in the AWF and Separation of Phenols by HPLC
AWF (50 μL) was combined with 350 μL double demineralized water and 50 μL Folin-Denis solution (Merck). After 3 min incubation, 100 μL saturated Na2CO3 solution was added. After 1 h incubation and centrifugation (1 min, 5,000g) absorption was measured at λ = 725 nm in a microplate reader (μQuant, BioTek Instruments GmbH). Concentrations were calculated in relation to p-coumaric acid standard solutions.
For the determination of the phenol composition in the AWF, phenols were extracted from the AWF. AWF (3 mL) was hydrolyzed in the presence of 150 μL HCL (8 m) and 200 μL acetonitril (ACN). After 1 h incubation at 80°C, 50 μL of saturated NaHCO3 was added. Samples were loaded on Bankerbond spe octadecyl C18 columns (Mallinckrodt Baker), which were previously equilibrated two times with 1 mL methanol and 500 μL double demineralized water. After loading, columns were washed with double demineralized water and samples were eluted with 500 μL 80% ACN/20% H3PO4 (0.1% [w/v]). HPLC analysis was carried out with a HPLC-DAD system (BioTek Instruments GmbH); precolumn: Phenomenex AJ0-4286 C18; column: Phenomenex Luna 5μ C18(2) (Phenomenex). The mobile phase used was: (A) ACN, and (B) H3PO4 (0.1% [w/v]). The solvent gradient changed as follows: (1) time 0 to 9 min, 0% A/100% B, flow 1.5 mL min−1; (2) time 9 to 22 min, 15% A/85% B, flow 1.5 mL min−1; (3) time 22 to 25 min, 30% A/70% B, flow 1.5 mL min−1; (4) time 25 to 27 min, 100% A/0% B, flow 1.5 mL min−1; and (5) time 27 to 36 min, 0% A/100% B, 1.5 mL min−1. Absorptions were measured at λ = 230, 266, 280, and 320 nm. Calibrations were carried out with standard solutions of p-hydroxybenzoic acid, p-coumaric acid, o-coumaric acid, chlorogenic acid, ferulic acid, synapinic acid, p-hydroxybenzaldehyde, catechin, salicylhydroxamic acid, salicylic acid, benzoic acid, vanillic acid, syringic acid, caffeic acid, syringaldehyde, protocatechenic acid, tannic acid, gentisic acid, and gallic acid (Sigma-Aldrich Chemie GmbH). Results show the chromatograms of three biological repetitions and three measurements combined using the Kontron system 3000 software (Kontron Instruments).
Mineral Analysis
For the analysis of Mn in the bulk-leaf tissue, center ribs were cut out of the middle leaflets of trifoliate leaves and 0.5 to 1 g leaf tissue was dried at 65°C. Drying was followed by dry ashing (480°C, 8 h) and dissolving the ash in 6 n HCl with 1.5% (w/v) hydroxylammonium chloride and dilution (1:10) with double demineralized water. AWF was diluted 1:10, and HCl and hydroxylammonium chloride were added to give final concentration of 0.6 n HCl and 0.15% (w/v) hydroxylammoniumchloride. Measurements were carried out by optical emission spectrometry, inductive coupled plasma (Spektro Flame, Spectro.
Statistical Analysis
Means and sds were from independent replications within an experiment and calculated by Excel 2000 (Microsoft). The numbers of replications are noted in the figure legends. Regression analysis, multiple analysis of variance (F test), and comparisons of means (Tukey test) were carried out by SAS 8e (SAS Institute). Levels of significance are indicated in graphs by *, **, and *** for P < 0.05, 0.01, and 0.001. Means with different letters are significantly different at P < 0.05 (Tukey).
Supplementary Material
This work was supported by the Deutsche Forschungsgemeinschaft (Special Research Programme 717, “The apoplast of higher plants” and Ho 931/17–1).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Walter Johannes Horst (horst@pflern.uni-hannover.de).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.070474.
References
- Aoba K (1986) Excess manganese disorder in fruit trees. Jpn Agric Res Q 20: 38–47 [Google Scholar]
- Archibald FS, Fridovich I (1982. a) The scavenging of superoxide radicals by manganous complexes: in vitro. Arch Biochem Biophys 214: 452–463 [DOI] [PubMed] [Google Scholar]
- Archibald FS, Fridovich I (1982. b) Investigations of the state of manganese in Lactobacillus plantarum. Arch Biochem Biophys 215: 589–596 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DI, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23: 517–532 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Burnell JN (1988) The biochemistry of manganese in plants. In RD Graham, RJ Hannam, NC Uren, eds, Manganese in Soils and Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 125–137
- Campa A (1991) Biological roles of plant peroxidases: known and potential functions. In J Evers, K Evers, MB Grisham, eds, Peroxidase in Chemistry and Biology Vol 2. CRS Press, Boca Raton, FL, pp 26–49
- Castillo FJ (1986) Extracellular peroxidases as markers of stress? In H Greppin, C Penel, T Gaspar, eds, Molecular and Physiological Aspects of Plant Peroxidases. University of Geneva, Geneva, pp 419–426
- Christensen JH, Bauw G, Welinder KG, van Montagu M, Boerjan W (1998) Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiol 118: 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza IRP, MacAdam JW (1998) A transient increase in apoplastic peroxidase activity precedes decrease in elongation rate of B73 maize (Zea mays) leaf blades. Physiol Plant 104: 556–562 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR (2003) Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 15: 1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner EF, Heupel A (1976) Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 130: 175–180 [DOI] [PubMed] [Google Scholar]
- Engelsma G (1972) A possible role of divalent manganese ions in the photoinduction of phenylalanine ammonialyase. Plant Physiol 50: 599–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecht-Christoffers MM, Braun H-P, Lemaitre-Guillier C, VanDorsselaer A, Horst WJ (2003. a) Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiol 133: 1935–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecht-Christoffers MM, Horst WJ (2005) Does apoplastic ascorbic acid enhance manganese tolerance of Vigna unguiculata and Phaseolus vulgaris? J Plant Nutr Soil Sci 168: 590–599 [Google Scholar]
- Fecht-Christoffers MM, Maier P, Horst WJ (2003. b) Apoplastic peroxidase and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiol Plant 117: 237–244 [Google Scholar]
- Foy CD (1984) Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soils. In F Adams, ed, Soil Acidity and Liming, Agron Monograph 12. ASA-CSSSA, Madison, WI, pp 57–97
- Gaspar T, Penel C, Castillo FJ, Greppin H (1985) A two-step control of basic and acidic peroxidases and its significance for growth and development. Physiol Plant 64: 418–423 [Google Scholar]
- Gelinas D, Postlethwait SN (1969) IAA oxidase inhibitors from normal and mutant maize plants. Plant Physiol 44: 1553–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Janse C, Elstner EF (1977) Involvement of malate, monophenols, and the superoxide radical in hydrogen peroxide formation by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 136: 271–276 [DOI] [PubMed] [Google Scholar]
- Halliwell B (1978) Lignin synthesis: the generation of hydrogen peroxide and superoxide by horseradish peroxidase and its stimulation by manganese(II) and phenols. Planta 140: 81–88 [DOI] [PubMed] [Google Scholar]
- Heim A, Brunner I, Frey B, Fossard E, Luster J (2001) Root exudation, organic acids, and element distribution in roots of Norway spruce seedlings treated with aluminum in hydrophonics. J Plant Nutr Soil Sci 164: 519–526 [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42: 462–468 [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of arabidopsis CAX2 in tobacco: altered metal accumulation and increased manganese tolerance. Plant Physiol 124: 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ (1988) The physiology of Mn toxicity. In RD Graham, RJ Hannam, NC Uren, eds, Manganese in Soils and Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 175–188
- Horst WJ, Fecht M, Naumann A, Wissemeier AH, Maier P (1999) Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) Walp. J Plant Nutr Soil Sci 162: 263–274 [Google Scholar]
- Horst WJ, Maier P (1999). Compartmentation of manganese in the vacuoles and in the apoplast of leaves in relation to genotypic manganese leaf-tissue tolerance in Vigna unguiculata (L.) Walp. In G Gissel-Nielsen, A Jensen, eds, Plant Nutrition—Molecular Biology and Genetics. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 223–234
- Kärkönen A, Koutaniemi S, Mustonen M, Syrjänen K, Brunow G, Kilpeläinen I, Teeri TH, Simola LK (2002) Lignification related enzymes in Picea abies suspension cultures. Physiol Plant 114: 343–353 [DOI] [PubMed] [Google Scholar]
- Kawano T, Kawano N, Muto S, Lapeyrie F (2002) Retardation and inhibition of the cation-induced superoxide generation in BY-2 tobacco cell suspension culture by Zn2+ and Mn2+. Physiol Plant 114: 395–404 [DOI] [PubMed] [Google Scholar]
- Kenten RH, Mann PJG (1950) The oxidation of manganese by peroxidase systems. Biochem J 46: 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenten RH, Mann PJG (1956) Manganese oxidation in the pea plant (Pisum sativum L.) grown under conditions of manganese toxicity. Biochem J 65: 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PS, Llungany M, Poschenrieder C, Gunsé B, Barceló J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52: 1339–1352 [PubMed] [Google Scholar]
- Klotz KL, Liu T-TY, Liu L, Lagrimini LM (1998) Expression of the tobacco anionic peroxidase gene is tissue-specific and developmentally regulated. Plant Mol Biol 36: 509–520 [DOI] [PubMed] [Google Scholar]
- Lagrimini LM (1991) Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol 96: 577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM, Vaughn J, Erb A, Miller SA (1993) Peroxidase overproduction in tomato: wound-induced polyphenol deposition and disease resistance. Hortic Sci 28: 218–221 [Google Scholar]
- Langheinrich U, Tischner R, Goldbold DL (1992) Influence of high Mn supply on Norway spruce (Picea abies (L.) Karst.) seedlings in relation to the nitrogen source. Tree Physiol 10: 259–271 [DOI] [PubMed] [Google Scholar]
- Lavid N, Schwartz A, Yarden O, Tel-Or E (2001) The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 212: 323–331 [DOI] [PubMed] [Google Scholar]
- Lee TT (1977) Role of phenolic inhibitors in peroxidase-mediated degradation of indole-3-acetic acid. Plant Physiol 59: 372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAdam JW, Sharp RE, Nelson CJ (1992) Peroxidase activity in the leaf elongation zone of tall fescue. II. Spatial distribution of apoplastic peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99: 879–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder M (1992) Compartmentation of peroxidase isoenzymes in plant cells. In C Penel, T Gaspar, H Greppin, eds, Plant Peroxidases 1980–1990, Topics and Detailed Literature on Molecular, Biochemical, and Physiological Aspects. University of Geneva, Geneva, pp 37–46
- Mäder M, Amberg-Fisher V (1982) Role of peroxidase in lignification of tobacco cells. I. Oxidation of nicotinamide adenine dinucleotide and formation of hydrogen peroxide by cell wall peroxidase. Plant Physiol 70: 1128–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder M, Füssl R (1982) Role of peroxidase in lignification of tobacco cells. II. Regulation by phenolic compounds. Plant Physiol 70: 1132–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder M, Nessel A, Bopp M (1977) Über die physiologische bedeutung der peroxidase-isoenzymgruppen des Tabaks anhand einiger biochemischer Eigenschaften. II. pH-Optima, Michaelis-Konstanten, maximale oxidationsraten. Z Pflanzenphysiol 82: 247–260 [Google Scholar]
- Mäder M, Nessel A, Schloss P (1986) Cell compartmentation and specific roles of isoenzymes. In H Greppin, C Penel, T Gaspar, eds, Molecular and Physiological Aspects of Plant Peroxidases. University of Geneva, Geneva, pp 247–260
- Mäder M, Ungemach J, Schloß P (1980) The role of peroxidase isoenzyme groups of Nicotiana tabacum in hydrogen peroxide formation. Planta 147: 467–470 [DOI] [PubMed] [Google Scholar]
- Maier P (1997) Bedeutung der Kompartimentierung von Mangan und organischen Säuren für die Mangantoleranz von Cowpea (Vigna unguiculata (L.) Walp). PhD thesis. University of Hannover, Hannover, Germany
- Neuhoff V, Stamm R, Eibl H (1985) Clear background and highly sensitive protein staining with coomassie blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis 6: 427–448 [Google Scholar]
- Neuhoff V, Stamm R, Pardowitz I, Arold N, Ehrhardt W, Taube D (1990) Essential problems in quantification of proteins following colloidal staining with coomassie brilliant blue dyes in polyacrylamide gels, and their solution. Electrophoresis 11: 101–117 [DOI] [PubMed] [Google Scholar]
- Pedreño MA, Ferrer MA, Gaspar Th, Munoz R, Ros Barcelo A (1995) The polyfunctionality of cell wall peroxidases avoids the necessity of an independent H2O2-generating system for phenolic coupling in the cell wall. Plant Perox Newslett 5. http://www.unige.ch/LABPV/perox.html (February 5, 1995)
- Pedreño MA, Sabater F, Muñoz R, Garcia-Carmona F (1987) Effect of different phenols on the NADH-oxidation catalysed by a peroxidase from lupin. Phytochemistry 26: 3133–3136 [Google Scholar]
- Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Picea avies L.). Plant Physiol 106: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros Barcelo A (1997) Lignification in plant cell walls. Int Rev Cytol 176: 87–132 [PubMed] [Google Scholar]
- Sato Y, Sugiyama M, Komamine A, Fukuda H (1995) Separation and characterization of the isoenzymes of wall-bound peroxidase from cultured Zinna cells during tracheary element differentiation. Planta 196: 141–147 [Google Scholar]
- Schaaf G, Catoni E, Fitz M, Schwacke R, von Wirén N, Frommer WB (2002) A putative role for the vacuolar calcium/manganese proton antiporter AtCAX2 in heavy metal detoxification. Plant Biol 4: 612–618 [Google Scholar]
- Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379 [DOI] [PubMed] [Google Scholar]
- Schlichting E, Sparrow LA (1988) Distribution and amelioration of manganese toxic soils. In RD Graham, RJ Hannam, NC Uren, eds, Manganese in Soils and Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 277–292
- Sirkar S, Amin JV (1974) The manganese toxicity of cotton. Plant Physiol 54: 539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich K, Ebermann R (1984) Investigation of hydrogen peroxide formation in plants. Phytochemistry 23: 2719–2722 [Google Scholar]
- Werhahn W, Braun HP (2002) Biochemical dissection of the mitochondrial proteome from Arabidopsis thaliana by three-dimensional gel electrophoresis. Electrophoresis 23: 640–646 [DOI] [PubMed] [Google Scholar]
- Wissemeier AH, Diening A, Hergenröder A, Horst WJ, Mix-Wagner G (1992) Callose formation as parameter for assessing genotypical plant tolerance of aluminium and manganese. Plant Soil 146: 67–75 [Google Scholar]
- Wissemeier AH, Horst WJ (1991) Simplified methods for screening cowpea cultivars for manganese leaf-tissue tolerance. Crop Sci 31: 435–439 [Google Scholar]
- Wissemeier AH, Horst WJ (1992) Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 143: 299–309 [Google Scholar]
- Wu Z, Liang F, Hong B, Young J, Sussmann MR, Harper JF, Sze H (2002) An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol 130: 128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki I, Piette LH (1963) The mechanism of aerobic oxidase reaction catalysed by peroxidase. Biochim Biophys Acta 77: 47–64 [DOI] [PubMed] [Google Scholar]
- Yamazaki I, Yokota K (1973) Oxidation states of peroxidase. Mol Cell Biochem 15: 39–52 [DOI] [PubMed] [Google Scholar]
- Yokota K, Yamazaki I (1977) Analysis and computer simulation of aerobic oxidation of reduced nicotinamide adenine dinucleotide catalysed by horseradish peroxidase. Biochemistry 16: 1913–1920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.