Abstract
Phytochelatin synthases (PCS) catalyze phytochelatin (PC) synthesis from glutathione (GSH) in the presence of certain metals. The resulting PC-metal complexes are transported into the vacuole, avoiding toxic effects on metabolism. Legumes have the unique capacity to partially or completely replace GSH by homoglutathione (hGSH) and PCs by homophytochelatins (hPCs). However, the synthesis of hPCs has received little attention. A search for PCS genes in the model legume Lotus (Lotus japonicus) resulted in the isolation of a cDNA clone encoding a protein (LjPCS1) highly homologous to a previously reported homophytochelatin synthase (hPCS) of Glycine max (GmhPCS1). Recombinant LjPCS1 and Arabidopsis (Arabidopsis thaliana) PCS1 (AtPCS1) were affinity purified and their polyhistidine-tags removed. AtPCS1 catalyzed hPC synthesis from hGSH alone at even higher rates than did LjPCS1, indicating that GmhPCS1 is not a genuine hPCS and that a low ratio of hPC to PC synthesis is an inherent feature of PCS1 enzymes. For both enzymes, hGSH is a good acceptor, but a poor donor, of γ-glutamylcysteine units. Purified AtPCS1 and LjPCS1 were activated (in decreasing order) by Cd2+, Zn2+, Cu2+, and Fe3+, but not by Co2+ or Ni2+, in the presence of 5 mm GSH and 50 μm metal ions. Activation of both enzymes by Fe3+ was proven by the complete inhibition of PC synthesis by the iron-specific chelator desferrioxamine. Plants of Arabidopsis and Lotus accumulated (h)PCs only in response to a large excess of Cu2+ and Zn2+, but to a much lower extent than did with Cd2+, indicating that (h)PC synthesis does not significantly contribute in vivo to copper, zinc, and iron detoxification.
Phytochelatins [PCs; (γGlu-Cys)2–11Gly] are Cys-rich polypeptides that play an essential role in the detoxification of some heavy metals in yeasts, plants, and other organisms (Grill et al., 1987; Cobbett and Goldsbrough, 2002). The synthesis of PCs from glutathione (GSH; γGlu-Cys-Gly) is catalyzed by phytochelatin synthase (PCS) and involves a transpeptidation reaction in which γGlu-Cys units of GSH are added to another GSH molecule or to an elongating PC polypeptide:
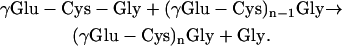 |
This reaction is strictly dependent on the presence of metals, but the mechanism of enzyme activation is a subject of debate (Grill et al., 1989; Vatamaniuk et al., 2000; Oven et al., 2002).
Legumes are unique among higher plants due to their ability to synthesize homoglutathione (hGSH; γGlu-Cys-βAla) and homophytochelatins [hPCs; (γGlu-Cys)2–11βAla] in addition to, or instead of, GSH and PCs, respectively. The thiol tripeptide hGSH is synthesized by a specific hGSH synthetase (Frendo et al., 2001; Matamoros et al., 2003), but the existence of homophytochelatin synthases (hPCS) in legumes is far from clear. It is well known that hPCs are formed by legume plants or cell suspension cultures when challenged with several metals and metaloids (Grill et al., 1986; Klapheck et al., 1995). However, only one report has examined the substrate specificity of the responsible enzyme (Oven et al., 2002). These authors concluded that the PCS1 of Glycine max is an hPCS (GmhPCS1), based on the finding that this enzyme, but not the PCS1 of Arabidopsis (Arabidopsis thaliana; AtPCS1), is able to catalyze hPC synthesis from hGSH as the sole substrate.
Another consideration of physiological interest is to ascertain what metals are able to activate PCS because there have been a number of contradictory results. A few studies have examined the effects of a broad range of metals on recombinant PCS enzymes either in purified form or in soluble crude extracts of the expression vector. Taking into account only those metals established as nutrients in plants, purified tagged AtPCS1 was found to catalyze PC synthesis in the presence of Cu2+, Zn2+, Mg2+, Ni2+, or Co2+ (Vatamaniuk et al., 2000). In sharp contrast, purified untagged AtPCS1 and GmhPCS1 did not show any activity with Mg2+, Ni2+, or Co2+ (Oven et al., 2002). Also, soluble extracts of Escherichia coli expressing AtPCS1 produced PCs when challenged with Cu2+ or Zn2+ (Ha et al., 1999). The same enzyme expressed in the yeast Schizosaccharomyces pombe had no activity with Zn2+ or Ni2+, but was activated by both metal ions when expressed in Saccharomyces cerevisiae (Cazalé and Clemens, 2001).
As part of our studies on the response of the model legume Lotus (Lotus japonicus) to heavy metals, we have isolated a full-length cDNA clone (accession no. AY633847) encoding a protein (LjPCS1) with high homology to PCS enzymes. Recombinant LjPCS1 was affinity purified and the untagged protein was compared with the previously described AtPCS1 (Vatamaniuk et al., 2000; Oven et al., 2002) in terms of substrate and metal specificities. Complementary experiments were performed to detect PCS activation in vivo by quantification of (h)PCs in Arabidopsis and Lotus plants treated with a metal excess. Our goal was to answer three questions of physiological relevance. First, is the PCS1 from legumes a genuine hPCS? Second, what metals known to be essential for plants are also able to activate PCS in vitro at low concentrations? Third, is there a correlation between the capacities of metals to activate PCS in vitro and to elicit PC synthesis in vivo? Using highly purified enzymes and strict controls with specific metal chelators, we provide conclusive answers to those questions, which, in some cases, are at odds with published results using identical biological material. Specifically, we demonstrate that LjPCS1 (and AtPCS1) catalyzes the synthesis of hPCs from hGSH alone but is not an authentic hPCS, and that both enzymes are activated, in decreasing order, by zinc (Zn), copper (Cu), and iron (Fe).
RESULTS
Sequence and Phylogenetic Analyses of LjPCS1
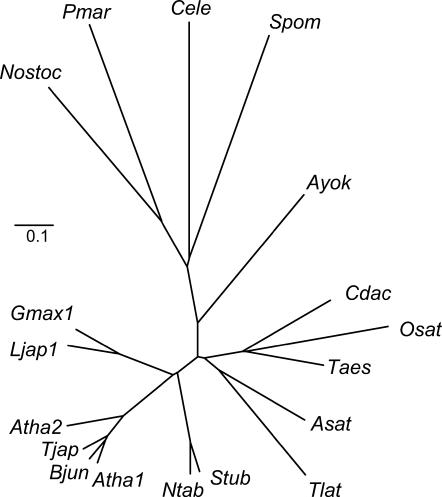
Lotus has been proposed as a model legume for genetic and molecular analyses because it is an autogamous plant with a small diploid genome, has a short generation time with a high seed yield, and is amenable for transformation (Handberg and Stougaard, 1992). Additional advantages of Lotus with respect to common crop legumes are that there is a large collection of mutagenized and tagged lines, its chloroplast genome has been completely sequenced, and its nuclear genome is being sequenced at a fast pace. A preliminary search in the databases of Lotus for expressed sequence tags encoding a putative PCS was unsuccessful, suggesting a low abundance of PCS transcripts. Therefore, gene-specific primers were designed in the framework of the Lotus genome sequencing project to screen cDNAs produced from total RNA of roots. As a result, a full-length cDNA clone encoding a protein with high homology to known plant PCS enzymes was isolated and characterized. The open reading frame is 1,506 bp long and encodes a protein (LjPCS1) with a predicted molecular mass of 55.5 kD, 501 amino acid residues, and a pI of 6.68. The nucleotide and amino acid sequences share 65% to 69% identity with AtPCS1 and 84% to 86% identity with GmhPCS1. The N-terminal (conserved) domain of LjPCS1 contains three amino acid residues, Cys-56, His-162, and Asp-180 (catalytic triad), that are essential for function (Vatamaniuk et al., 2004; Vivares et al., 2005; Rea, 2006). The protein is very rich in Cys residues (4.2%) and its C-terminal (variable) domain has a Cys2-X3-Cys-X2-Cys motif that is present in the PCS from most higher plants (Cobbett and Goldsbrough, 2002). An unrooted phylogenetic tree was constructed including the majority of the predicted PCS sequences available in the databases (Fig. 1). The tree revealed separate clusters, paralleling taxonomic distance, for the PCS of cyanobacteria, nematodes, yeasts, ferns, and the families of vascular plants Poaceae, Alliaceae-Typhaceae, Solanaceae, Brassicaceae, and Leguminosae. Sequence and phylogenetic analyses of LjPCS1 and GmhPCS1 also indicated that, most probably, the two proteins are functional homologs. To gain insight into the function of LjPCS1, the enzyme was heterologously expressed and purified to near homogeneity, and its properties were compared with those of an identically produced AtPCS1.
Figure 1.
Phylogenetic analysis of PCS proteins from cyanobacteria, nematodes, fungi, and plants. The unrooted tree was constructed using the neighbor-joining method (ClustalW) with 1,000 bootstrap replicates. Branch lengths are proportional to genetic distance, which is indicated by a bar (0.1 substitutions per site). GenBank accession numbers are as follows (in parentheses): Asat (Allium sativum, AAO13809), Atha1 (AtPCS1, AAD41794), Atha2 (AtPCS2, AAK94671), Ayok (Athyrium yokoscense, BAB64932), Bjun (Brassica juncea, CAC37692), Cdac (Cynodon dactylon, AAO13810), Cele (Caenorhabditis elegans, NP496475), Gmax (GmhPCS1, AAL78384), Ljap1 (LjPCS1, AY633847), Ntab (Nicotiana tabacum, AAO74500), Nostoc (Nostoc, NP485018), Osat (Oryza sativa, AAO13349), Pmar (Prochlorococcus marinus, NP894844), Taes (Triticum aestivum, AAD50592), Tjap (Thlaspi japonicum, BAB93119), Tlat (Typha latifolia, AAG22095), Spom (S. pombe, CAA92263), and Stub (Solanum tuberosum, CAD68109).
Purification of Recombinant Proteins
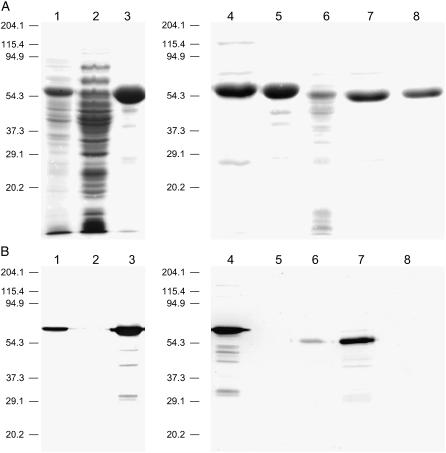
An essential part of this work was to produce large amounts of highly purified and active enzymes for biochemical analysis. An identical expression vector was used to produce recombinant LjPCS1 and AtPCS1 containing an N-terminal (His)6-tag. Optimal conditions for protein purification and tag removal were critical because some inconsistencies found on the metal activation of recombinant AtPCS1 might be due to the use of differently tagged (Vatamaniuk et al., 2000; Beck et al., 2003) or untagged (Oven et al., 2002) proteins. Purification of AtPCS1 and LjPCS1 by immobilized metal affinity chromatography was monitored by SDS-PAGE (Fig. 2A) and immunoblots (Fig. 2B).
Figure 2.
Purification of heterologously expressed PCS enzymes from model plants by immobilized metal affinity chromatography. A, SDS-PAGE analysis of fractions obtained during purification of AtPCS1 and LjPCS1. Gels were stained conventionally with Coomassie Brilliant Blue R-250. Lanes: 1, desalted crude extract of recombinant AtPCS1; 2, proteins eluted with 50 mm imidazole; 3, AtPCS1 eluted with 250 mm imidazole; 4, AtPCS1 preparation used to obtain 5, prior to thrombin digestion; 5, AtPCS1 after tag removal with thrombin; 6, desalted crude extract of recombinant LjPCS1; 7, LjPCS1 eluted with 250 mm imidazole; and 8, LjPCS1 after tag removal with thrombin. Lanes were loaded with 20 μg of protein. B, Immunoblot analysis of fractions shown in Figure 2A, using anti-polyHis monoclonal antibody and an alkaline phosphatase-based detection system (Sigma). Lanes were loaded with 2 μg of protein.
Both analyses also confirmed the complete removal of the tag and the absence of significant protein degradation during overnight incubation with thrombin. Protein purity was at least 96% as assessed by densitometric analysis. Typical specific activities for both the untagged and tagged preparations of AtPCS1 and LjPCS1, using 5 mm GSH as the substrate and 50 μm Cd2+ as the activating metal ion, were in the range of 80 to 100 nmol of total PCs produced per minute per milligram of protein. For calculations of enzyme activity, only the sum of the three major polypeptides (PC2–4), or of their homologs (hPC2–4), was considered.
Substrate Specificity of PCS Enzymes
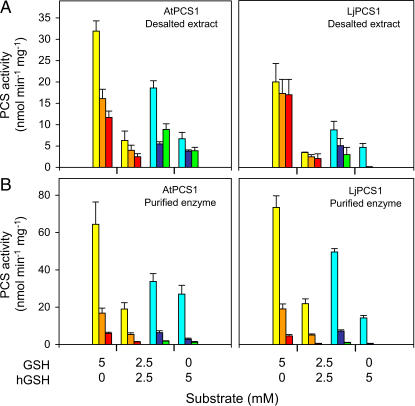
To determine the specificity of AtPCS1 and LjPCS1 for GSH and hGSH, preliminary experiments were conducted using desalted crude enzyme preparations, 500 μm Cd2+, and varying concentrations of the thiol tripeptides (Fig. 3A). This study was important because Oven et al. (2002) had reported that AtPCS1 was completely inactive in catalyzing hPC synthesis from hGSH alone, whereas GmhPCS1 was able to do it, albeit at low rates. In the presence of 5 mm GSH and 500 μm Cd2+, AtPCS1 and LjPCS1 produced approximately 60 nmol of total PCs per minute per milligram of protein. As expected, AtPCS1 produced decreasing amounts of PC2, PC3, and PC4. During the assay period, many AtPCS1 preparations were also able to produce PC5 (49% of PC4), PC6 (9% of PC4), and, less frequently, even higher order polypeptides. However, LjPCS1 produced similar amounts of the three major polypeptides and, in a few cases, PC5 (17% of PC4).
Figure 3.
Substrate specificity of PCS enzymes from model plants. A, Desalted crude extracts of AtPCS1 (200 μg of protein) and LjPCS1 (120 μg of protein) were assayed (30 min) with 500 μm Cd2+ and the stated concentrations of (h)GSH. B, Purified AtPCS1 (60 μg of protein) and LjPCS1 (60 μg of protein) were assayed (30 min) with 50 μm Cd2+ and the stated concentrations of (h)GSH. The polypeptides PC2 (yellow), PC3 (orange), PC4 (red), hPC2 (light blue), hPC3 (dark blue), and hPC4 (green) were quantified by HPLC with postcolumn derivatization. Values are given in nmol of (h)PCs produced (GSH equivalents) per minute per milligram of protein and represent means ± se of four to six enzyme preparations.
Desalted preparations of AtPCS1 and LjPCS1 produced at least 2-fold more hPCs than PCs when a combination of 2.5 mm GSH and 2.5 mm hGSH was used as the substrate and 500 μm Cd2+ as the activating metal ion (Fig. 3A). As expected, AtPCS1 produced greater amounts of hPC2 than of hPC3 and hPC4, and occasionally longer polypeptides such as hPC5 (40% of hPC4) and hPC6 (5% of hPC4). LjPCS1 also produced decreasing amounts of the three major hPC polypeptides but negligible amounts of hPC5. Most interestingly, AtPCS1 and LjPCS1 were able to synthesize hPCs with hGSH as the only substrate (Fig. 3A). Furthermore, AtPCS1 synthesized 3-fold more hPCs than did LjPCS1. The amounts of total hPCs produced by AtPCS1 and LjPCS1 with 5 mm hGSH were, respectively, 24% and 9% of the amounts of total PCs formed with 5 mm GSH (Fig. 3A).
The results with desalted crude enzyme preparations were confirmed with the corresponding purified, untagged proteins (Fig. 3B). For these experiments, the concentration of Cd2+ was lowered to 50 μm, which was optimal for purified enzymes under our assay conditions. When a combination of 2.5 mm GSH and 2.5 mm hGSH was used as the substrate, AtPCS1 and LjPCS1 produced 2-fold more hPCs than PCs. When 5 mm hGSH was used as the sole substrate, AtPCS1 and LjPCS1 produced 31 and 15 nmol of total hPCs per minute per milligram of protein, respectively. The amounts of total hPCs formed with 5 mm hGSH relative to those of total PCs formed with 5 mm GSH were 36% for AtPCS1 and 15% for LjPCS1 (Fig. 3B).
Activation of PCS Enzymes by Physiologically Relevant Metals
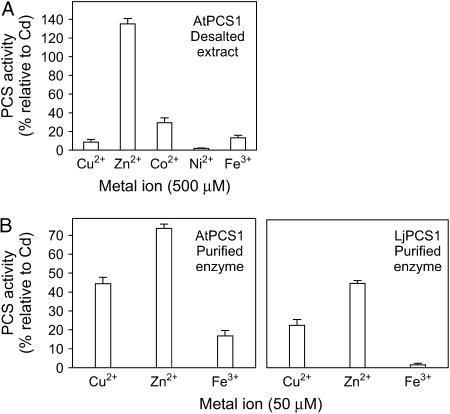
Metal activation of AtPCS1 was initially examined by assaying the activity of desalted soluble extracts with all metals known to be essential nutrients in plants (Fig. 4A). In all experiments, Cd2+ was included as a positive control to verify the quality of the enzyme preparations and as a reference value to normalize PCS activities. Controls omitting metal ions were run in parallel. AtPCS1 activity was not detectable when K+ (KCl), Mg2+ (MgCl2·6H2O), Ca2+ (CaCl2·2H2O), Mn2+ (MnSO4·H2O), Mo6+ (Na2MoO4·2H2O), or B3+ (H3BO3) was included in the assay medium at concentrations of 500 μm. In contrast, the activity was low but significant (1.9%) with Ni2+ (NiSO4·6H2O); moderate (9%–29%) with Cu2+ (CuCl2·2H2O), Co2+ (CoCl2·6H2O), or Fe3+ [Fe(NO3)3·9H2O]; and very high (135%) with Zn2+ (ZnSO4·7H2O) at concentrations of 500 μm (Fig. 4A).
Figure 4.
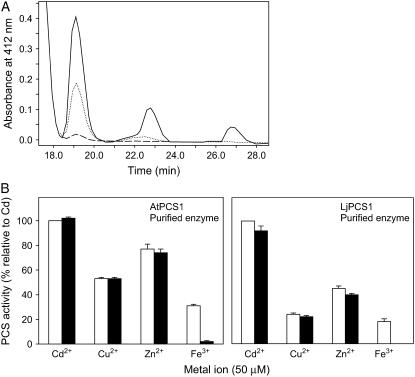
Activation of PCS enzymes from model plants by physiologically relevant metals. A, Desalted crude extracts of AtPCS1 (200 μg of protein) were assayed (30 min) with 500 μm metal ions and 5 mm GSH. Values represent means ± se of three to six enzyme preparations and are expressed in percent relative to the PCS activities with Cd2+. The 100% value corresponds to a specific activity of 56 ± 7 nmol of total PCs (GSH equivalents) produced per minute per milligram of protein. B, Purified AtPCS1 (60 μg of protein) and LjPCS1 (60 μg of protein) were assayed (30 min) with 50 μm metal ions and 5 mm GSH. Values represent means ± se of four to eight enzyme preparations and are expressed in percent relative to the PCS activities with Cd2+. The 100% values correspond to specific activities of 87 ± 3 (AtPCS1) and 89 ± 14 (LjPCS1) nmol of total PCs (GSH equivalents) produced min−1 mg−1 of protein.
The finding that Fe3+ is an activator of the enzyme was totally unexpected and the identity of the PC2 produced was accordingly verified by mass spectrometry. Also, another Fe3+ salt (FeCl3·6H2O) and two Fe2+salts (FeSO4·7H2O and FeCl2·4H2O) were found to activate AtPCS1 to a similar extent. To discard any contamination of the Fe(NO3)3·9H2O salt with adventitious metals or metaloids, a semiquantitative analysis of 68 elements was performed by inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry. Only aluminum and chromium could be detected as contaminants of the Fe3+ salt. Because these metals were present at trace levels (20–80 μg g−1) and are unable to activate PCS (Oven et al., 2002), they cannot account for the observed Fe-dependent PCS activity.
A second set of experiments was carried out with affinity-purified AtPCS1 and LjPCS1 enzymes using a concentration of 50 μm of metal ions (Fig. 4B). Similar results of metal activation were obtained for the purified tagged and untagged enzymes (data not shown), ruling out any possible interaction of metals with the (His)6-tag. This is consistent with the fact that Ni2+ and Co2+, which are typically employed in metal affinity chromatography because of their ability to strongly bind to His, did not activate the enzymes. Again, Zn2+ was the best activator among the physiologically relevant metals, although AtPCS1 and LjPCS1 activities were, respectively, 74% and 45% of those obtained with Cd2+. The corresponding activities with Cu2+ were 44% and 23% and those with Fe3+ were 17% and 2% (Fig. 4B). As was the case with desalted extracts, the purified untagged enzymes showed similar activations with Fe2+ and Fe3+.
Two additional experiments were performed to verify that the AtPCS1 activity observed with Fe2+ or Fe3+ was genuine. First, to exclude the possibility that Fe would be acting as an activator by displacing other metals that might be bound to the enzyme, two aliquots of purified AtPCS1 were dialyzed overnight against buffer alone or against buffer containing the metal-chelating resin Chelex, respectively. The enzyme activity was preserved by the dithiothreitol (DTT) added to the buffer during the dialysis and was similar regardless of the Chelex treatment. Second, addition to the assay medium of 500 μm EDTA, a general metal chelator, abolished the reaction initiated by Cd2+, Cu2+, Zn2+, Fe2+, or Fe3+ (Fig. 5). In contrast, addition of 500 μm desferrioxamine, a highly Fe-specific chelator (Gower et al., 1989), failed to inhibit the activation of PCS by Cd2+, Cu2+, or Zn2+, but completely prevented the activation by Fe2+ or Fe3+ (Fig. 5). These results provided a final proof for the Fe-dependent activation of PCS.
Figure 5.
Activation of PCS enzymes from model plants by Fe. A, Representative HPLC chromatogram showing activation of purified AtPCS1 by 5 mm GSH and either 50 μm Cd2+ (solid line) or 50 μm Fe3+ (dotted line). The latter reaction is completely inhibited by 500 μm desferrioxamine (dashed line). Note the synthesis of PC2–4 with Cd2+ and of PC2–3 with Fe3+ after the 30-min incubation period. B, Purified AtPCS1 (60 μg of protein) and LjPCS1 (60 μg of protein) were assayed with 50 μm metal ion, 5 mm GSH, and either 0 (white bars) or 500 μm (black bars) of desferrioxamine. AtPCS1 was incubated for 30 min and LjPCS1 for 60 min. All reactions were completely inhibited by 500 μm EDTA. Values are means ± se of two to three enzyme preparations and are expressed in percent relative to the PCS activities with Cd2+. The 100% values correspond to specific activities of 91 ± 4 (AtPCS1) and 68 ± 4 (LjPCS1) nmol of total PCs (GSH equivalents) produced per minute per milligram of protein.
Synthesis of PCs and hPCs in Plants
Experiments were also conducted to demonstrate (h)PC synthesis in vivo using the same metals found to activate AtPCS1 and LjPCS1 in vitro (Fig. 6). Concentrations up to 100-fold of Cu2+ or Zn2+ and up to 20-fold of Fe3+ higher than those present in the nutrient solution were given to Arabidopsis and Lotus plants grown in petri dishes or in pots, respectively. Again, Cd2+ was used as a positive control for (h)PC synthesis.
Figure 6.
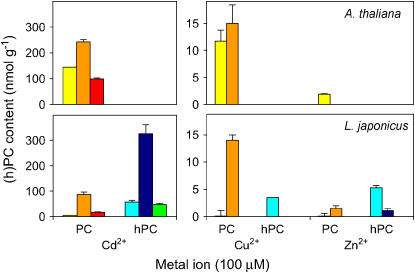
Synthesis of PCs and hPCs in model plants in response to metals. Arabidopsis and Lotus plants were treated for 4 d with 100 μm Cd2+, 100 μm Cu2+, 100 μm Zn2+, or 500 μm Fe3+. Figure shows (h)PC contents in whole plants of Arabidopsis and in roots of Lotus. Control (untreated) and Fe3+-treated plants had no detectable (h)PCs. Color codes for the (h)PC polypeptides are as in Figure 3. Values are means ± se of three to four plants and are expressed in nanomoles of (h)PCs (GSH equivalents) per gram of fresh weight.
Whole plants of Arabidopsis and leaves and roots of Lotus were harvested after 4 d of metal treatment and were used for (h)PC analysis. Comparison of both plant species in their ability to synthesize PCs or hPCs was of interest because Arabidopsis plants produce only GSH, whereas Lotus leaves and roots produce 97% hGSH and 3% GSH (Matamoros et al., 2003). Metal treatments did not cause any symptoms of toxicity in Lotus plants. In contrast, Cu2+ (100 μm) and Fe3+ (500 μm) had minor and moderate detrimental effects, respectively, on the growth of Arabidopsis plants, whereas Zn2+ (100 μm) had a stimulatory effect. No (h)PCs were detected in leaves of Lotus for any of the metal treatments. Application of 100 μm Cd2+ led to the accumulation (in decreasing order) of PC3, PC2, and PC4 in whole plants of Arabidopsis and of PC3, PC4, and PC2 in roots of Lotus. However, in the latter species, the total amount of hPCs exceeded the total PC content. In particular, there was a large accumulation of hPC3 and lower amounts of hPC2 and hPC4 in the roots. Treatment with 100 μm of Cu2+ or Zn2+ also induced PC synthesis in both plant species, albeit the amounts were in the range of 5% to 13% with Cu2+ and 0.5% to 1.5% with Zn2+ relative to those with Cd2+ (Fig. 6). Interestingly, in roots of Lotus, Zn2+ induced preferentially hPC synthesis, as occurred with Cd2+, whereas Cu2+ had a major effect on PC synthesis. Decreasing the concentration of Cu2+ and Zn2+ to 20 μm also lowered the content of (h)PCs. Treatment with Fe3+ (200–500 μm) or Ni2+ (100 μm) had no effect (<0.4% relative to Cd2+) on (h)PC accumulation (data not shown).
DISCUSSION
Legumes are a particularly attractive plant material with which to study PCS genes and proteins because they can synthesize hGSH and hPCs in a species-specific and organ-dependent manner (Grill et al., 1986; Klapheck et al., 1995; Matamoros et al., 2003). The pathway of (h)PC biosynthesis involves, in addition to PCS, the enzymes γGlu-Cys synthetase, GSH synthetase, and hGSH synthetase. We have fully characterized the corresponding three genes of Lotus and demonstrated that GSH and hGSH are produced by specific GSH and hGSH synthetases (Matamoros et al., 2003). To complete the molecular study of the (h)PC biosynthetic pathway, we set out to characterize genes encoding PCS in Lotus. A full-length cDNA clone was isolated that encodes LjPCS1, a Cys-rich protein with a theoretical mass of 55.5 kD and 84% to 86% identity with GmhPCS1, the only other PCS so far characterized from legumes (Oven et al., 2002). However, G. max produces exclusively hPCs (Grill et al., 1986; Oven et al., 2002), whereas Lotus is able to synthesize both PCs and hPCs. Possible explanations include differences in the availability of GSH and hGSH in the two legumes or in the capacity of their PCS enzymes to produce each type of polypeptide. Consequently, we decided to characterize in detail LjPCS1 and compare its properties with those of AtPCS1, a typical PC-producing enzyme (Ha et al., 1999; Vatamaniuk et al., 2000; Cazalé and Clemens, 2001; Oven et al., 2002).
LjPCS1 contains the amino acid residues known to be essential for activity and, indeed, when expressed in E. coli, catalyzed the synthesis of PCs from GSH. The assay of LjPCS1 purified by immobilized metal affinity chromatography confirmed the results obtained with E. coli soluble extracts, indicating that the enzyme is a genuine PCS. The substrate specificity of LjPCS1 and, for comparison, of AtPCS1 was examined using purified enzymes, which were obtained with the same expression vector and were assayed under identical conditions. The results of the assay of AtPCS1 and LjPCS1 with GSH, hGSH, or a combination of both thiols can be interpreted in the light of the proposed catalytic mechanism of PCS (Vatamaniuk et al., 2004; Vivares et al., 2005). The enzyme has two substrate binding sites, the donor site, located at Cys-56 in AtPCS1 and LjPCS1, and the acceptor site, yet to be defined. It is thought that a GSH molecule binds to the donor site forming an acyl-enzyme intermediate, which in turn provides the γGlu-Cys unit to a second GSH (or elongating PC) molecule, which is bound to the acceptor site (Vivares et al., 2005; Rea, 2006). The observations that AtPCS1 and LjPCS1 are able to synthesize hPCs from hGSH alone at low rates, but considerably larger amounts of hPCs than PCs from equimolar concentrations of GSH and hGSH, reveal that both enzymes can bind hGSH preferentially, but not exclusively, at the acceptor site. In other words, hGSH is a good acceptor but a poor donor of γGlu-Cys units compared with GSH.
The finding that AtPCS1 catalyzes hPC synthesis from hGSH as the sole substrate (even at higher rates than LjPCS1) is in open contradiction with previous results by other authors using the same AtPCS1 clone (Oven et al., 2002). We can only suggest, as possible reasons, an insufficient sensitivity of their method to quantify hPCs or a limitation of enzyme activity under their assay conditions. We conclude that hPC synthesis is an intrinsic biochemical feature of PCS1 enzymes rather than a peculiarity of those of legumes, and that GmhPCS1, previously characterized as an hPCS (Oven et al., 2002), is not such an enzyme but a typical PCS. In fact, as occurred with AtPCS1 and LjPCS1, GmhPCS1 catalyzed hPC synthesis from hGSH at much lower rates than PC synthesis from GSH. We cannot discard, however, the occurrence of several PCS in legumes with distinct specificities for GSH and hGSH. In this context, we propose that a genuine hPCS, if it exists at all, should be significantly more efficient in catalyzing hPC synthesis than PC synthesis from their respective thiol precursors.
The effects of metals on the activation of purified PCS have only been investigated in a few cases with some controversial results (Vatamaniuk et al., 2000; Oven et al., 2002; Beck et al., 2003). We conclude from our studies, using purified untagged AtPCS1 and LjPCS1, that Cu2+ and Zn2+, but not Mg2+, Ni2+, or Co2+, are enzyme activators at low concentrations (50 μm). Qualitatively, these results support those of Oven et al. (2002) and Beck et al. (2003), and disagree with those of Vatamaniuk et al. (2000). The latter authors found low but significant activation of FLAG-tagged AtPCS1 by Mg2+ and Ni2+, and a greater stimulatory effect of Cu2+ than Zn2+. In our enzyme preparations, however, the AtPCS1 and LjPCS1 activities with Zn2+, the best physiological metal activator, are approximately 2-fold greater than those with Cu2+ at 50 μm.
A completely unexpected finding in this work is that Fe is able to activate AtPCS1 and LjPCS1. Results were similar regardless of the Fe2+ and Fe3+ salts used, and elemental analysis ruled out any possible contamination of the salts with known metal activators of PCS. Definitive proof for an Fe-mediated activation of PCS was obtained by using desferrioxamine, a siderophore that binds one atom of Fe3+ with a stability constant of 1031 (Gower et al., 1989). Although desferrioxamine specifically binds the ferric form, it exhibits a potent ferroxidase activity on Fe2+ ions (Gower et al., 1989). This would explain why PCS activation by Fe2+ was also completely inhibited by desferrioxamine. A previous study with purified AtPCS1 failed to demonstrate enzyme activation by Fe2+ (Oven et al., 2002). We cannot offer an explanation for this discrepancy, but it is worth noting that significant enzyme activation occurred after 30 min for AtPCS1 (31% relative to Cd2+) but only after 60 min for LjPCS1 (18% relative to Cd2+). In contrast, LjPCS1 activities with Cd2+, Cu2+, or Zn2+ were already maximal after 30 min. The observations that the activation rates with Fe3+ are different for AtPCS1 and LjPCS1, and slower than those found for other metal ions, can be explained by a two-step mechanism involving the rapid formation of Fe3+-(h)GSH thiolates and their slow binding, as cosubstrates, to the acceptor site of the enzymes (Vatamaniuk et al., 2000). In fact, it has been estimated that most of the Cd2+ (>98%) and Zn2+ (>80%) supplied in the enzyme assays is present as metal-thiolates (Vatamaniuk et al., 2000). This is also probably the case for Fe3+, taking into account the stability constants of the metal thiolate complexes, the relative concentrations of (h)GSH (3–5 mm) and metal ions (25–50 μm), and the pH (8.0) of the assays. Nevertheless, we cannot discard a slow direct binding of Fe3+ to the enzymes, probably in Cys residues located in the C-terminal domain. In the PCS proteins of higher plants this domain is dispensable for catalysis and is involved in metal sensing, as shown by studies with truncated proteins obtained by mutagenesis (Cobbett and Goldsbrough, 2002) or partial proteolysis (Ruotolo et al., 2004).
Several metals of physiological interest induce PC accumulation in intact plants and cell suspension cultures. Cells of Rauvolfia serpentina (Zenk, 1996) and Lycopersicon esculentum (Chen et al., 1997) accumulated PCs in response to an excess of Cu2+ or Zn2+. In contrast, PC and hPC accumulation in plants of Cicer arietinum was observed after challenge with Cd2+ or As5+, but not with Cu2+ or Zn2+ (Gupta et al., 2004). These differences may be attributed to variations in metal concentrations, in the duration of metal treatment, and in the accessibility of metals to PCS. We have found that Arabidopsis and Lotus plants accumulate only low amounts of (h)PCs in response to moderate or high concentrations (20–100 μm) of Cu2+ or Zn2+. Because these metal ions, especially Zn2+, are strong activators of AtPCS1 and LjPCS1 in vitro, we have to conclude that (h)PC synthesis is not a primary mechanism for Cu2+ or Zn2+ detoxification. This conclusion is consistent with previous results showing that the PC-deficient (cad1) mutant and wild-type Arabidopsis plants are similarly sensitive to Cu2+ and Zn2+ toxicity (Ha et al., 1999; Cobbett and Goldsbrough, 2002). A stronger case occurs for Fe3+, which did not induce (h)PC accumulation in plants but activated both PCS enzymes. The lack of correlation between enzyme activation in vitro and (h)PC accumulation in intact plants is best explained by the inability of metal ions to reach PCS due to processes such as adsorption to cell walls, accumulation in the apoplast, long-distance transport, and binding to metallothioneins, in the case of Cu and possibly Zn (Cobbett and Goldsbrough, 2002), and to ferritin, in the case of Fe (Briat and Lobréaux, 1997). This explanation is supported by the interesting, and subsequently overlooked, observation that high concentrations (100–500 μm) of Ni2+ or Fe2+ induce PC accumulation in cultured cells of L. esculentum, an experimental system in which metal ions can readily reach PCS in vivo (Chen et al., 1997). It is becoming clear that PCs and PCS enzymes are more ubiquitous in living organisms than previously envisaged (Rea, 2006) and, hence, that they may have roles other than the detoxification of nonessential heavy metals, which are mainly of anthropogenic origin. In this respect, our results cannot rule out an involvement of (h)PCs in the homeostasis of Zn, Cu, and even Fe, processes in which subtle changes of metal concentration and low rates of PC synthesis are expected. In particular, the remarkable activation of PCS by Zn observed in vitro is suggestive of such a regulatory role. Also, emerging additional functions for PCs and PCS enzymes, such as detoxification of xenobiotic compounds (Beck et al., 2003) or scavenging of reactive oxygen species (Tsuji et al., 2002), merit further investigation.
MATERIALS AND METHODS
Chemicals and Biochemicals
All chemicals and biochemicals were of the highest purity available. NaCl, KCl, CaCl2·2H2O, MgCl2·6H2O, H3BO3, MnSO4·H2O, and ZnSO4·7H2O were purchased from Panreac. CdCl2, CuCl2·2H2O, NiSO4·6H2O, FeCl2·4H2O, FeCl3·6H2O, FeSO4·7H2O, Fe(NO3)3·9H2O, desferrioxamine (deferoxamine mesylate), and GSH were from Sigma-Aldrich, Na2MoO4·2H2O from Fisher, CoCl2·6H2O from Mallinckrodt, hGSH from Bachem, and Chelex-100 resin (200–400 mesh, Na+ form) from Bio-Rad.
Plant Material and Growth Conditions
Lotus (Lotus japonicus cv Gifu B-129) seeds were scarified, germinated for 2 d in petri dishes, and transferred to pots containing vermiculite under controlled environment conditions (Matamoros et al., 2003). Plants were fed BD nutrient solution (Broughton and Dilworth, 1971) supplemented with 5 mm NH4NO3· Salt concentrations in the BD medium (pH 6.5) were 1 mm CaCl2·2H2O, 0.5 mm KH2PO4, 0.25 mm MgSO4·7H2O, 0.25 mm K2SO4, 1 μm MnSO4·H2O, 2 μm H3BO3, 0.5 μm ZnSO4·7H2O, 0.2 μm CuSO4·5H2O, 0.1 μm CoCl2·6H2O, 0.1 μm Na2MoO4·2H2O, and 27 μm Fe (in the form of Sequestrene). After approximately 45 d, pots were separated at random in six groups and plants were given distilled water (control), 100 μm CdCl2, 100 μm CuCl2·2H2O, 100 μm ZnSO4·7H2O, 100 μm NiSO4·6H2O, or 500 μm FeCl3·6H2O, respectively, for 4 d. Roots were harvested in liquid nitrogen and stored at −80°C until analysis.
Arabidopsis (Arabidopsis thaliana; ecotype Col-0) seeds were surface sterilized, washed, and plated on a medium containing 1.5% (w/v) Phytagar and half-strength Murashige and Skoog medium (pH 5.6), in which the amount of ZnSO4 had been reduced to 0.1 μm. After stratification at 4°C for 3 d, seedlings were grown for 15 d in a controlled environment chamber at 23°C with a 16-h photoperiod. Plants were then transferred to plates containing the same medium supplemented with water (control), 100 μm CdCl2, 100 μm CuSO4·5H2O, 100 μm ZnSO4·7H2O, or 500 μm FeCl3·6H2O. After 4 d, plants were washed, harvested in liquid nitrogen, and stored at −80°C until analysis.
Quantification of PCs and hPCs
The (h)PCs were extracted from 60 mg of roots with 120 μL of 0.1% trifluoroacetic acid containing 0.5 mm diethylenetriaminepentaacetic acid and were quantified by HPLC with postcolumn derivatization (Grill et al., 1987) with modifications. The (h)PCs were separated on two C18 (4.6 × 250 mm; 5 μm) columns (Baker) connected in series, with a linear gradient (A, 0.1% trifluoroacetic acid; B, 20% acetonitrile and 0.1% trifluoroacetic acid) using the following program (3 min, 50% B; 30 min, 100% B; 35 min, 100% B; 38 min, 0% B) at a flow rate of 0.8 mL min−1. The (h)PCs eluting from the column were derivatized with 75 μm 5,5′-dithio-bis(2-nitrobenzoic acid) in 80 mm potassium phosphate (pH 7.8) in a reaction loop (total flow rate of 1.6 mL min−1 residence time of 1.56 min) at 50°C. Dinitrophenyl derivatives were detected at 412 nm (PDA996 detector; Waters) and identified by coelution with PC standards provided by Prof. Zenk (Halle, Germany) or with chemically synthesized hPC standards (Biosynthan).
Isolation and Cloning of LjPCS1
Total RNA from Lotus roots was isolated with the RNAqueous kit (Ambion), treated with DNase I (Roche), and reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Promega). The complete open reading frame of LjPCS1 was isolated by PCR using high-fidelity Platinum Taq DNA polymerase (Invitrogen) and specific primers (forward, AACATATGGCGATGGCGGGGTTG; reverse, TACTCGAGCTAAGACAAAGGTACACC), based on a genomic sequence obtained within the frameshift of the Lotus sequencing project (Kazusa DNA Research Institute, Japan). The PCR program comprised an initial denaturation step at 94°C for 5 min, followed by 40 cycles of 30 s at 94°C, 40 s at 61°C, and 2 min at 68°C, and a final elongation step at 68°C for 10 min. The resulting 1.5-kb product was cloned in pCRII-TOPO (Invitrogen) and subsequently in the pET-28a(+) expression vector (Novagen) using the NdeI and XhoI restriction sites.
Production, Purification, and Assay of Recombinant PCS
Transformed Escherichia coli BL21(DE3) cells were incubated overnight at 37°C in 3 mL of Luria-Bertani broth. Then, 1.5 mL of the subculture was added to 500 mL of broth, which was further incubated at 37°C until an A600 of 1.0 was reached. Protein expression was induced by overnight incubation at 18°C with 0.1 mm isopropylthio-β-galactoside. Cells were collected by centrifugation and lysed by sonication in buffer A, consisting of 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 2.5 mm CaCl2, 10% glycerol, 50 mm imidazole, and 10 mm β-mercaptoethanol. The soluble extract was loaded on a nickel affinity column (HiTrap Chelating; Amersham Biosciences), the column was washed with buffer A, and the recombinant protein was eluted with buffer A containing 250 mm imidazole. The use of 20 mm imidazole in the washing buffer or 500 mm imidazole in the eluting buffer did not increase the yield of recombinant proteins but rather resulted in contamination with unwanted proteins. The enzyme was loaded on a HiTrap desalting column (Amersham Biosciences), eluted with buffer A containing 1 mm β-mercaptoethanol but no imidazole, and concentrated 4-fold with centricon-10 devices (Amicon).
To remove the (His)6-tag from the purified protein, a second addition of 2.5 mm CaCl2 was made to the protein (approximately 0.5 mg) preparation, and this was incubated with 2 units of biotinylated thrombin (Novagen) at 20°C for 16 h. Thrombin was then removed with streptavidine agarose beads as recommended by the manufacturer and β-mercaptoethanol was added at a final concentration of 2 mm. The preparation was loaded on a nickel affinity column and the untagged protein was eluted (first 1.5 mL) with buffer B, which comprised 20 mm Tris-HCl (pH 8.0), 500 mm NaCl, 10% glycerol, 10 mm β-mercaptoethanol, and 20 mm imidazole. The protein was loaded on a desalting column, eluted with buffer B containing 1 mm β-mercaptoethanol but no imidazole, and concentrated.
The PCS activity was determined by quantification of the synthesized PCs and hPCs. Briefly, the reaction mixture contained 100 mm Tris-HCl (pH 8.0), 1 mm β-mercaptoethanol, 50 or 500 μm metal ion, 2.5 or 5 mm GSH and/or hGSH, and 50 μL extract, in a final volume of 100 μL. The reaction was allowed to proceed for 30 or 60 min at 35°C and was stopped by addition of 15 μL of 5% trifluoroacetic acid. The extract was cleared by centrifugation and filtered, and 50 μL was injected on the HPLC.
PCS Experiments with Chelex and Desferrioxamine
To remove trace amounts of metals that might be bound to PCS, two aliquots of purified AtPCS1 were dialyzed (8-kD cutoff membranes; Spectra/Por; Spectrum), respectively, against a buffer comprising 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, and 25 mm DTT, or against the same buffer plus 5% (w/v) Chelex-100 resin. Dialysis was left to proceed overnight at 4°C, with stirring, and DTT was replaced by 10 mm β-mercaptoethanol by further dialysis for 2 h. The enzyme preparations were then loaded on a HiTrap desalting column as described above, concentrated 4-fold, and assayed for PCS activity. The effect of metal chelators on purified AtPCS1 and LjPCS1 was examined using concentrations of 50 μm metal ion, 5 mm GSH, and 500 μm EDTA or desferrioxamine, as described in the legend to Figure 5.
Other Analyses
Inductively coupled plasma atomic emission spectrometry (Perkin-Elmer Optima 3200RL) and inductively coupled plasma mass spectrometry (Perkin-Elmer ELAN6000) were used for semiquantitative detection of trace amounts of other elements in Fe(NO3)3·9H2O. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Voyager DE-PRO; Applied Biosystems) and nano-electrospray tandem mass spectrometry (Finnigan LCQ ion trap mass spectrometer; ThermoQuest, Finnigan MAT) were used to verify PC and hPC structures.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AY633847.
Acknowledgments
We thank Dr. M. Oven, Prof. T. Kutchan, and Prof. M. H. Zenk for providing the AtPCS1 clone, Dr. A. Beck and Prof. E. Grill for helpful advice on the HPLC analysis of (h)PCs, and Dr. J. Abian for mass spectrometry analyses of (h)PCs. J.L. and L.N. are the recipients of fellowships (B041–2004 and BES–2003–1462) from Gobierno de Aragón and Ministerio de Educación y Ciencia (MEC), respectively. M.A.M. acknowledges a postdoctoral contract (Ramón y Cajal program) from MEC. This work is part of the Ph.D. thesis of J.L., supervised by M.A.M. and M.B.
This work was supported by the Ministerio de Educación y Ciencia-Fondos Europeos de Desarrollo Regional (grant nos. AGL2002–2876 and AGL2005–1404) and by Gobierno de Aragón-Fondo Social Europeo (group E33).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Manuel Becana (becana@eead.csic.es).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073635.
References
- Beck A, Lendzian K, Oven M, Christmann A, Grill E (2003) Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates. Phytochemistry 62: 423–431 [DOI] [PubMed] [Google Scholar]
- Briat J-F, Lobréaux S (1997) Iron transport and storage in plants. Trends Plant Sci 2: 187–193 [Google Scholar]
- Broughton WJ, Dilworth MJ (1971) Control of leghemoglobin synthesis in snake beans. Biochem J 125: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalé A-C, Clemens S (2001) Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Lett 507: 215–219 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou J, Goldsbrough PB (1997) Characterization of phytochelatin synthase from tomato. Physiol Plant 101: 165–172 [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Frendo P, Hernández Jiménez MJ, Mathieu C, Duret L, Gallesi D, Van de Sype G, Hérouart D, Puppo A (2001) A Medicago truncatula homoglutathione synthetase is derived from glutathione synthetase by gene duplication. Plant Physiol 126: 1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower JD, Healing G, Green CJ (1989) Determination of desferrioxamine-available iron in biological tissues by high-pressure liquid chromatography. Anal Biochem 180: 126–130 [DOI] [PubMed] [Google Scholar]
- Grill E, Gekeler W, Winnacker EL, Zenk MH (1986) Homo-phytochelatins are heavy metal-binding peptides of homo-glutathione containing Fabales. FEBS Lett 205: 47–50 [Google Scholar]
- Grill E, Löeffler S, Winnacker E-L, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86: 6838–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker E-L, Zenk MH (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84: 439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta DK, Tohoyama H, Joho M, Inouhe M (2004) Changes in the levels of phytochelatins and related metal-binding peptides in chickpea seedlings exposed to arsenic and different heavy metal ions. J Plant Res 117: 253–256 [DOI] [PubMed] [Google Scholar]
- Ha S-B, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS (1999) Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 11: 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487–496 [Google Scholar]
- Klapheck S, Schlunz S, Bergmann L (1995) Synthesis of phytochelatins and homo-phytochelatins in Pisum sativum L. Plant Physiol 107: 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Clemente MR, Sato S, Asamizu E, Tabata S, Ramos J, Moran JF, Stiller J, Gresshoff PM, Becana M (2003) Molecular analysis of the pathway for the synthesis of thiol tripeptides in the model legume Lotus japonicus. Mol Plant Microbe Interact 16: 1039–1046 [DOI] [PubMed] [Google Scholar]
- Oven M, Page JE, Zenk MH, Kutchan TM (2002) Molecular characterization of the homo-phytochelatin synthase of soybean Glycine max. J Biol Chem 277: 4747–4754 [DOI] [PubMed] [Google Scholar]
- Rea PA (2006) Phytochelatin synthase, papain's cousin, in stereo. Proc Natl Acad Sci USA 103: 507–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotolo R, Peracchi A, Bolchi A, Infusini G, Amoresano A, Ottonello S (2004) Domain organization of phytochelatin synthase. J Biol Chem 279: 14686–14693 [DOI] [PubMed] [Google Scholar]
- Tsuji N, Hirayanagi N, Okada M, Miyasaka H, Hirata K, Zenk MH, Miyamoto K (2002) Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochem Biophys Res Commun 293: 653–659 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lang A, Chalasani S, Demkiv LO, Rea PA (2004) Phytochelatin synthase, a dipeptidyltransferase that undergoes multisite acylation with γ-glutamylcysteine during catalysis. J Biol Chem 279: 22449–22460 [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y, Rea PA (2000) Mechanism of heavy metal ion activation of phytochelatin (PC) synthase. J Biol Chem 275: 31451–31459 [DOI] [PubMed] [Google Scholar]
- Vivares D, Arnoux P, Pignol D (2005) A papain-like enzyme at work: native and acyl-enzyme intermediate structures in phytochelatin synthesis. Proc Natl Acad Sci USA 102: 18848–18853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk MH (1996) Heavy metal detoxification in higher plants—a review. Gene 179: 21–30 [DOI] [PubMed] [Google Scholar]