Abstract
Extracellular ATP can serve as a signaling agent in animal cells, and, as suggested by recent reports, may also do so in plant cells. In animal cells it induces the production of reactive oxygen species through the mediation of NADPH oxidase. Similarly, here we report that in leaves of Arabidopsis (Arabidopsis thaliana), applied ATP, but not AMP or phosphate, induces the accumulation of superoxide (O2−) in a biphasic, dose-dependent manner, with a threshold at 500 nm ATP. This effect did not require ATP hydrolysis for it was mimicked by ATPγS. ATP also induced increased levels of Arabidopsis respiratory burst oxidase homolog D (AtrbohD) mRNA, but ATP-treated plants that had disrupted AtrbohD and AtrbohF genes did not accumulate O2−, indicating that NADPH oxidases are responsible for the induced O2− accumulation. Inhibitors of mammalian P2-type ATP receptors abolished ATP-induced O2− production, suggesting that the ATP effects may be mediated through P2-like receptors in plants. Cytosolic Ca2+ and calmodulin are likely to help transduce the ATP responses, as they do in animal cells, because a Ca2+ channel blocker, a Ca2+ chelator, and calmodulin antagonist all reduced ATP-induced O2− accumulation. Furthermore, ATP treatment enhanced the expression of genes that are induced by wounds and other stresses. The ATP measured at wound sites averaged 40 μm, well above the level needed to induce O2− accumulation and gene expression changes. Transgenic plants overexpressing an apyrase gene had reduced O2− production in response to applied ATP and wounding. Together, these data suggest a possible role for extracellular ATP as a signal potentially in wound and stress responses.
Extracellular ATP (eATP) is a well-characterized signaling agent in mammals. It induces the respiratory burst in phagocytes, and it exerts this effect through P2 receptors (Ralevic and Burnstock, 1998; Di Virgilio et al., 2001) and a signaling chain that typically includes increased cytosolic Ca2+ concentration ([Ca2+]cyt) as an early step (Kuroki and Minakami, 1989; Dichmann et al., 2000). These receptors mediate diverse responses in animals including platelet aggregation, the inflammatory response, neurotransmission, and apoptosis (Ralevic and Burnstock, 1998; Di Virgilio et al., 2001). Recent reports indicate that there may be eATP signaling in plants. Low microgram concentrations of eATP induce increases in [Ca2+]cyt (Demidchik et al., 2003) and membrane depolarization (Lew and Dearnaley, 2000) in Arabidopsis (Arabidopsis thaliana) roots. At higher concentrations, eATP induces increases in [Ca2+]cyt and downstream gene expression changes associated with stress and wounding in intact seedling tissues (Jeter et al., 2004), and it inhibits auxin transport, root gravitropism (Tang et al., 2003), and pollen germination (Steinebrunner et al., 2003) in Arabidopsis. The destruction of eATP appears to induce programmed cell death in leaves of several plant species (Chivasa et al., 2005).
Among the signaling changes induced by eATP in animal cells is enhanced production of reactive oxygen species (ROS; Dichmann et al., 2000; Pines et al., 2005). ROS have been implicated in the responses of a wide variety of plants to both biotic and abiotic stresses (Apel and Hirt, 2004). Wounding and herbivory stimulate increased levels of ROS (Felton et al., 1994; Orozco-Cardenas and Ryan, 1999) along with an enhanced expression of defense genes such as those of protease inhibitors (Orozco-Cardenas et al., 2001). Pathogen infections induce the oxidative burst, a large accumulation of ROS that occurs in two phases beginning within minutes after infection, and this response is central to the defense response of plants to pathogens (Lamb and Dixon, 1997; Rodriguez and Redman, 2005). Various abiotic stresses including salt, drought, ozone, and cold, also induce the accumulation of ROS (Xiong et al., 2002).
The two best-characterized ROS are superoxide (O2−) and hydrogen peroxide (H2O2), but also included in this chemical category are hydroxyl radical, singlet oxygen, and hypochlorous acid (Henderson and Chappell, 1996). NADPH oxidase catalyzes the conversion of O2 to O2−, and its activation mostly accounts for the large consumption of oxygen that characterizes the respiratory burst in mammalian phagocytic cells (Vignais, 2002).
NADPH oxidase homologs have been implicated as key players in the production of O2− in plants (Mittler et al., 2004). Diphenyleneiodonium (DPI), a suicide substrate inhibitor of mammalian NADPH oxidases, blocks the oxidative burst in rose (Rosa damascena) and soybean (Glycine max) cells (Auh and Murphy, 1995; Rajasekhar et al., 1999). Several NADPH oxidase subunit gp91phox homologs have been identified in plants; for example, 10 in Arabidopsis (Keller et al., 1998; Foreman et al., 2003; Kwak et al., 2003), one in rice (Oryza sativa; Groom et al., 1996), and two in tomato (Lycopersicon esculentum; Amicucci et al., 1999; Sagi et al., 2004).
Plants with reduced or disrupted gp91phox homologs have compromised responses to biotic and abiotic stress and have a reduced capability to accumulate ROS. Antisense lines of tomato Lerboh1 had a reduced ROS accumulation and compromised wound response, failing to produce wild-type levels of protease inhibitor II after being wounded (Sagi et al., 2004). Arabidopsis plants disrupted in gp91phox homologs Arabidopsis respiratory burst oxidase homolog D (AtrbohD) and AtrbohF, have reduced ROS production and cell death after treatment with the avirulent bacterium Pseudomonas syringae pv tomato DC3000 (Torres et al., 2002), and they have diminished guard cell responses to abscisic acid (ABA; Kwak et al., 2003). In Nicotiana benthamiana, NbrbohA and NbrbohB genes were required for accumulation of ROS and resistance to Phytophtora infestans (Yoshioka et al., 2003). These data show that NADPH oxidase homologs in plants are important for the accumulation of ROS and subsequent responses of plants to various stresses.
Physical injury to plants can occur as a result of herbivory or environmental stresses such as wind, rain, or hail (Leon et al., 2001). Wounding enhances the expression of a multitude of genes that function in the repair of damaged tissues and in defense mechanisms to prevent further damage (Reymond et al., 2000). Characteristics of the wound response include an increased level of oligosaccharides, such as oligogalacturonic acid (OGA), production of ROS (Orozco-Cardenas and Ryan, 1999; Orozco-Cardenas et al., 2001), increases in cytosolic Ca2+ levels (Knight et al., 1993) and activation of calmodulin (CaM; Leon et al., 1998), induction of mitogen-activated protein kinase signaling (Rakwal and Agrawal, 2003), and increased production of the phytohormones jasmonic acid (Creelman et al., 1992; Reymond and Farmer, 1998) and ethylene (Nishiuchi et al., 2002; Cabrera and Saltveit, 2003). In solanaceous plants, systemin, an 18-amino acid polypeptide wound signal, leads to the induction of proteinase inhibitor genes, but an equivalent pathway has not been found in Arabidopsis (Leon et al., 2001).
Many aspects of the plant defense response are analogous to the animal immune response (Bergey et al., 1996; Staskawicz et al., 2001). The signaling role of eATP in ROS production in animals, its newly discovered role in inducing increased [Ca2+]cyt in plants, plus the expectation that wounded plant cells would release ATP into their apoplastic space raise the possibility that eATP could induce ROS production and participate in wound signaling in plants. To test whether a similar burst of ROS production is occurring in response to eATP in plants and whether this accumulation is due to NADPH oxidase, we tested wild-type and atrbohD/F double mutant Arabidopsis plants for O2− accumulation when treated with eATP. We show that submicromolar concentrations of eATP do induce a significant accumulation of O2− in a dose-dependent fashion and that there is a biphasic accumulation in response to eATP. This accumulation of O2− is dependent on homologs of the NADPH oxidase subunits gp91phox, AtrbohD, and AtrbohF.
RESULTS
ATP and ADP Induce O2− Accumulation
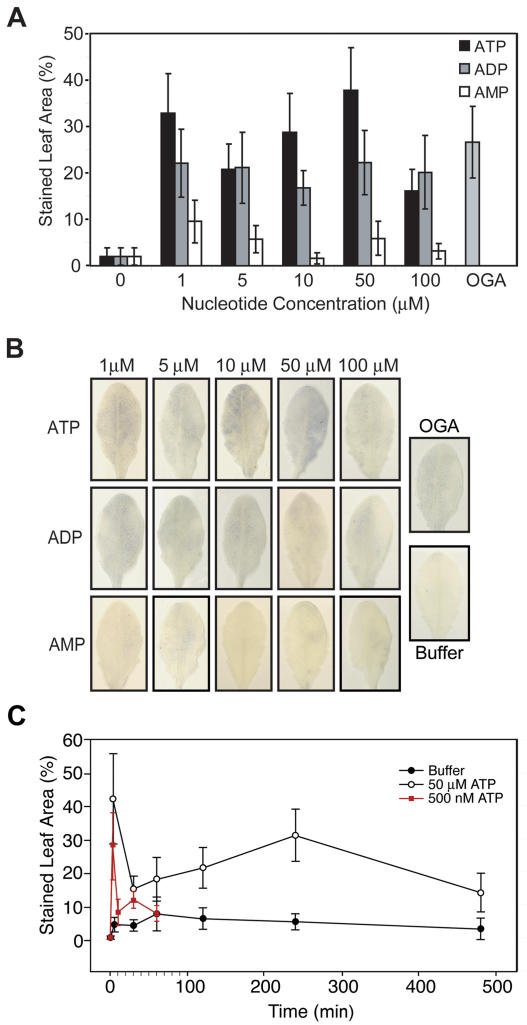
In response to the range of ATP concentrations tested, there were two distinct peaks of increased O2− accumulation, one at 1 μm ATP and the other at 50 μm ATP (Fig. 1A). All concentrations of ATP tested induced significantly higher O2− accumulation than the phosphate buffer (PB) control. The positive control of OGA, which is known to induce an oxidative burst in Arabidopsis (Hu et al., 2004), also induced a statistically significant increase over the buffer control. Across the concentrations tested, ATP-induced O2− accumulation was generally greater than ADP-induced O2− accumulation, while AMP-induced O2− accumulation was significantly less than that of either ATP or ADP (Fig. 1, A and B).
Figure 1.
eATP and extracellular ADP induce O2− accumulation. A, O2− accumulation in Arabidopsis leaves 1 h after treatments with eATP, extracellular ADP, and extracellular AMP. The error bars represent sd (n ≥ 8; two independent experiments). All ATP and ADP treatments are statistically significant compared to the buffer or AMP treatments (P ≤ 0.001). B, Images of representative leaves treated with various nucleotides, OGA, or buffer. C, Time course for O2− accumulation following treatment with 50 μm ATP (black line, white circles; n ≥ 10) or 500 nm ATP (red line, red squares; n = 10) or buffer only (black line, black circles; n = 10).
The O2− accumulation in response to infiltration of 50 μm ATP peaked at two distinct time points, 3 min and 4 h (Fig. 1C). At all of the time points measured, there was a significant accumulation of O2− in response to ATP treatment as compared to the PB control (P ≤ 0.0009). To determine whether the O2− assay was in fact detecting O2− in the leaves, 2 mm xanthine was added together with 5 units of xanthine oxidase to evolve O2−, and the O2− was detected using the same method. All times that were tested with xanthine and xanthine oxidase had significant increases in O2− compared to a buffer control (P ≤ 0.0002; data not shown), and the pattern of Nitroblue tetrazolium staining was similar to that observed after ATP treatment.
A test of the threshold of the response to eATP revealed that 250 nm ATP induces no response, but 500 nm ATP induces a significant production of O2− that is at least equal to that induced by 1 μm ATP (data not shown). The kinetics of the response to 500 nm ATP for the first 60 min closely parallels the kinetic pattern induced by 50 μm ATP (Fig. 1C).
ATP-Induced O2− Accumulation Depends on NADPH Oxidase
Several authors have shown that homologs of mammalian NADPH oxidase subunits are responsible for stress-induced production of O2− in plants (Torres et al., 2002; Kwak et al., 2003; Yoshioka et al., 2003; Sagi et al., 2004). To determine whether ATP-induced ROS accumulation could be attributed to NADPH oxidase production of O2−, we tested ATP effects on mutants with double knockouts of genes encoding the NADPH oxidase subunit homologs AtrbohD and AtrbohF. These mutants failed to accumulate O2− in response to ATP application (Table I; P = 0.89). Consistent with this finding, the suicide substrate inhibitor of mammalian NADPH oxidase, DPI, diminished the ATP-induced O2− accumulation to the level of the negative control (P = 0.70; data not shown). For the DPI experiment a dimethyl sulfoxide-only control was added and it gave the same results as the buffer-only control.
Table I.
Test of O2− response in atrbohD/F plants
| Treatment
|
Stained Leaf Area
|
|
|---|---|---|
| Wild-Type | atrbohD/F | |
| % | ||
| Buffer | 8.2 ± 7.1 | 13.2 ± 8.3 |
| Buffer ± 50 μm ATP | 39.1 ± 12.6a | 12.8 ± 5.2 |
Differs from all other mean values (P < 0.001); all n ≥ 7.
Presence of eATP at Arabidopsis Wound Sites
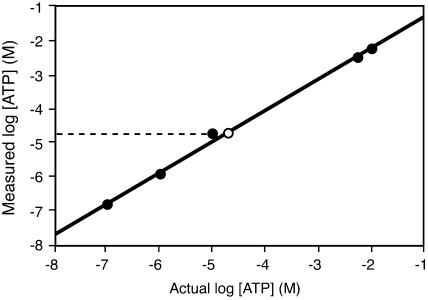
To determine a possible physiological source of eATP that could induce ROS accumulation in plants, we measured the concentration of ATP in the extracellular fluid present at Arabidopsis wound sites. The sampling and measuring procedure used here was linear over a range from 100 nm to 10 mm ATP and closely matched the actual values of the ATP standards over that range (Fig. 2). Using the same procedure, we measured a mean value of 40 ± 22 μm [ATP] from seven different pooled samples collected from Arabidopsis rosette leaf wound sites (Fig. 2). The values for these seven samples ranged between 25 and 45 μm.
Figure 2.
Measurement of [ATP] at Arabidopsis wound sites. The average of the calculated concentrations of the known samples (black circles) are plotted against the known concentration of the samples (n ≤ 3). The regression equation of the measured versus actual concentrations (y = [0.9150 × x] − 0.3875; r2 = 0.997) was used to correct the average measured ATP concentration (white circle) at Arabidopsis wound sites (n = 7). ATP concentrations are shown as log molar values. The sd (not shown) for the 40 μm mean value recorded was ± 22 μm.
Plants Overexpressing Apyrase Have Reduced O2− Production in Response to eATP and Wounding
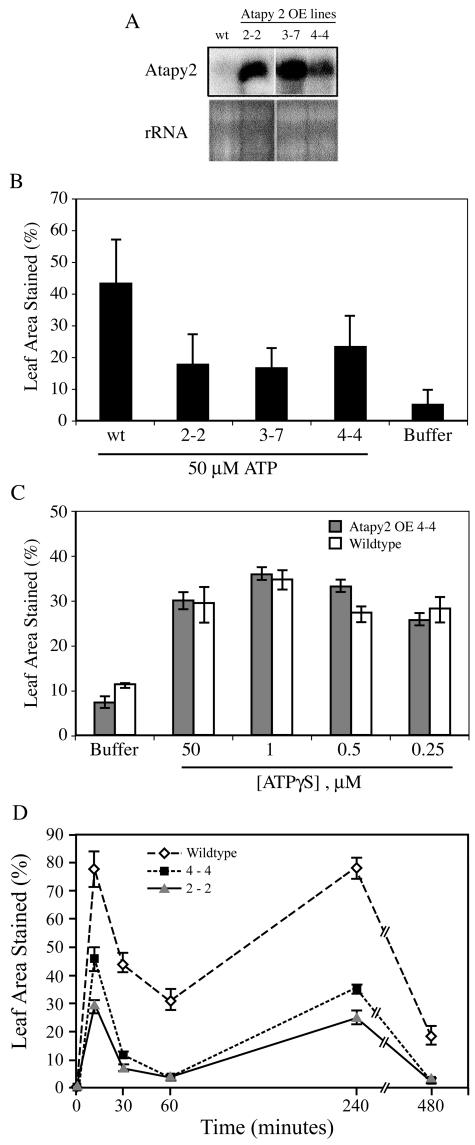
Plants, like animals, have ectoapyrase enzymes that regulate the concentration of eATP (Thomas et al., 2000). The two Arabidopsis apyrases that are structurally the most similar to the pea (Pisum sativum) ectoapyrase psNTP9 are AtAPY1 and AtAPY2 (Steinebrunner et al., 2000), and, like psNTP9, both appear to have signal peptides at their N-terminal end. Three different transgenic lines overexpressing (OE) AtAPY2 (Fig. 3A) all show lowered O2− responses to applied ATP (Fig. 3B). As judged by immunoblot analysis, these lines show enhanced expression of the apyrase protein (S. Reichler, T. Butterfield, and S. Roux, unpublished data), but their phenotype does not differ from that of wild-type plants under the growth conditions used.
Figure 3.
Responses of plants overexpressing apyrase (OE). A, Northern analysis of the expression level of AtAPY2 in three independent lines of transgenic plants overexpressing (OE) AtAPY2 and in wild-type plants. The size of the transcripts expressed by the OE plants is approximately 1.4 kb. B, Three different lines of OE plants produce less O2− than wild-type plants in response to applied ATP. C, OE plants (line 4-4) produce the same level of O2− as wild-type plants in response to ATPγS, an effective agonist of P2 receptors that is not hydrolyzed by apyrase or other phosphatases. D, Wound-induced accumulation of O2−, measured at various time points after a wound is reduced in plants OE an apyrase gene (AtAPY2). The first time point is 3 min after the wound, but the symbols are displaced somewhat to the right to more clearly separate them from the left border.
Transgenic line 4-4 was tested for its response to ATPγS, a P2 receptor agonist that, like ATP, induces increase in [Ca2+]cyt, but, unlike ATP, cannot be hydrolyzed by apyrase or by phosphatases (Jeter et al., 2004). At the concentrations tested, the peak response of O2− production in both wild-type and apyrase-OE plants was at 1 μm, and the threshold response of both was at or below 250 nm (Fig. 3C). Wild-type and OE plants did not differ significantly in their O2− production response to ATPγS.
Both wounding and eATP application to unwounded leaves induced a biphasic response of O2− production (Fig. 3D), but the O2− response of transgenic lines 4-4 and 2-2 to wounding was muted relative to that of wild-type plants (Fig. 3D).
Other Nucleotides Induce O2− Accumulation
Many animal P2 receptors have broad nucleotide specificity (Ralevic and Burnstock, 1998). At 50 μm concentrations ATP, UTP, and GTP induced O2− production about equally (P > 0.22), but significantly greater than the buffer control (P < 10−5; Table II). The effect of 50 μm CTP on production was significantly less than that of the other nucleotides tested (P < 0.001) and was only marginally greater than that of the buffer control (P = 0.04; Table II).
Table II.
Specificity of nucleotide effects (50 μm) on O2− production
Values with different superscript letters differ from all other mean values (P < 0.05); all n ≥ 6.
| Treatment | Stained Leaf Area |
|---|---|
| % | |
| Buffer | 6.1a ± 2.7 |
| ATP | 35.4b ± 13.8 |
| GTP | 29.9b ± 5.3 |
| UTP | 34.7b ± 10.2 |
| CTP | 14.9c ± 8.9 |
P2 Receptor Inhibitors and Adenosine Reduce eATP-Induced O2− Accumulation
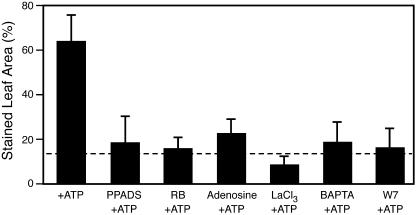
Two inhibitors of the P2 receptors that mediate eATP signaling in animals, pyridoxalphosphate-6-azophenyl-2′, 4′-disulphonic acid (PPADS) and reactive blue 2 (RB2; Ralevic and Burnstock, 1998) reduced the ATP-induced O2− to levels similar to the control (Fig. 4; P ≥ 0.25). The O2− production responses to PPADS and RB2 alone were not significantly different from the PB control (P ≥ 0.69; data not shown).
Figure 4.
ATP-induced O2− accumulation is significantly reduced by antagonists of P2 receptors, adenosine, and antagonists of Ca2+ signaling. Leaves were assayed for O2− 1 h after treatment. The error bars represent sd. For treatment with inhibitor plus ATP compared to ATP alone, P is <0.0001; n ≥ 8. The dashed line marks the level of O2− accumulation in the buffer control.
In neutrophils, adenosine, a product of ATP turnover, inhibits O2− production through the activation of A2 adenosine receptors (Cronstein et al., 1985; Bengtsson et al., 1996). To determine whether or not adenosine can inhibit eATP-induced O2− accumulation, we cotreated plants with 10 μm adenosine and 50 μm ATP and measured O2− accumulation (Fig. 4). Cotreatment with adenosine reduced eATP-induced O2− accumulation to levels similar to the buffer-only control (Fig. 4; P > 0.05). Adenosine treatment alone was not significantly different from the PB control (P > 0.05; data not shown).
Ca2+ Mediation Is Involved in eATP-Induced ROS Accumulation
Canonical P2 receptor signaling and wound signaling involve downstream Ca2+ signaling. When they were applied in addition to ATP, both 1,2-Bis(2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetraacetic acid, which chelates extracellular Ca2+, and LaCl3, which blocks Ca2+ channels, reduced ATP-induced O2− accumulation to levels similar to control (Fig. 4; P ≥ 0.25). Neither agent alone induced effects significantly different from the PB control (P > 0.05; data not shown).
To further study the involvement of Ca2+ mediation of ATP-induced O2− accumulation, we used a CaM antagonist, N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide (W7), to block the action of CaM. Addition of W7 to the ATP treatment reduced O2− accumulation compared to ATP treatment in the absence of W7 (P < 0.0001; Fig. 4). N-(6-Aminohexyl)-1-naphthalenesulfonamide (W5), which is a much less potent CaM antagonist, was ineffective in blocking ATP-induced O2− accumulation (P > 0.05; data not shown). Responses to W5 and W7 alone also were not significantly different from the PB control (P > 0.05; data not shown).
ATP Induces Expression of Genes Also Induced by ROS and Wounding
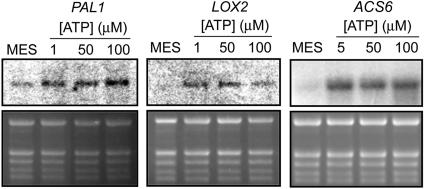
Applied ATP induced increased abundance of transcripts for the Phe ammonia-lyase gene 1 (PAL1), which is also induced by ROS (Levine et al., 1994), with maximal effect at 100 μm (Fig. 5). It also induced an increased transcript abundance for genes encoding lipoxygenase 2 (LOX2) and 1-aminocyclopropane-1-carboxylate synthase 6 (ACS6), which are biosynthetic enzymes for jasmonic acid and ethylene, two hormones that can mediate wound and defense responses (Turner et al., 2002; Wang et al., 2002; Fig. 5).
Figure 5.
Northern analyses of eATP-induced changes in transcript levels of PAL1, LOX2, and ACS6. Transcript levels for PAL1 were measured at 3 h for LOX2 at 90 min and for ACS6 at 30 min. The sizes of the transcripts for PAL1, LOX2, and ACS6 are approximately 2.2 kb, approximately 2.7 kb, and approximately 1.5 kb, respectively. These experiments were done twice with similar results. Top section, northern analysis of the abundance of the three transcripts assayed; bottom section, rRNA loading controls.
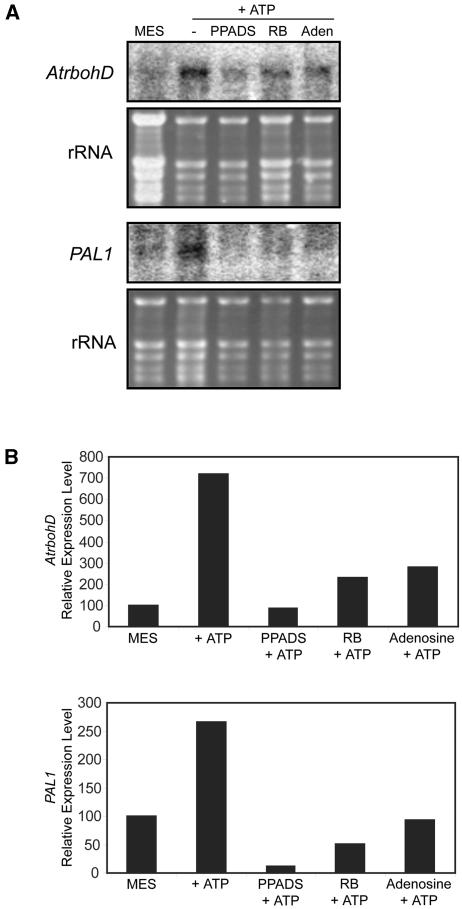
P2 Receptor Inhibitors Block eATP-Induced Changes in Gene Expression
We also examined the effect of P2 receptor inhibitors and adenosine on eATP-induced changes in gene expression (Fig. 6). Pretreatment of seedlings with 250 μm PPADS, 30 μm RB2, or 10 μm adenosine reduced the eATP-induced expression of AtrbohD and of PAL1, a gene whose expression can be induced by ROS (Fig. 6, A and B).
Figure 6.
Levels of AtrbohD and PAL transcripts induced by eATP are reduced by antagonists of P2 receptors and adenosine. A, Northern analysis of the level of AtrbohD messages 60 min after various treatments and of PAL messages 90 min after various treatments. B, Relative expression levels of the messages for AtrbohD and PAL1 after the bands were adjusted using the loading controls. These experiments were done in duplicate with similar results. The inhibitor concentrations used, as in Figure 4, were: 250 μm PPADS, 30 μm RB2, and 10 μm adenosine.
DISCUSSION
Parallels in Animal and Plant Stress Signaling
When activated by a pathogen, phagocytic blood cells have a respiratory burst that results in the production of ROS (Vignais, 2002). This respiratory burst can be induced by eATP (Kuroki and Minakami, 1989; Dichmann et al., 2000), catalyzed by NADPH oxidase activity in the plasma membrane (Vignais, 2002). Homologues to the phagocytic NADPH oxidase gp91phox subunit have been identified in plants and are responsible for the production of ROS in response to infection (Torres et al., 2002; Yoshioka et al., 2003), wounding (Sagi et al., 2004), and ABA (Kwak et al., 2003). Many aspects of the plant defense response are analogous to the animal immune response (Bergey et al., 1996; Staskawicz et al., 2001) and our data indicate that in plants, as in animals, eATP may be an important signaling molecule involved in the induction of ROS and downstream stress responses.
Availability and Regulation of ATP as an Extracellular Signal
The low (submicromolar) concentrations at which ATP elicits O2− accumulations in leaves suggests that eATP is most likely acting as a signal in leaf wound responses, just as when it induces increased [Ca2+]cyt in root cells (Demidchik et al., 2003). The most obvious mechanism for ATP release into the plant extracellular matrix (ECM) would be wounding. Cytoplasmic ATP concentrations in plant cells typically range between 0.5 and 1 mm, depending on their metabolic state (Gout et al., 1992; Borisjuk et al., 2003). Phloem sap may also have near millimolar ATP (Geigenberger et al., 1993). During wounding, at the moment of cell or phloem rupture, the surrounding tissue would be exposed to concentrations of ATP in this range.
The samples for directly measuring the [ATP] in the extracellular fluid at wound sites were typically collected <3 min after wounding, coincident with the first peak in O2− accumulation. Immediately after wounding, we would expect the initial [ATP] present in the ECM to be higher than the low micromolar level reported here. However, our measurement of an average of 40 μm ATP remaining in the extracellular fluid within 3 min after wounding suggests that levels of ATP within the range that induces O2− accumulation persist for some period of time after wounding.
There are additional mechanisms for ATP release into the ECM. Plasma membrane proteins from the ATP-binding cassette transporter family can release ATP into the ECM, and the overexpression of an ATP-binding cassette transporter family member MDR1 in Arabidopsis resulted in increased levels of ATP available on the surface of leaves compared to wild-type plants (Thomas et al., 2000). Secretion of vesicles containing ATP (Dubyak and El-Moatassim, 1993; Joseph et al., 2003) and the release of ATP in response to pathogens (McNamara et al., 2001) have been shown to be a source of eATP in animal systems, and these processes could operate in plants as well. Plant cells release ATP in response to various abiotic stresses, such as osmotic stress and cold stress, and mechanical stimulation although the mechanism mediating this release is unknown (Jeter et al., 2004).
Enhanced Expression of Apyrase Suppresses Nucleotide-Induced O2− Production
Our observation that transgenic plants OE apyrase show muted O2− production responses to applied ATP and to wounding suggests that increased apyrase expression lowers the effective [ATP] in the vicinity of the postulated receptors that respond to this agonist. Related studies in animal cells suggest that P2 nucleotide receptors and ectoapyrases occur together in a cell surface microenvironment, and that they compete there for the nucleotides that are released from cells during stress or mechanical stimulation (Joseph et al., 2003; Alvarado-Castillo et al., 2005). Key first steps toward testing whether this pairing occurs also in plants will be the identification of P2-like receptors in Arabidopsis, and the development of assays that can report the [ATP] in wall spaces just outside the cell membrane.
As observed in both animal systems and Arabidopsis (Jeter et al., 2004), applied ATPγS can mimic ATP in inducing cell responses, and it mimics ATP in inducing the production of O2− (Fig. 3C). Because ATPγS cannot be hydrolyzed by ectophosphatases in the ECM, it should be effective at lower doses than ATP in inducing responses and this has been observed in growth responses (Tang et al., 2003). Likewise, although 250 nm is below the threshold for ATP-induced O2− production, this concentration of ATPγS induces a significant O2− response (Fig. 3C). However, the effectiveness of ATPγS, unlike that of ATP, is not altered by overexpression of apyrase, as expected, since ATPγS is not a substrate for apyrase (Fig. 3C).
Our results showing the effects of apyrase overexpression on nucleotide-induced O2− production beg the question whether enhanced apyrase expression will also affect the ability of wound-released ATP to induce O2− production. Figure 3D answers this question in the affirmative, providing further support for the hypothesis that the ATP released at wound sites participates importantly in early steps of the wound-signaling cascade.
The fact that adenosine inhibits ATP-induced O2− production and changes in gene expression, suggests that it may act as a negative regulator of eATP signaling in plants as it does in animals. Ectophosphatases such as apyrase can hydrolyze ATP and ADP to AMP, and 5′ nucleotidase hydrolyzes AMP to adenosine (Zimmerman, 1996), so the duration of eATP signaling could be tightly regulated through the combined effects of degradation of eATP and subsequent negative feedback through adenosine.
Biphasic Accumulation of O2−
In the absence of any pathogens, elicitors, wounding, ozone, or mechanical stress, two temporally distinct peaks of eATP-induced O2− accumulation were observed (Fig. 1C). Biphasic peaks have been described in response to avirulent pathogens, ozone, wounding, and mechanical stress (Lamb and Dixon, 1997; Johnson et al., 2003; Razem and Bernards, 2003). In response to OGA, virulent pathogens, and avirulent pathogens, plants produce a nonspecific, weak, and transient burst of ROS fairly quickly after treatment, usually within 1 h called phase I (Lamb and Dixon, 1997). However, in the case of an avirulent pathogen, plants have a more prolonged and massive accumulation hours after inoculation, usually between 3 and 6 h, called phase II (Lamb and Dixon, 1997). Additionally, mechanical stress and wounding induce multiple peaks of O2− production, possibly associated with wound healing (Johnson et al., 2003; Razem and Bernards, 2003). The biphasic peak that we observed in response to ATP suggests a possible role for eATP in response to a stress, such as wounding, mechanical stress, or infection, although additional studies will have to be done to show a role for ATP in any of these responses. The possibility exists that the second peak of O2− accumulation may be a result of a second release of ATP to the ECM, which could occur during the growth associated with the wound-healing process. Plant cell growth typically requires the delivery of ATP-containing secretory vesicles to the plasma membrane, and the fusion of these vesicles with the membrane would release their ATP contents into the wall.
Role of NADPH Oxidase Subunits for O2− Accumulation
Our finding that the atrbohD/F mutant did not accumulate O2− in response to eATP treatment (Table I) indicates that the NADPH oxidase subunits, AtrbohD and AtrbohF, are required for the production of O2− induced by eATP. Also supporting this conclusion is our finding that the suicide substrate inhibitor of mammalian NADPH oxidase, DPI, suppressed the ATP-induced O2− accumulation. The AtrbohD and AtrbohF subunits and NADPH oxidase activity also appear to be needed for ROS-dependent ABA signaling in Arabidopsis (Kwak et al., 2003). It is likely that O2− accumulation is due to the production of O2− rather than decreased dismutation to H2O2, because the half life of O2− is 2 to 4 μs, and we measured the accumulation of O2− an hour after treatment. The fact that the atrbohD/F mutant showed reduced O2− accumulation after eATP treatment is also consistent with the conclusion that eATP affects O2− production rather than a decrease in dismutation to H2O2.
eATP Signal Transduction
In animal systems, nucleotides fulfill their roles as signals through binding to P2 receptors. Our observation that nucleotides induce O2− production and that two P2 receptor inhibitors, PPADS and RB2, prevent ATP induction of O2− and ATP-induced changes in gene expression is consistent with the hypothesis that in plants, eATP can act as a signal through interaction with a receptor at least functionally similar to the P2 receptors in animals. Moreover, animal P2 receptors are typically activated by a rather broad range of nucleotides (Ralevic and Burnstock, 1998) and our observation that ATP, GTP, UTP, and, to a lesser extent, CTP all induce significant O2− production (Table II) would suggest that potential receptors in plants would also be broadly responsive to nucleotides. However, to date, no P2-like receptors have been identified in plants. Clearly, the identification of some membrane-bound receptor with an external binding site for nucleotides would be necessary before eATP could be confirmed as a signaling molecule in plants.
P2 receptor signaling is mediated through [Ca2+]cyt increases in animals, and Ca2+ is an intermediate signal leading to the ATP-induced respiratory burst (Kuroki and Minakami, 1989; Dichmann et al., 2000). ATP also induces increases in [Ca2+]cyt in Arabidopsis (Demidchik et al., 2003; Jeter et al., 2004) and our data indicate that extracellular stores of Ca2+ are necessary for ATP-induced O2− accumulation (Fig. 4). Additionally, because W7 blocks induction of O2− accumulation by eATP treatment, these data suggest CaM activation is an intermediate signal in eATP-induced O2− accumulation.
Twelve mammalian P2 receptors with diverse affinities for different nucleotides have been characterized (Di Virgilio et al., 2001). The existence of multiple P2-like receptors in plants could help explain the two distinct peaks of O2− accumulation in response to different concentrations of ATP as well as the relatively less effective but still significant activity of ADP. In addition, the different homologs of the NADPH oxidase subunit gp91phox in Arabidopsis may respond differently to different concentrations of ATP or ADP.
Applied ATP induces the accumulation of transcripts of genes that are also induced by wounding or pathogen infection. Given the release of ATP into the ECM during wounding and the potential for ATP release in response to pathogen attack, our findings suggest that eATP could be an early signaling agent in the stimulus-response pathway leading from wounding and pathogen attack to increased mRNA levels for wound- and defense-response genes such as PAL1, LOX2, and ACS6. PAL1 is a gene involved in the wound or defense response of plants and expression of this gene is induced by ROS, which is accumulated downstream of physical injury or pathogen infection (Levine et al., 1994; Reymond et al., 2000; Desikan et al., 2001). ACS6 encodes the rate-limiting enzyme in the biosynthesis of ethylene (Wang et al., 2002) and, as recorded in Genevestigator (Zimmermann et al., 2004), is up-regulated 7-fold by ROS. LOX2 encodes a lipoxygenase critical for jasmonic acid synthesis, and its expression is induced by wounding (Bell and Mullet, 1993). The fact that eATP induces the expression of these genes that are implicated in wound signaling suggests that it could act upstream of jasmonic acid and ethylene in mediating the response of plants to wounding or possibly pathogen infection. Whether eATP induces the expression of all these genes through the mediation of O2− remains to be tested.
The report of Jeter et al. (2004) that the ability of ATP to induce the increased accumulation of transcripts for stress-regulated genes can be suppressed by gadolinium, a calcium channel blocker, highlighted the potential role of Δ[Ca2+]cyt in mediating the effects of ATP on gene expression. Similarly, our finding that PPADS and two other antagonists of P2-like receptors block the accumulation of transcripts for AtrbohD and PAL lends support to the idea that these ATP effects could be mediated by a detection system with pharmacological properties similar to the animal P2 receptors.
Previous studies have reported that O2− can induce programmed cell death (Levine et al., 1994; Jabs et al., 1996) and Chivasa et al. (2005) have found that the removal of ATP from the ECM also induces this response. Stimuli that induce O2− production do not always lead to the programmed cell death of the responding cells (Dorey et al., 1999; Mur et al., 2005) and Torres et al. (2005) report that NADPH oxidase-derived ROS actually prevent the spread of cell death in pathogen-challenged Arabidopsis leaves. Chivasa et al. (2005) do not claim that the programmed cell death response to the removal of eATP is mediated by O2− production. Moreover, whereas the programmed cell death response required more than 16 h to be detected, the O2− response to the presence of eATP is induced within minutes, and this timing difference makes comparison of the two findings problematic.
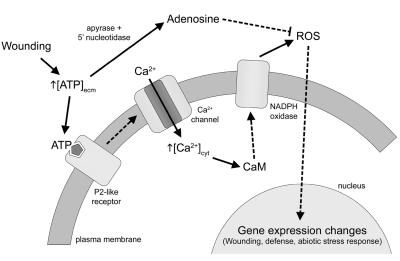
Hypothetical Pathway Linking eATP to O2− Production
We propose a speculative model for the induction of O2− production by eATP and its tight control by NADPH oxidase homologs in Arabidopsis (Fig. 7). The model depicts the ATP receptor and the linked calcium channel as conceptually two separate entities, but the receptor could also be a P2X type, which is itself a ligand-gated calcium channel. The model predicts that following a wound or other stimulus resulting in the disruption of the plasma membrane (Mehdy et al., 1996; Orozco-Cardenas et al., 2001) the ATP released binds to P2-like receptors, which leads to the mobilization of Ca2+, CaM activation, increased activity of NADPH oxidase, and increased O2− production, and it proposes that this sequence of events is terminated by the breakdown of ATP by ectoenzymes, such as apyrase and 5′ nucleotidase to adenosine, which antagonizes the ATP effects (Fig. 7; Zimmerman, 1996). Based on well-established precedents in animals, we speculate that the role of eATP as a signaling molecule in plants may go beyond the mediation of diverse responses to abiotic and biotic stresses to include functions in diverse developmental processes.
Figure 7.
Model for eATP signaling in Arabidopsis. The dashed lines indicate the likelihood of multiple intervening steps; the solid lines indicate a direct link between the two steps.
MATERIALS AND METHODS
Plant Material
For O2− assays, Arabidopsis (Arabidopsis thaliana) ecotype Wassilewskija or atrbohD/F double mutant and ecotype Columbia-0 were grown on Metro Mix 350 (Hummert) under continuous light for 4 to 5 weeks. The seeds for the atrbohD/F double mutant were obtained from J. Kwak (Kwak et al., 2003). For gene expression analysis, Arabidopsis seeds were sown on 1.5% agar plates containing Murashige and Skoog basal salt mixture (Sigma), 1% Suc, and 1× B5 vitamin mixture (1 mg/L nicotinic acid, 10 mg/L thiamine·HCl, 1 mg/L pyrodoxine·HCl, and 100 mg/L m-inositol). The seedlings were grown with the plates oriented vertically for 10 to 14 d under continuous light (54 μmol m−2 s−1). All experiments were performed on plants of the same age and grown under the same conditions.
O2− Detection
O2− accumulation was detected according to Jabs et al. (1996) by the reduction of Nitroblue tetrazolium (Fisher or Sigma). Leaves were boiled in 96% ethanol to clear the leaves (Sang et al., 2001) and stored in 70% ethanol until further analysis. This method was chosen over methods that detect H2O2 because there are other sources of H2O2 that are not dismutation products of O2−, so measuring O2− accumulation directly is a better indication of the NADPH oxidase activity.
ATP, ADP, and AMP Dose Treatments
Rosette leaves were pressure infiltrated using a syringe with no needle with 1, 5, 10, 50, or 100 μm ATP, ADP, or AMP. PB alone was infiltrated as the negative control and OGA (10 μg/mL) as the positive control. All leaves were incubated for 1 h at room temperature. We chose 1 h to ensure uniformity of treatment conditions. The leaves were cut off immediately and immersed in 10 mm potassium buffer and 10 mm NaN3, then O2− was detected as described above in “O2− Detection.” OGA was obtained from M. Mehdy.
For RNA analysis, whole seedlings were submerged gently in 10 mL of MES pH 5.7 (0.5 g/L) alone, and 1, 5, 50, or 100 μm ATP. ATP solutions were dissolved in MES pH 5.7. After submerging the seedlings, they were vacuum infiltrated for 30 s and the vacuum was broken quickly. The seedlings were treated for 30 min and the solutions were poured out. The seedlings were collected at 30, 60, 90, and 180 min after the initial treatment and immediately frozen in liquid nitrogen and stored at −80°C for RNA isolation.
Time Course
Arabidopsis rosette leaves were infiltrated with 50 μm ATP dissolved in PB pH 7.5 (0.16 mm KH2PO4, 1.1 mm K2HPO4), PB alone, or xanthine (2 mm; Sigma)/xanthine oxidase (5 units; Sigma) as the positive control. Leaves were cut from the plant after 3, 30, 60, 120, 240, and 480 min and immediately immersed in 10 mm potassium buffer and 10 mm NaN3. O2− was detected as described in “O2− Detection.”
atrbohD/F Double Knockout ATP Treatments
Full-grown atrbohD/F double knockout rosette leaves or wild-type Columbia-0 rosette leaves were infiltrated with either 50 μm ATP in PB or PB alone. Leaves were incubated at room temperature for 1 h, cut off the plants, and immersed in 10 mm potassium buffer and 10 mm NaN3. O2− was detected as described above in “O2− Detection.”
ATP Measurements at Wound Sites
Rosette leaves of mature Arabidopsis plants were detached, placed on a microscope slide, and wounded with a micropipette. Wounds were typically 3 to 4 mm long at the edge of the leaf and cut completely through the leaf.
Fluid from the wound site was collected with a micropipette positioned with a manual micromanipulator. The volume of fluid collected was calculated from the height of column of fluid in the micropipette and the measured dimensions of the tip of the micropipette. Fluid volumes typically ranged between 0.1 and 7.0 nL. Immediately after collection, the tip of the micropipette was snapped off in a 1.5 mL microcentrifuge tube and plunged in liquid N2. Typically, less than 3 min elapsed between wounding and freezing of the collected sample. A new wound site was created for each fluid collection, although the same leaf was used for more than one wound. Two to four collections were pooled together in the same microcentrifuge tube and stored at −80°C for [ATP] determination.
The concentration of ATP present in each of the samples was determined using a bioluminescent detection reagent (ENLITEN rLuciferase/Luciferin; Promega). Frozen pooled collections were resuspended in 10 mL of buffer (10 mm HEPES, pH 7.7), vortexed, spun down quickly, and transferred to a 12 × 50 mm test tube for measurement. Fifty microliters of rLuciferase/Luciferin reagent was added to the resuspended sample and luminescence was measured in a luminometer (Turner Designs 20/20; Turner BioSystems) using a 2 s delay and a 10 s integration. The amount of ATP present in the sample was calculated from the measured relative light units using a standard curve spanning the relative light unit range obtained from the samples.
The accuracy of this approach was validated by sampling from source pipettes containing known ATP standards. The methods for collection and concentration determination were the same as described above for the wound samples and the calculated concentrations were compared against the known concentrations of the sample.
Inhibitor Treatments
Rosette leaves were infiltrated with PB alone, 50 μm ATP, 50 μm ATP plus inhibitor, or inhibitor alone. The leaves were incubated at room temperature for 1 h, cut, and immersed in 10 mm potassium buffer and 10 mm NaN3. O2− was detected as described in “O2− Detection.” The inhibitors used were 250 μm DPI, 250 μm PPADS, 30 μm RB2, 10 μm adenosine, 1 mm lanthanum chloride (LaCl3), 1 mm 1,2-Bis(2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetraacetic acid, 50 μm W7, and 50 μm W5. All inhibitors were dissolved in distilled deionized water except DPI, which was dissolved in dimethyl sulfoxide, and for this treatment a dimethyl sulfoxide-only control was added. All inhibitors were obtained from Sigma except for W5 (Calbiochem).
Seedlings were pretreated with inhibitor or MES. Ten milliliter solutions of MES or inhibitor (PPADS, RB, or adenosine) were poured gently into the petri dishes containing seedlings. They were briefly vacuum infiltrated (30 s) and the vacuum was broken quickly. The seedlings incubated in the solutions for 10 min, and the solutions were poured out. Treatments of either 50 μm ATP or MES for the negative control were added to the seedlings. The seedlings were again vacuum infiltrated for a brief time (30 s) and incubated in the solutions for 30 min. The solutions were poured out, and the seedlings were collected at 30, 60, 90, and 180 min. The seedlings were immediately frozen in liquid nitrogen and stored at −80°C for RNA isolation.
RNA Isolation and Northern Analysis
RNA was isolated using the RNeasy kit (Qiagen) according to the manufacturer's instructions. Ten micrograms of RNA was denatured by incubation with a glyoxyl loading dye (Ambion) for 30 min at 50°C. All of the RNA was separated by electrophoresis and transferred onto a Bright star nylon membrane (Ambion). The RNA was cross-linked to the membrane using short UV light for 2 min. The northern analysis was done using the northern Max-Gly kit (Ambion) according to the manufacturer's instructions. Radioactivity was detected using a Phosphorimager (model 445SI; Molecular Dynamics). Specific cDNA probes for AtrbohD, PAL1, LOX2, or ACS6 were hybridized to the membranes. Probes were randomly labeled with dCTP-α32P (NEN-Perkin Elmer) using the DECAprime II kit (Ambion) according to the manufacturer's instructions. The primers used for AtrbohD (AF055357) were 5′-CACGCACTCAAAGGTCTCAAG-3′ (forward) and 5′-CAGACGAAAGCTTTGATGCC-3′ (reverse). The primers used for PAL1 (NM_129260) were 5′-GGAGCTCCCATTCCAATATG-3′ (forward) and 5′-GAAGAAGGTATGATTCACAC-3′ (reverse). The primers for LOX2 (L23968) were 5′-TATTGTAGAGAGTCCTTGTCG-3′ (forward) and 5′-GACCAAGTTATGCCCTCCAG-3′ (reverse). The primers for ACS6 (NM_117199) were 5′-GGTTAAAGGCCAAAGCCGGT-3′ (forward) and 5′-GGCGAATGAGGCGAGAAGAA-3′ (reverse). For ACS6, dCTP-α32P was directly incorporated into the cDNA during PCR.
Construction of Transgenic Lines OE Atapy2
The AtAPY2 cDNA (GenBank accession no. AF141671) existed as an insert in the TA cloning site of the vector pCR2.1 (Invitrogen; Steinebrunner et al., 2000). The cDNA was released as an EcoRI fragment and ligated into the binary vector pLBJ21 in sense orientation downstream of the 35S promoter from Cauliflower mosaic virus. Several independent transgenic lines of the ecotype Wassilewskija were selected on kanamycin.
Computer Analysis
All leaves were analyzed with ImageJ (http://rsb.info.nih.gov/ij/index.html). Stained areas were measured by measuring pixels of staining and divided by total leaf area to normalize from leaf to leaf. This ratio was multiplied by 100 to obtain the percentage of stained leaf area.
The northern analysis with inhibitor treatments were also analyzed using Image J. The areas of the bands from the northern image were measured, as well as the bands from the equal loading gel. The expression levels were adjusted according to the equal loading bands. The MES buffer treatment was designated as 100 and the other treatments are relative to the MES buffer. The resulting graphs show the relative expression levels.
Statistical analyses were done using the Student's t-test in Microsoft Excel. Error bars are all sd.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF141671 (AtAPY1), AF055357 (AtrbohD), L23968 (LOX2), NM_129260 (PAL1), and NM_117199 (ACS6).
Acknowledgments
We thank Dr. M. Mehdy for the OGA and for thoughtful discussions about the manuscript and research, T. Butterfield for independent confirmation of [ATP] at wound sites, and Dr. W. Tang for sharing primers and labeled probe for ACS6.
This work was supported by grants from the National Science Foundation (IBN–0080363 and IBN–0344221 to S.J.R.) and from the National Aeronautics and Space Administration (NGT5–50371 to S.C.S.). C.J.S. was supported in part by a grant to June M. Kwak from the National Research Initiative of the U.S. Department of Agriculture (2004–35100–14909).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Stanley J. Roux (sroux@uts.cc.utexas.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073072.
References
- Alvarado-Castillo C, Harden TK, Boyer JL (2005) Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol 67: 114–122 [DOI] [PubMed] [Google Scholar]
- Amicucci E, Gaschler K, Ward JM (1999) NADPH oxidase genes from tomato (Lycopersicon esculentum) and curly leaf pondweed (Potamogeton crispus). Plant Biol 1: 524–528 [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Auh CK, Murphy TM (1995) Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol 107: 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Mullet JE (1993) Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol 103: 1133–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T, Zalavary S, Stendahl O, Grenegard M (1996) Release of oxygen metabolites from chemoattractant-stimulated neutrophils is inhibited by resting platelets: role of extracellular adenosine and actin polymerization. Blood 87: 4411–4423 [PubMed] [Google Scholar]
- Bergey DR, Howe GA, Ryan CA (1996) Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA 93: 12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Walenta S, Panitz R, Wobus U, Weber H (2003) Energy status and its control on embryogenesis of legumes: ATP distribution within Vicia faba embryos is developmentally regulated and correlated with photosynthetic capacity. Plant J 36: 318–330 [DOI] [PubMed] [Google Scholar]
- Cabrera RM, Saltveit ME (2003) Survey of wound-induced ethylene production by excised root segments. Physiol Plant 119: 203–210 [Google Scholar]
- Chivasa S, Ndimba B, Simon WJ, Lindsey K, Slabas AR (2005) Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 17: 3019–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE (1992) Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA 89: 4938–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Rosenstein ED, Kramer SB, Weissman G, Hirschhorn R (1985) Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils: Adenosine acts via an A2 receptor on human neutrophils. J Immunol 135: 1366–1371 [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM (2003) Is ATP a signaling agent in plants? Plant Physiol 133: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, Luttman W, Virchow C, Di Virgilio F, Norgauer J (2000) Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca2+ mobilization and actin reorganization in human eosinophils. Blood 95: 973–978 [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97: 587–600 [DOI] [PubMed] [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S (1999) Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol 121: 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C (1993) Signal transduction via P-2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol 265: C577–C606 [DOI] [PubMed] [Google Scholar]
- Felton GW, Summers CB, Mueller AJ (1994) Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J Chem Ecol 20: 639–650 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M (1993) Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta 190: 446–453 [Google Scholar]
- Gout E, Bligny R, Douce R (1992) Regulation of intracellular pH values in higher plant cells. J Biol Chem 267: 13903–13909 [PubMed] [Google Scholar]
- Groom QL, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG (1996) rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J 10: 515–522 [DOI] [PubMed] [Google Scholar]
- Henderson LM, Chappell JB (1996) NADPH oxidase of neutrophils. Biochim Biophys Acta 1273: 87–107 [DOI] [PubMed] [Google Scholar]
- Hu XY, Neill SJ, Cai WM, Tang ZC (2004) Induction of defence gene expression by oligogalacturonic acid requires increases in both cytosolic calcium and hydrogen peroxide in Arabidopsis thaliana. Cell Res 14: 234–240 [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273: 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16: 2652–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Doherty SJ, Croy RRD (2003) Biphasic superoxide generation in potato tubers: a self-amplifying response to stress. Plant Physiol 131: 1440–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR (2003) Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278: 23331–23342 [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Read ND, Campbell AK, Trewavas AJ (1993) Imaging calcium dynamics in living plants using semi-synthetic recombinant aequorins. J Cell Biol 121: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M, Minakami S (1989) Extracellular ATP triggers superoxide production in human neutrophils. Biochem Biophys Res Commun 162: 377–380 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Titarenko E, Sanchez-Serrano JJ (1998) Jasmonic acid-dependent and independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana. Mol Gen Genet 258: 412–419 [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Sanchez-Serrano JJ (2001) Wound signalling in plants. J Exp Bot 52: 1–9 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon RA, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Lew RR, Dearnaley JDW (2000) Extracellular nucleotide effects on the electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci 153: 1–6 [Google Scholar]
- McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C (2001) ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci USA 98: 9086–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW (1996) The role of activated oxygen species in plant disease resistance. Physiol Plant 98: 365–374 [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Draper J (2005) In planta measurements of oxidative bursts elicited by avirulent and virulent bacterial pathogens suggests that H2O2 is insufficient to elicit cell death in tobacco. Plant Cell Environ 28: 548–561 [Google Scholar]
- Nishiuchi T, Suzuki K, Kitajima S, Sato F, Shinshi H (2002) Wounding activates immediate early transcription of genes for ERFs in tobacco plants. Plant Mol Biol 49: 473–482 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for induction of defense genes in tomato plants in response to wounding, systemin, methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G (2005) Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res 33: 4379–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar VK, Lamb C, Dixon RA (1999) Early events in the signal pathway for the oxidative burst in soybean cells exposed to avirulent Pseudomonas syringae pv glycinea. Plant Physiol 120: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakwal R, Agrawal GK (2003) Wound signaling-coordination of the octadecanoid and MAPK pathways. Plant Physiol Biochem 41: 855–861 [Google Scholar]
- Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492 [PubMed] [Google Scholar]
- Razem FA, Bernards MA (2003) Reactive oxygen species production in association with suberization: evidence for an NADPH-dependent oxidase. J Exp Bot 54: 935–941 [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404–411 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Redman R (2005) Balancing the generation and elimination of reactive oxygen species. Proc Natl Acad Sci USA 102: 3175–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16: 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Cui D, Wang X (2001) Phospholipase D and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiol 126: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz BJ, Mudgett MB, Dangl JL, Galen JE (2001) Common and contrasting themes of plant and animal diseases. Science 292: 2285–2289 [DOI] [PubMed] [Google Scholar]
- Steinebrunner I, Jeter C, Song C, Roux SJ (2000) Molecular and biochemical comparison of two different apyrases from Arabidopsis thaliana. Plant Physiol Biochem 38: 913–922 [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ (2003) Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 131: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Brady SR, Sun Y, Muday GK, Roux SJ (2003) Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol 131: 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ (2000) A role for ectophosphatase in xenobiotic resistance. Plant Cell 12: 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp 91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais PV (2002) The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell (Suppl) 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell (Suppl) 14: S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H (1996) Extracellular purine metabolism. Drug Dev Res 39: 337–352 [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]