Abstract
It has been shown that fluorescence resonance energy transfer (FRET)-based PCR, including the TaqMan assay and molecular beacons, has potential for rapid detection of pathogens. In these promising real-time detection assays a single internal oligonucleotide probe labeled on both the 5′ (reporter) and 3′ (quencher) ends is used for selective generation of fluorescence. In this paper, we describe the use of a previously reported novel probe design for FRET-based PCR detection of Listeria monocytogenes in pure culture and in a model food commodity. In the assay described here an asymmetric probe set is used; this probe set consists of a long 5′ fluorescein-labeled reporter probe and a short, complementary 3′ DABCYL-labeled quencher oligonucleotide, which are used in a 5′ nuclease amplification and detection assay. By using the listeriolysin O (hly) and p60 (iap) genes as amplification targets, the performance of two primer-probe sets in amplification and subsequent detection of target DNA was evaluated. In studies performed with pure cultures of L. monocytogenes, the PCR profiles indicated that the relative change in fluorescence intensity was correlated with both the initial number of cells and the accumulation of specific amplicons for both hly and iap gene fragments. Experiments were also done to determine the applicability of the method to the detection of L. monocytogenes by targeting hly DNA and its short-lived mRNA product in a model food commodity. Twenty-five-milliliter samples of reconstituted nonfat dry milk (NFDM) were seeded with L. monocytogenes and processed to concentrate the bacteria by centrifugation, and this was followed by nucleic acid extraction and amplification with hly-specific primers. Endpoint detection of PCR and reverse transcription-PCR amplicons could be achieved at inoculum levels of 103 and 104 CFU of L. monocytogenes/25 ml of NFDM, respectively. This study demonstrated that this asymmetric FRET-based amplification and detection protocol provides an alternative approach for endpoint detection of nucleic acid amplification products as applied to detection of pathogens in a model food.
With the introduction of molecular biological detection methods, such as the PCR, food microbiologists had high hopes that methods would soon be available to overcome some of the common difficulties associated with the detection of pathogens in foods. Of particular interest was the potential to replace time-consuming cultural enrichment steps with specific nucleic acid sequence enrichment, thereby decreasing the total detection time. There have been many reports of PCR-based assays for detection of food-borne pathogens (for a review see reference 27), and several companies market these systems, some of which have been certified recently by the Association of Official Analytical Chemists (23, 24). Unfortunately, all of these methods require prior cultural enrichment, and hence the term rapid remains, in part, a misnomer.
An important rate-limiting step for the PCR lies in the fact that amplification products are detected most commonly by postamplification agarose gel electrophoresis and/or DNA hybridization. In an effort to reduce the time necessary for detection and confirmation, a number of investigators have described methods to simplify or even eliminate the need to perform postamplification assays. For example, molybdate (7) and some intercalating agents (9) have been used to measure the degree of DNA amplification, but these protocols are limited because they do not consider amplicon sequence specificity. Other investigators have focused their efforts on adapting the fluorescence resonance energy transfer (FRET) phenomenon to the detection of specific PCR amplicons, including AmpliSensor (5), molecular beacons (16, 25), Amplifluor (17), Scorpions primers (26), and adjacent hybridization probes (29). Of particular note in recent years is the TaqMan assay (Applied Biosystems, Foster City, Calif.), which has been applied to the detection of food-borne pathogens, including Listeria monocytogenes (1, 6, 8, 19) and Escherichia coli O157:H7 (20, 21, 22, 28).
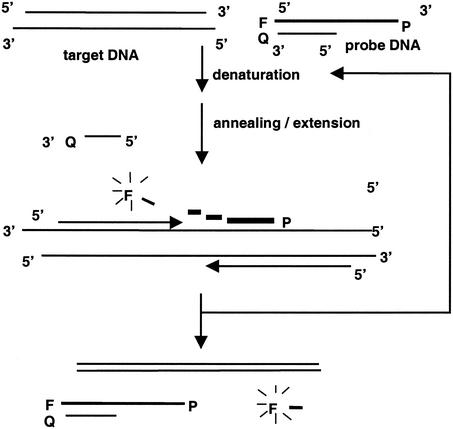
Although many variations of the FRET concept have been reported, in most of them a single oligonucleotide labeled on both the 5′ (reporter) and 3′ (quencher) ends is used for selective generation of fluorescence. In an effort to reduce the development and assay costs associated with FRET-based PCR assays, we (14) described the use of an inexpensive asymmetric probe set which consists of a long 5′ fluorescein-labeled (reporter) oligonucleotide and a short 3′ 4-[4′-dimethylaminophenylazo]benzoic acid-labeled (quencher) oligonucleotide (Fig. 1). These probes are able to form an asymmetrical double-stranded conformation at room temperature such that the 5′ fluorescein-labeled probe is quenched by the nearby quencher oligonucleotide. During the normal annealing-primer extension step of the PCR, the reporter probe molecules are available to hybridize with the target amplicons, and the fluorescein label is digested by the 5′ nuclease activity of Taq DNA polymerase (10). After the DNA amplification is complete, the residual reporter probe reanneals to the short quencher oligonucleotide at room temperature. The increase in fluorescence at the end of the assay indicates that specific amplicons are produced. Although this assay is not a real-time assay in the sense that the detection of PCR products occurs simultaneously as the PCR is progressing, it is, however, an endpoint detection assay in that specific PCR products can be detected by fluorescence immediately following termination of the PCR. As such, this protocol takes advantage of the lower cost of single labeling (approximately $225 for 250 nmol of the single-label probe versus $400 for the same amount of the dual-label probe) and provides a promising alternative for rapid and efficient endpoint detection of PCR amplicons in a single reaction tube.
FIG. 1.
Detection of PCR products by using 5′ nuclease activity and an asymmetric fluorogenic probe set. P, probe; F, fluorescein label; Q, quencher.
The purpose of this study was to adapt the previously described asymmetric FRET-based PCR assay to amplification and detection of L. monocytogenes in a model food commodity. The listeriolysin O (hly) and p60 (iap) genes were chosen as the amplification targets. The performance of primer-probe sets in amplification and subsequent detection of target DNA was evaluated. Experiments were also done to determine the applicability of the method for detection of L. monocytogenes by targeting hly DNA and its short-lived mRNA product in a model food commodity.
MATERIALS AND METHODS
Bacterial strains.
Six Listeria strains (L. monocytogenes ATCC 19115, L. innocua ATCC 33091, L. ivanovii ATCC 19119, L. grayi ATCC 25401, L. seeligeri ATCC 35967, and L. welshimeri ATCC 35897) were used in various experiments to evaluate gene amplification efficiency and primer specificity. A single colony of each Listeria strain was resuspended in 0.3 ml of double-distilled water, and 1 μl of each cell suspension was used in initial PCR optimization experiments, as well as for evaluation of primer specificity.
Isolation of L. monocytogenes nucleic acids from NFDM.
Twenty-four-milliliter samples of reconstituted nonfat dry milk (NFDM) were each inoculated with 1 ml of a serially diluted overnight culture of L. monocytogenes to obtain a final concentration of 1 × 102 to 1 × 105 CFU/25 ml. The samples were pretreated by adding 1.5 ml of 25% (wt/vol) sodium citrate with 10 min of shaking at room temperature, as previously reported (15). After this, cells were collected by centrifugation at 10,000 × g for 10 min at 4°C and extracted for DNA or RNA isolation.
For isolation of DNA, the pellet was resuspended in 0.1 ml of 1% sodium dodecyl sulfate and solubilized by adding 0.4 ml of 60% (wt/vol) guanidinium thiocyanate. The DNA was extracted by adding 0.5 ml of phenol-chloroform-isoamyl alcohol (25:24:1) and was precipitated by using isopropanol. The air-dried DNA pellet was resuspended in 25 μl of distilled water; 10 μl of a reconstituted DNA suspension was used in each PCR amplification.
For RNA extraction, the cell pellet was resuspended in 0.1 ml of 1% sodium dodecyl sulfate-0.5% diethyl pyrocarbonate and solubilized by adding 0.4 ml of 60% (wt/vol) guanidinium thiocyanate and 40 μl of 3 M sodium acetate (pH 4.8). The RNA was extracted by adding 0.4 ml of water-saturated phenol and 0.2 ml of chloroform. To enhance the efficiency of recovery of RNA after isopropanol precipitation, 10 μg of yeast tRNA (Sigma, St. Louis, Mo.) was added as a coprecipitant. The air-dried RNA pellet was resuspended in 30 μl of distilled water; 5 μl of reconstituted RNA was used in each reverse transcription (RT) reaction. Because of the special design of the RT-PCR primer set (13), RNase-free DNase treatment of RNA extracts was not necessary prior to RT.
Oligonucleotide primers and probes.
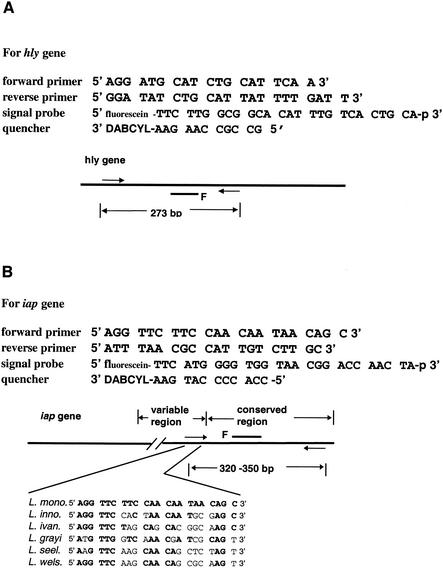
The sequences of amplification primers and detection probes and the sizes of amplicons are shown in Fig. 2. The oligonucleotides were designed based on DNA sequence data from GenBank and information previously published by Bassler et al. (1) and Bubert et al. (3). To reduce false-positive signals in RT-PCR due to the presence of residual genomic DNA, the reverse primer used for RT-PCR targeting of the hly mRNA was modified by incorporating three consecutive mismatched bases near its 3′ terminus, as previously reported (13). The oligonucleotide primers and fluorescein-labeled probes were synthesized by Integrated DNA Technologies, Inc. (Coralville, Iowa).
FIG. 2.
Oligonucleotide primer and probe sets used for amplification of hly and iap gene-specific fragments by PCR and RT-PCR. The hly-specific primer-probe set was designed on the basis of data obtained from GenBank (accession numbers U25443, U25446, U25449, and U25452) and previously published information (1). The iap-specific primer set was also designed on the basis of data obtained from GenBank (accession numbers X52268, M80348, M80349, M80350, M80351, M80352, and M80353) and previous reports (3).
Synthesis of cDNA and amplification of target nucleic acids.
A 10-μl RT mixture containing 4 U of avian myeloblastosis virus reverse transcriptase, 10 U of RNasin (Promega, Madison, Wis.), 2 μl of 5× RT buffer (250 mM Tris [pH 8.3], 150 mM KCl, 40 mM MgCl2, 20 mM dithiothreitol), 5 μl of RNA from NFDM samples, 0.25 mM dATP, 0.25 mM dCTP, 0.25 mM dGTP, 0.25 mM dTTP, and 1 pmol of RT primer was incubated at 45°C for 1 h and then at 94°C for 5 min to inactive the enzymes.
For amplification of target gene fragments, 40 μl of a PCR solution was mixed with 10 μl of a genomic DNA extract or RT mixture for further amplification. The 50-μl PCR mixture, which contained 1× PCR buffer, 3 mM MgCl2, 0.2 mM dATP, 0.2 mM dCTP, 0.2 mM dGTP, 0.4 mM dUTP (PCR) or 0.3 mM dUTP plus 0.05 mM dTTP (RT-PCR), 10 pmol of each forward and reverse primer, 5 pmol of each reporter and quencher probe, 1 U of Taq DNA polymerase, 1 U of anti-Taq antibody (Invitrogen, Carlsbad, Calif.), and 0.2 U of uracil-N-glycosylase, was used for amplification. To digest possible carryover contaminating DNA by uracil-N-glycosylase and to denature the target template, 1 cycle of 45°C for 5 min and 94°C for 5 min was used. After this, the DNA was amplified by using 40 cycles of 94°C for 30 s, 60°C (in some experiments, an annealing temperature of 58°C was also tested) for 30 s, and 72°C for 45 s. The PCR was carried out with a GeneAmp PCR system 9600 (Applied Biosystems). Each PCR tube was removed from the thermal cycler after completion of amplification and was equilibrated to room temperature. Forty microliters of each PCR solution was transferred into a 96-well microtiter plate, and the fluorescence was measured (excitation wavelength, 485 nm; emission wavelength, 530 nm) with a CytoFluor II fluorescence plate reader (PerSeptive Biosystems, Framingham, Mass.). Amplification was confirmed by agarose (1%) gel electrophoresis and ethidium bromide (EtBr) staining.
RESULTS
Detection of L. monocytogenes DNA from pure cultures.
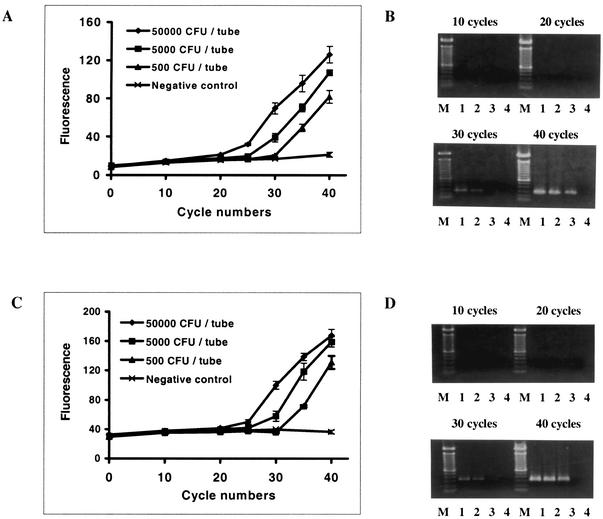
In a previous study (14), we reported that the amplification profile obtained with the asymmetric probe set-coupled PCR indicated that there was an overall increase in fluorescence at the end of the assay that reflected the initial copy number of positive control plasmid DNA. In this study, L. monocytogenes was used as the template for direct amplification of target DNA fragments. The PCR profiles (Fig. 3A and C) and agarose gel electrophoresis (Fig. 3B and D) revealed changes in fluorescence intensity that were correlated with the initial number of cells and with the accumulation of specific amplicons for both hly and iap gene fragments, and the detection limits were ≥500 CFU/reaction mixture.
FIG. 3.
Amplification of hly (A and B) and iap (C and D) genes from pure cultures of L. monocytogenes. (A and C) PCR profiles for hly gene amplification and iap gene amplification, respectively, as measured with a fluorescence plate reader. The values are means ± standard deviations for three replicate samples. (B and D) Agarose (1%) gel electrophoresis and EtBr staining of PCR products for hly gene amplification (273 bp) and iap gene amplification (330 bp), respectively. Lane M, 100-bp DNA ladder; lanes 1 to 4, PCR products amplified from suspensions corresponding to 5 × 104, 5 × 103, 5 × 102, and 0 CFU of L. monocytogenes per reaction mixture, respectively.
Primer specificity tests.
The hly and iap primer-probe sets were further tested for their ability to discriminate L. monocytogenes from five different species in the genus Listeria (Table 1). The hly gene fragment-specific primer set showed excellent specificity for L. monocytogenes. This primer set generated the specific amplicon solely from the DNA of L. monocytogenes at annealing temperatures as low as 58°C. At the same annealing temperature, the iap-specific primer-probe set produced a very faint nonspecific 440-bp amplicon for DNA obtained from an L. ivanovii culture (data not shown). When the DNA sequences of L. ivanovii (GenBank accession number M80350) were compared to the sequence of the forward iap primer, it was found that the nonspecific amplification was most likely caused by a short region exhibiting a high degree of sequence similarity (10 of 12 bases from the 3′ end of primer) with this primer. However, this weak nonspecific amplification could be eliminated by increasing the annealing temperature to 60°C (Table 1).
TABLE 1.
Primer specificity for amplification of gene fragments from L. monocytogenes compared to other species of the genus Listeria
| Gene target | Annealing temp (°C) | Strain | Amplicon size (bp) | Fluorescencea | Gelb |
|---|---|---|---|---|---|
| hly | 58 | L. monocytogenes ATCC 19115 | 273 | 115 | + |
| L. innocua ATCC 33091 | 28 | − | |||
| L. ivanovii ATCC 19119 | 27 | − | |||
| L. grayi ATCC 25401 | 30 | − | |||
| L. seeligeri ATCC 35967 | 32 | ||||
| L. welshimeri ATCC 35897 | 30 | − | |||
| Water (negative control) | 26 | − | |||
| iap | 60 | L. monocytogenes ATCC 19115 | 330 | 189 | + |
| L. innocua ATCC 33091 | 45 | − | |||
| L. ivanovii ATCC 19119 | 43 | − | |||
| L. grayi ATCC 25401 | 44 | − | |||
| L. seeligeri ATCC 35967 | 43 | − | |||
| L. welshimeri ATCC 35897 | 46 | − | |||
| Water (negative control) | 40 | − |
The values are fluorescence values (excitation wavelength, 485 nm; emission wavelength. 530 nm) obtained with a CytoFluor II plate reader.
+ or − indicates the presence or absence, respectively, of bands of the expected size as determined by agarose gel electrophoresis and EtBr staining.
Amplification of L. monocytogenes DNA from NFDM samples.
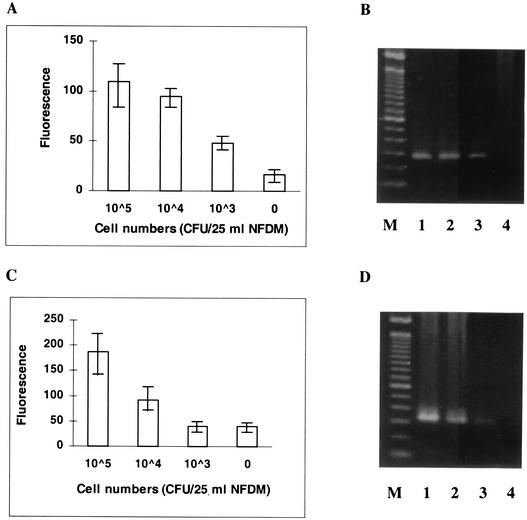
As described above, DNA of L. monocytogenes could be amplified, detected, and confirmed efficiently from pure cultures of L. monocytogenes by using the asymmetric probe set. However, amplification and confirmation of the same gene fragments from a more complex sample matrix remain challenging due to the presence of potential inhibitors and competitors of the PCR. Amplification of DNA isolated from L. monocytogenes obtained from seeded NFDM samples was tested as a model system. The results showed that the target DNA extracted from 1,000 CFU seeded in 25 ml of reconstituted NFDM could be specifically amplified and detected either by agarose gel electrophoresis or by changes in fluorescence intensity by using the hly-specific primer-probe set (Fig. 4A and B). The iap-specific primer-probe set generated higher background fluorescence (noise), and use of this set decreased detection sensitivity by at least 1 log (Fig. 4C and D).
FIG. 4.
Detection of L. monocytogenes hly (A and B) and iap (C and D) genes (genomic DNA) from food samples. (A and C) Fluorescence from hly-specific amplification and iap-specific amplification, respectively. The values are means ± standard deviations for three replicate samples. (B and D) Agarose (1%) gel electrophoresis and EtBr staining of PCR amplicons for hly gene amplification (273 bp) and iap gene amplification (330 bp), respectively. Lane M, 100-bp DNA ladder; lanes 1 to 4, PCR products amplified from DNA extracts corresponding to 105, 104, 103, and 0 CFU of L. monocytogenes/25-ml NFDM sample, respectively.
Amplification of L. monocytogenes mRNA from NFDM samples.
Recent studies have demonstrated a better correlation between the presence and amplification of bacterial mRNA and cell viability (12, 19). In this study, we used a modified DNase treatment-free RT protocol (13) along with the asymmetric probe set to detect bacterial mRNA in the NFDM food matrix. The results revealed that the hly mRNA obtained from an NFDM sample containing 10,000 CFU/25 ml could be detected and that false-positive signals generated by the amplification of residual DNA were inhibited (Fig. 5). Furthermore, yeast tRNA, the coprecipitant added to improve the recovery the mRNA, did not interfere with the amplification of specific cDNA under these RT-PCR amplification conditions (data not shown). These experiments also demonstrated the ability of the asymmetric probe set to confirm the presence of specific amplicons (273 bp) even in the presence of relatively high copy numbers of nonspecific amplicons (approximately 200 bp) (Fig. 5B, lanes 1 to 5).
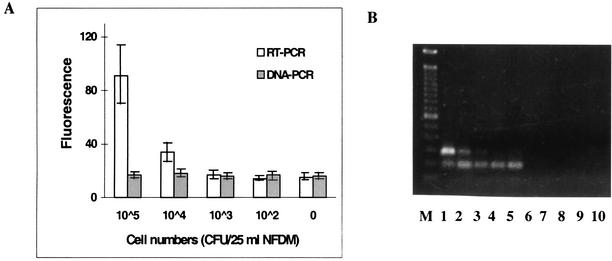
FIG. 5.
Detection of L. monocytogenes hly gene product (mRNA) from food samples. (A) Fluorescence from hly-specific amplification of RNA extracts obtained from seeded 25-ml NFDM samples amplified by RT-PCR (open bars) and PCR (shaded bars). The values are means ± standard deviations for three replicate samples. (B) Agarose (1%) gel electrophoresis and EtBr staining of amplicons (273 bp). Lane M contained a 100-bp DNA ladder. The amplicons of RT-PCR (lanes 1 to 5) and PCR (lanes 6 to 10) were obtained by amplification performed with RNA extracts corresponding to 105, 104, 103, 102, and 0 CFU of L. monocytogenes/25-ml NFDM sample, respectively.
DISCUSSION
In the first report on the use of a fluorogenic PCR-based assay for the detection of L. monocytogenes, Bassler et al. (1) described the 5′ nuclease TaqMan assay targeting the hly gene, which detected as few as 50 to 100 CFU per amplification reaction mixture in less than 3 h. In another report Nogva et al. cited detection limits as low as 6 to 60 CFU per reaction mixture (18), although these authors noted that the DNA extraction efficiency can negatively affect test sensitivity when the test is performed with cell concentrations that are <500 CFU per reaction mixture. Norton and Batt (19) described a single-tube reverse transcriptase fluorogenic probe-based TaqMan assay targeting hlyA mRNA of L. monocytogenes, although no specific detection limits were cited. Most recently, Hein et al. (8) described use of the iap gene to discriminate between L. monocytogenes and L. innocua in a quantitative PCR assay, demonstrating a detection limit of six gene copies. These studies and others indicate that the most commonly used PCR amplification targets for the detection of L. monocytogenes are the listeriolysin O gene (hlyA) and the protein p60 gene (iap). Although Klein and Juneja (12) reported that targeting the iap gene-specific mRNA yielded the best detection limits with RT-PCR (compared to mRNA from the hlyA and prfA genes), we obtained better detection limits by targeting the hlyA gene product. With PCR detection limits for pure cultures in the range of 500 CFU/reaction mixture, our assay is slightly less sensitive than those described by other workers (1, 18). However, the detection limits and the amplification specificity of both PCR and RT-PCR have been improved recently by adjustments to PCR conditions and nucleic acid extraction protocols in our laboratory (data not shown), and we are hopeful that with further optimization, the detection performance can be further improved. Furthermore, we have also illustrated that our method is applicable to detection of mRNA and can be applied to a model food matrix.
There are still important issues that must be addressed if rapid fluorogenically based detection of food-borne pathogens is to become a routine reality. Critical methodological issues include, but are not limited to, the following: (i) the need to utilize large realistic sample sizes (≥25 ml or ≥25 g) to obtain bacterial nucleic acid, compared to requisite PCR amplification volumes (10 to 50 μl); (ii) the presence of low levels of contaminating pathogens; (iii) the need to assure that nonviable cells are not detected; and (iv) the need to confirm PCR amplification products with more lengthy DNA hybridization assays (2, 27). While in many studies the workers have reported development of PCR and RT-PCR protocols for pure cultures, few researchers have addressed the critical issue of food sample preparation prior to detection. As with the commercial PCR detection kits for food-borne pathogens (23, 24), in almost all other studies the workers also report the need for cultural enrichment prior to nucleic acid extraction and detection in order to achieve detection limits approaching 1 CFU/25-g food sample (4, 12, 18, 20). In the case of fluorogenic PCR detection of food-borne pathogens, the usual approach is either to extract the nucleic acids from a 1-ml portion of an enrichment culture (4, 6) or to precede the extraction with a paramagnetic capture step (18). For instance, Nogva et al. (18) reported fluorogenic PCR detection limits of 60 to 6,000 CFU of L. monocytogenes when the amplifications were preceded by a paramagnetic concentration step done on 1.4 ml of artificially inoculated skim or whole milk. Our approach is unique in that we begin with 25-ml samples and concentrate the bacteria by centrifugation, which is followed by nucleic acid extraction and amplification. Using this type of approach, we obtained detection limits of 103 to 104 CFU of L. monocytogenes per 25-ml sample of our model food commodity without prior cultural enrichment. By targeting mRNA rather than DNA and incorporating the fluorogenic endpoint detection strategy, our approach addresses several of the issues cited above. We recognize that the reconstituted NFDM matrix is compositionally simple and that additional work in matrix processing to concentrate pathogens and to remove inhibitors is critical (15). A next logical step would be to address such matrix issues by adapting this type of technology to the detection of L. monocytogenes in at-risk products (cheeses, processed meats) or environmental samples.
Recent studies have demonstrated a better correlation between the presence and amplification of bacterial mRNA and cell viability (12, 19). When mRNA is used as a target template, RNA extraction is considered necessary to avoid coamplification of genomic DNA. Furthermore, a DNase treatment prior to RT is usually employed to further avoid false-positive signals that might occur due to the contamination of RNA extracts with small amounts of genomic DNA. However, when samples contain low copy numbers of target RNA, the optional DNA removal and subsequent purification steps can cause further loss of RNA, resulting in a decrease in RT-PCR detection limits and overall test sensitivity (11). To address this issue, we (13) described an RT-PCR method designed to reduce false-positive results due to coamplification of contaminating genomic DNA using a Bacillus cereus model. Briefly, when a DNA oligonucleotide primer containing three consecutive mismatched bases near its 3′ terminus was constructed and RT was performed at a low temperature (40 or 45°C), the cDNA produced contained the mismatched sequence. When this was followed by PCR amplification at high annealing temperatures (≥60°C), the amplification of contaminating genomic DNA was hindered relative to the amplification of the cDNA. In the present study, we were able to design RT-PCR primers targeting the hly gene mRNA of L. monocytogenes using the same general format. This effectively eliminated the need to perform DNase treatment in order to avoid false-positive signals from residual genomic DNA, further simplifying the assay design and most likely improving the detection limit.
For rapid detection of specific amplicons, FRET-based PCR with internal amplicon-specific probes remains an excellent choice. Unlike protocols in which molybdate (7), intercalating agents (9), and fluorescein-labeled amplification primers (17) are used, in which detection is not based on sequence specificity, FRET-based PCR offers exquisite specificity and allows discrimination of nonspecific amplicons from specific amplicons. While the TaqMan assay and molecular beacon approaches are the most popular protocols, they use a dual-label oligonucleotide rather than two singly labeled complementary oligonucleotides for the selective generation of fluorescence. This theoretically simplifies FRET-based assays but also adds expense. The design described here takes advantage of the fact that the cost of single labeling is about one-half the cost of dual labeling ($225 versus $400 for 250-nmol portions of the two probes, respectively), which results in reduced cost in assay development and also maintains the simplicity provided by dual-label probes. For example, a minor change in the sequence or length of the probe during assay optimization would normally necessitate the purchase of a new dual-label probe. In the case of our asymmetric probe design, the use of two separate modules (i.e., a signal module [fluorescein-labeled primer] and a switch module [quencher-labeled short oligonucleotide]) means that only a new signal module would be needed under the same circumstances. Furthermore, the assay can be completed with any PCR thermocycler and fluorescence reader that are routinely available, which means that it is not necessary to purchase expensive real-time amplification equipment. This concept makes our design a good choice for assay development and optimization for a research laboratory on a limited budget.
In this study we demonstrated that 103 to 104 CFU of L. monocytogenes DNA or mRNA in 25 ml of NFDM could be amplified and detected by an asymmetric FRET-based amplification protocol with endpoint detection in about 2.5 h. The ease of use, high specificity, and relatively low cost of this method make it a promising alternative to other fluorescent PCR protocols. However, before any of the methods can be practically used in routine assays of low numbers of pathogens in foods, issues such as pathogen concentration, nucleic acid recovery, and inhibitor removal must be dealt with. Indeed, technical breakthroughs in these issues will be critical to the success of future nucleic acid amplification methods. Currently, research and modification of preamplification processing, amplification optimization, and protocol integration are under way in our laboratory and other laboratories.
Acknowledgments
This study was funded by Dairy Management, Inc., through the Southeast Dairy Foods Research Center.
The use of trade names in this paper does not imply endorsement by the North Carolina Agricultural Research Service or criticism of similar products not mentioned.
Footnotes
Paper no. FSR 02-14 of the Journal Series of the Department of Food Science, North Carolina State University, Raleigh.
REFERENCES
- 1.Bassler, H. A., S. J. A. Flood, K. J. Livak, J. Marmaro, R. Knorr, and C. A. Batt. 1995. Use of fluorogenic probe in a PCR-based assay for the detection of Listeria monocytogenes. Appl. Environ. Microbiol. 61:3724-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bej, A. K., and M. H. Mahbubani. 1994. Detection of food borne microbial pathogens, p. 341-365. In PCR technology: current innovations. CRC Press, Boca Raton, Fla.
- 3.Bubert, A., S. Kohler, and W. Goebel. 1992. The homologous and heterologous regions within the iap gene allow genus- and species-specific identification of Listeria spp. by polymerase chain reaction. Appl. Environ. Microbiol. 58:2625-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, S., A. Yee, M. Griffiths, C. Larkin, C. T. Yamashiro, R. Behari, C. Paszko-Kolva, K. Rahn, and S. A. DeGrandis. 1997. The evaluation of a fluorgenic polymerase chain reaction assay for the detection of Salmonella species in food commodities. Int. J. Food Microbiol. 35:239-250. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S., R. Xu, A. Yee, K. Y. Wu, C.-N. Wang, S. Read, and S. A. De Grandis. 1998. An automated fluorescent PCR method for detection of Shiga toxin-producing Escherichia coli in foods. Appl. Environ. Microbiol. 64:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, T., C. Frazier, J. Tuttle, S. Flood, L. Yagi, C. T. Yamashiro, R. Behari, C. Paszko, and R. Cano. 1998. Rapid detection of Listeria monocytogenes in dairy samples utilizing a PCR-based fluorgenic 5′ nuclease assay. J. Ind. Microbiol. Biotechnol. 21:167-174. [Google Scholar]
- 7.Gibson, N. J., C. R. Newton, and S. Little. 1997. A colorimetric assay for phosphate to measure amplicon accumulation in polymerase chain reaction. Anal. Biochem. 254:18-22. [DOI] [PubMed] [Google Scholar]
- 8.Hein, I., D. Klein, A. Lehner, A. Bubert, E. Brandl, and M. Wagner. 2001. Detection and quantification of the iap gene of Listeria monocytogenes and Listeria innocua by a new real-time quantitative PCR assay. Res. Microbiol. 152:37-46. [DOI] [PubMed] [Google Scholar]
- 9.Higuchi, R., G. Dollinger, P. S. Walsh, and R. Griffith. 1992. Simultaneous amplification and detection of specific DNA sequences. Bio/Technology 10:413-417. [DOI] [PubMed] [Google Scholar]
- 10.Holland, P. M., R. D. Abramson, R. Watson, and D. H. Gelfand. 1991. Detection of specific polymerase chain reaction product by utilization the 5′→3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 88:7276-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivarsson, K., and B. Weijdegard. 1998. Evaluation of the effects of DNase treatment on signal specificity in RT-PCR and in situ RT-PCR. BioTechniques 25:630-638. [DOI] [PubMed] [Google Scholar]
- 12.Klein, P. G., and V. K. Juneja. 1997. Sensitive detection of viable Listeria monocytogenes by reverse transcription-PCR. Appl. Environ. Microbiol. 63:4441-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo, K., and L.-A. Jaykus. 2000. Selective amplification of bacterial RNA: use of a DNA primer containing mismatched bases near its 3′ terminus to reduce false positive signals. Lett. Appl. Microbiol. 31:187-192. [DOI] [PubMed] [Google Scholar]
- 14.Koo, K., and L.-A. Jaykus. 2000. Modified method to detect PCR products by 5′ nuclease activity and an asymmetric fluorogenic probe set. BioTechniques 29:690-694. [DOI] [PubMed] [Google Scholar]
- 15.Lucore, L. A., M. A. Cullison, and L.-A. Jaykus. 2000. Immobilization with metal hydroxides as a means to concentrate food-borne bacteria for detection by cultural and molecular methods. Appl Environ Microbiol. 66:1769-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKillip, J. L., and M. Drake. 2000. Molecular beacon polymerase chain reaction detection of Escherichia coli O157:H7 in milk. J. Food Prot. 63:855-859. [DOI] [PubMed] [Google Scholar]
- 17.Nazarenko, I. A., S. K. Bhatnagar, and R. J. Hohman. 1997. A closed tube format for amplification and detection of DNA based on energy transfer. Nucleic Acids Res. 25:2516-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogva, H. K., K. Rudi, K. Naterstad, A. Holck, and D. Lillehaug. 2000. Application of a 5′-nuclease PCR for quantitative detection of Listeria monocytogenes in pure cultures, water, skim milk, and unpasteurized whole milk. Appl. Environ. Microbiol. 66:4266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton, D., and C. A. Batt. 1999. Detection of viable Listeria monocytogenes with a 5′ nuclease PCR assay. Appl. Environ. Microbiol. 65:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberst, R. D., M. P. Hays, L. K. Bohra, R. K. Phebus, C. T. Yamashiro, C. Paszko-Kolva, S. J. A. Flood, J. M. Sargeant, and J. R. Gillespie. 1998. PCR-based DNA amplification and presumptive detection of Escherichia coli O157:H7 with an internal fluorogenic probe and the 5′ nuclease (TaqMan) assay. Appl. Environ. Microbiol. 64:3389-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma, V. K., E. A. Dean-Nystrom, and T. A. Casey. 1999. Semi-automated fluorgenic PCR assays (TaqMan) for rapid detection of Escherichia coli O157:H7 and other Shiga toxigenic E. coli. Mol. Cell. Probes 13:291-302. [DOI] [PubMed] [Google Scholar]
- 22.Sharma, V. K., and S. A. Carlson. 2000. Simultaneous detection of Salmonella strains and Escherichia coli O157:H7 with fluorogenic PCR and single-enrichment-broth culture. Appl. Environ. Microbiol. 66:5472-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shearer, A. E., C. M. Strapp, and R. D. Joerger. 2001. Evaluation of a polymerase chain reaction-based system for detection of Salmonella enteritidis, Escherichia coli O157:H7, Listeria spp., and Listeria monocytogenes on fresh fruits and vegetables. J. Food Prot. 64:788-795. [DOI] [PubMed] [Google Scholar]
- 24.Stewart, D., and S. M. Gendel. 1998. Specificity of the BAX polymerase chain reaction system for detection of the foodborne pathogen Listeria monocytogenes. J. AOAC Int. 81:817-822. [PubMed] [Google Scholar]
- 25.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14:303-308. [DOI] [PubMed] [Google Scholar]
- 26.Whitcombe, D., J. Theaker, S. P. Guy, T. Brown, and S. Little. 1999. Detection of PCR products using self-probing amplicons and fluorescence. Nat. Biotechnol. 17:804-807. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, I. G. 1997. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 63:3741-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witham, P. K., C. T. Yamashiro, K. J. Livak, and C. A. Batt. 1996. A PCR-based assay for the detection of Escherichia coli Shiga-like toxin genes in ground beef. Appl. Environ. Microbiol. 62:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittwer, C. T., M. G. Herrmann, A. A. Moss, and R. P. Rasmussen. 1997. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques 22:130-138. [DOI] [PubMed] [Google Scholar]