Abstract
Many divalent salts (e.g., calcium, iron, zinc), have important nutritional value and are used to fortify food or as dietary supplements. Sensory characterization of some divalent salts in aqueous solutions by untrained judges has been reported in the psychophysical literature, but formal sensory evaluation by trained panels is lacking. To provide this information, a trained descriptive panel evaluated the sensory characteristics of 10 divalent salts including ferrous sulfate, chloride and gluconate; calcium chloride, lactate and glycerophosphate; zinc sulfate and chloride; and magnesium sulfate and chloride. Among the compounds tested, iron compounds were highest in metallic taste; zinc compounds had higher astringency and a glutamate-like sensation; and bitterness was pronounced for magnesium and calcium salts. Bitterness was affected by the anion in ferrous and calcium salts. Results from the trained panelists were largely consistent with the psychophysical literature using untrained judges, but provided a more comprehensive set of oral sensory attributes.
INTRODUCTION
The sensory characteristics of inorganic salts are of general interest due to their use as food flavors and as important nutrients in the diet. Unfortunately, with the exception of sodium and lithium chlorides which are uniquely salty tasting, most other salts have complex tastes and are often characterized by bitterness and other undesirable sensations such as astringency (Murphy et al. 1981; van der Klaauw and Smith 1994; Keast 2003; Lawless et al. 2003). Characteristics such as bitterness of divalent salts can be modified by anions. Organic anions such as lactate, gluconate or glycerophosphate have been shown to reduce the bitterness of calcium salts relative to calcium chloride (Tordoff 1996; Lawless et al. 2003) and thus have been used in calcium fortified food.
Calcium fortification of many food items and beverages is an increasingly common practice. Insufficient consumption of dietary calcium can contribute to calcium deficiency and osteoporosis, a condition affecting 20-30 million Americans and a major cause of bone fractures among the elderly (Williams 1999). Iron deficiency and anemia are the most common nutritional deficiencies in developing countries affecting billions of people worldwide (Yip 2001). In the U.S.A., iron deficiency is estimated to affect 11% of females of childbearing age and 9% of children below the age of 2, for whom anemia can impair cognitive development (Looker et al. 1997). In spite of the nutritional significance of these multivalent ions, little has been published concerning the sensory properties of calcium and iron salts.
In most psychophysical studies, terms are generally not defined for subjects in order to avoid biasing them toward a particular response. Rather their native and culturally shared definitions are relied upon for phenomenological description of their sensory world. Although well-meaning, this approach may fail to elucidate sensory properties for which the panelists have no common vocabulary or for which their conceptual frame of reference is fuzzy. Furthermore, terms are often preselected by the experimenter and may reflect that person's opinion of the words needed to describe the sensations. In taste psychophysics, this has sometimes led to a restriction of allowable responses to the classical four basic tastes. Important oral sensory characteristics such as metallic, umami or astringency may be overlooked. In contrast, the approach of applied descriptive analysis is to provide training and references to build a consensus vocabulary and to exhaustively and quantitatively profile the sensory attributes of products (Meilgaard et al. 1991; Gacula 1997). This is based on a psychophysical model, in which attributes vary in unidimensional intensity (Lawless 1999), but diverges from psychophysical practice in having more extensive processes of consensual vocabulary development, panel training and concept alignment. The goal of this study was to examine the sensory properties of several divalent salts of cations with important nutritional implications and to do so by means of a trained descriptive analysis panel.
MATERIALS AND METHODS
Panelists
Panelists were recruited from the Cornell University community. Twenty-four volunteers completed a prescreening questionnaire concerning availability and general health and then participated in two screening test sessions (described further below). Fifteen were asked to continue into training based on their total scores on the screening tests. Of these 15, seven completed training and participated in the formal evaluation sessions with the 10 test compounds. All seven were tasters of PROP (6-n-propylthiouracil), non-smokers, had no reported anosmia, had at least some college education and were fluent English speakers. Two subjects were male. Ages were 21-58 years with a mean of 34. Panelists received compensation for training and testing. All gave informed consent. Procedures were reviewed and approved by the Cornell University Committee on Human Subjects.
Stimuli
The test stimuli (Table 1) consisted of 10 solutions in deionized water, including ferrous sulfate, chloride and gluconate, calcium chloride, lactate and glycerophosphate, zinc sulfate and chloride, and magnesium sulfate and chloride. Test samples were prepared daily to prevent oxidation. Formulae, molecular weights, sources and concentrations are shown in Table 1. Concentrations were chosen to produce approximately equal overall perceived intensity, as found in preliminary testing (Yang 2004). During screening, the following taste samples were given for identification: sucrose (2% w/v), NaCl (0.2% w/v), citric acid (0.07% w/v), quinine HCl (0.0036% w/v), monosodium glutamate (0.1% w/v), aluminum ammonium sulfate (0.09% w/v) and capsaicin (2 ppm). For PROP screening, the following concentrations of 6-n-propylthiouracil were given: 0.00018 M and 0.000018 M. They were rated on a 9-point category scale (not bitter to extremely bitter) and subjects rating the lower concentration as eight or nine were considered supertasters; all others qualified as tasters. Taste stimuli were presented in 20-mL samples in 120-mL plastic cups, sipped and expectorated, except for the capsaicin solution, which was swabbed on the upper lip with a cotton swab, and a clean copper penny, which was simply tasted and expectorated. The following odorants were given for identification: diacetyl (buttery), benzaldehyde (almond or cherry), anize (licorice), lavender, vanilla extract and 1-octene-3-one (mushroom, earthy, metallic). Odorants were presented on fragrance testing strips kept in screw-cap amber jars. They were examined by unscrewing the caps, sniffing the air within the jar and replacing the cap. During training, the following reference standards were presented: 0.15 M sucrose (sweet), 0.32 M NaCl (salty), 0.19 M citric acid (sour), 0.09 mM quinine HCl (bitter), 0.01 M monosodium glutamate (umami) and 0.002 M aluminum ammonium sulfate (astringent). In addition to the penny used in screening, a variety of reference standards were examined as potential examples of metallic sensations, as shown below in results (see Table 2). Further details may be found in Yang (2004).
TABLE 1.
STIMULI
| Stimulus | Formula | Source | M. W. | Concentration |
|---|---|---|---|---|
| Calcium chloride | CaCl22H2O | Aldrich Chemical | 147.02 | 0.02 M |
| Calcium glycerophosphate | C3H7CaO6P | Fluka Chemika | 210.14 | 0.05 M |
| Calcium lactate | CaC6H10O6 | Fluka Chemika | 218.23 | 0.10 M |
| Ferrous chloride | FeCl24H2O | J. T. Baker | 198.81 | 0.03 M |
| Ferrous sulfate | FeSO47H2O | J. T. Baker | 278.02 | 0.05 M |
| Ferrous gluconate | FeC12H22O142H2O | Alfa Aesar | 482.19 | 0.05 M |
| Magnesium chloride | MgCl26H2O | Fisher Chemicals | 203.31 | 0.03 M |
| Magnesium sulfate | MgSO47H2O | EM Industries | 246.48 | 0.15 M |
| Zinc chloride | ZnCl22H2O | J. T. Baker | 136.30 | 0.01 M |
| Zinc sulfate | ZnSO47H2O | J. T. Baker | 287.56 | 0.02 M |
TABLE 2.
PHYSICAL AND FOOD REFERENCES FOR METALLIC SENSATIONS
| Physical references | Food references |
|---|---|
| Copper metal | Tuna (water packed) |
| Stainless steel | Tuna (oil packed) |
| Steel | Canned sardines |
| Aluminum | Canned anchovies |
| Rusted steel | Canned diced tomatoes |
| Aluminum food cans | Evaporated milk |
| Used can opener | Evaporated skim milk |
| Stainless spoons | Canned infant formula |
| Keys | Fortified supplement drinks |
| Copper penny | Energy bars |
| Copper penny, zinc core exposed | Pineapple juice concentrate |
| Mineral water | Canned green beans |
| Tap water | Canned spinach |
| Stale bottled water | Multivitamins |
| Deionized water | Ferrous sulfate iron supplement |
| Ferrous gluconate iron supplement |
Screening and Training
Candidates performed five screening exercises in two sessions: taste identification, a scaling exercise and PROP screening in the first session and odor identification and description of a complex unfamiliar product in the second session. The scaling exercise involved estimation of proportions of different geometric figures that were shaded (Meilgaard et al. 1991). The unfamiliar complex product was Asian bitter melon tea. The 15 candidates with the highest total scores in identification, scaling and description were invited for training.
Training involved 19 one-hour sessions for vocabulary development and three additional sessions for performance assessment. During the first session, panelists were given a short introduction to descriptive analysis as a sensory method. Taste samples were given for description as listed above. Six sessions were then used to explore metallic sensations. Proposed references are shown below in results. The list of generated terms was circulated and panelists were encouraged to add or delete attributes as they felt necessary. Once attributes were decided upon, the group ranked various samples as highest or lowest in each attribute. Later, scores were assigned on 15-point scales, anchored “none” to “very strong.” Inspection of high and low reference materials was done after two holiday breaks. At the end of training, 15 attributes had been collected and were included in the final test ballot.
Performance assessment was done after four and five training sessions. In the first performance monitoring, panelists evaluated calcium gycerophosphate, zinc sulfate and a deionized water sample. During the second session they evaluated ferrous gluconate, calcium lactate and magnesium chloride. During the third performance monitoring session they evaluated ferrous sulfate, magnesium sulfate and citric acid. Analysis of variance was performed to see whether the panel could discriminate among samples as shown by significant F-ratios for samples. Interactions were examined to assess reproducibility (judge by replication) and concept alignment (judge by sample interactions). Five of the attributes were low on the scale, showed no significant sample differences and are not reported below. They were sweet, salty, tingle, spicy aftertaste and sweet aftertaste.
Test Sessions
Evaluations were done on the 10 test solutions in the sensory evaluation laboratory of the Food Science Department at Cornell University. Evaluations were done in individual booths under red light to disguise any visual differences. Compusense five (version 4.4.8, Compusense, Inc., Guelph, Ontario, Canada) was used to randomize presentation orders, apply random three-digit codes and to collect judgments. Testing was conducted over 6 days to provide three replicate tests of the samples, five samples per day. Stimuli were sipped, held in the mouth until all attributes were rated and then expectorated. Panelists then rinsed with deionized water and a 0.05 M sucrose solution (Zacarias et al. 2001) and again with deionized water. A mandatory minimum two-minute delay was programmed into the ballot sequence. Unsalted crackers were also available for palate cleansing.
Data Analysis
Analysis of variance was performed using SAS® PROC GLM. There were four main effects: panelist, replication, stimulus and session. All six two-way interactions were retained in the model. Panelists were treated as a random variable (mixed model Type III F-tests). Comparisons across product pairs were tested with Least Significant Difference (LSD) tests. Principal components analysis was performed using SYSTAT® 5.5 with varimax rotation.
RESULTS
Potential reference standards evaluated by the panelists for the concept of metallic sensations are shown in Table 2. The development of consensus for a concept of metallic sensations was facilitated by using a variety of both commercial food products and chemical references. At the end of training, they chose to use their experience with the entire reference set as a definition of metallic taste rather than a single reference standard, a process of abstraction and generalization (O'Mahony et al. 1990).
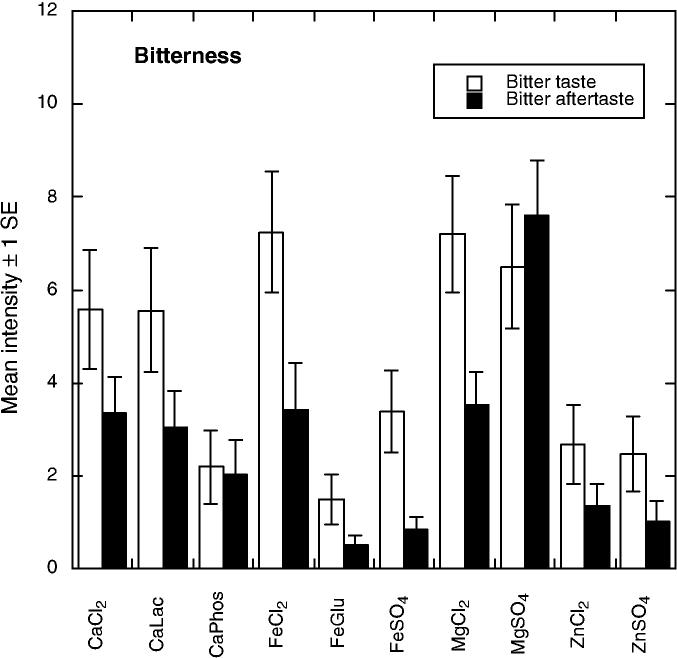
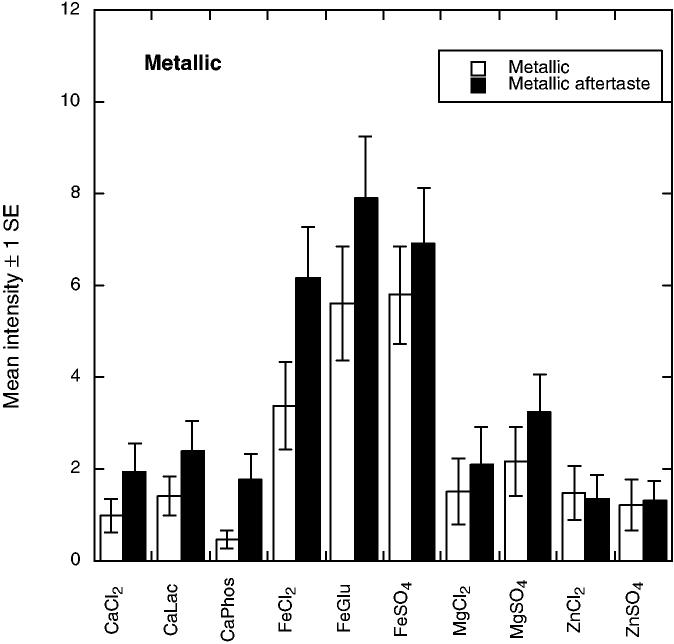
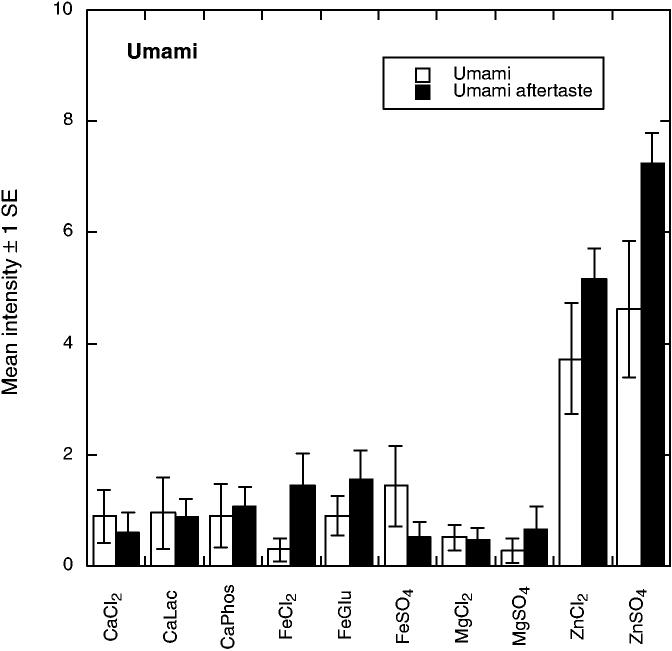
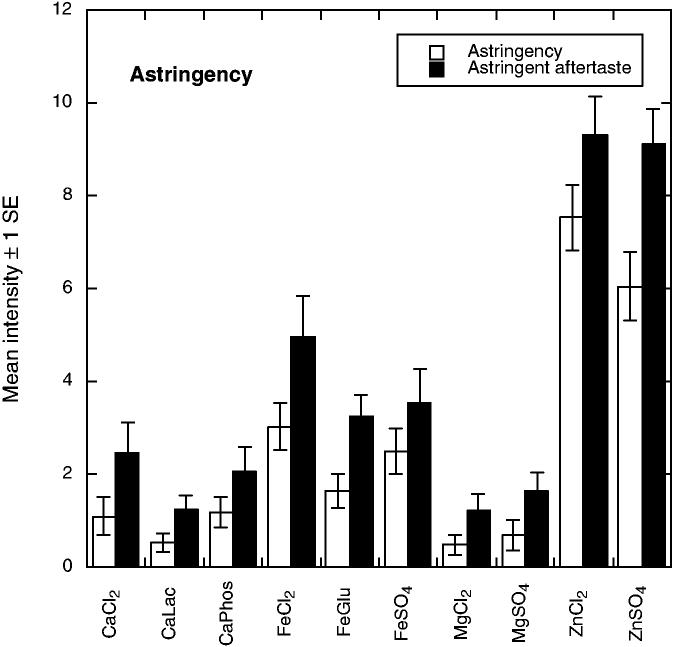
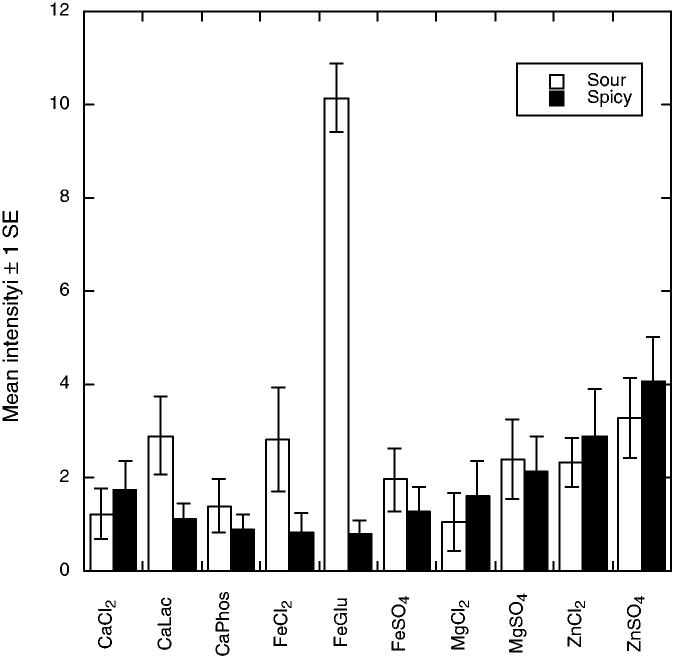
There were 10 significant attributes showing differences among the compounds. As shown in Fig. 1, bitter tastes (F9,55 = 3.11, P = 0.0043) were pronounced for the calcium and magnesium salts and for ferrous chloride. Bitter aftertaste was most pronounced for MgSO4 (F9,56 = 6.26 P < 0.001). Evidence for anionic inhibition of bitterness was seen in that calcium phosphate was lower in bitterness than the other CaCl2 and ferrous gluconate was less bitter than FeCl2, effects seen in previous work with calcium salts (Tordoff 1996; Lawless et al. 2003). Figure 2 shows that the iron compounds were most pronounced in metallic taste (F9,55 = 3.99, P < 0.001) and metallic aftertaste (F9,55 = 5.05, P < 0.001). Panelists found the zinc compounds to have a glutamate-like taste or mouthfeeling, which they labeled as umami (see Fig. 3). Samples differed in umami sensation (F9,55 = 3.07, P = 0.0048) and umami aftertaste (F9,55 = 8.71, P < 0.001). Zinc compounds were also higher in astringency (F9,57 = 29.61, P < 0.001) and astringent aftertaste (F9,56 = 19.48, P < 0.001) as shown in Fig. 4. Figure 5 shows the mean values for the two remaining scales with significant sample differences, sour (F9,56 = 7.60, P < 0.001) and a spicy/irritative sensation similar to that from capsaicin (F9,56 = 2.28, P < 0.05). The major difference in these attributes was a sour taste from ferrous gluconate, as discussed below.
FIG. 1.
MEAN PERCEIVED BITTERNESS AND BITTER AFTERTASTE (± 1 STANDARD ERROR) FOR THE 10 COMPOUNDS. SCALES WERE 15-POINTS WITH “NONE” AND “VERY STRONG” AS ANCHORS
FIG. 2.
MEAN PERCEIVED METALLIC SENSATION AND METALLIC AFTERTASTE (± 1 STANDARD ERROR) FOR THE 10 COMPOUNDS
FIG. 3.
MEAN PERCEIVED UMAMI SENSATION AND UMAMI AFTERTASTE (± 1 STANDARD ERROR) FOR THE 10 COMPOUNDS
FIG. 4.
MEAN PERCEIVED ASTRINGENCY AND RESIDUAL ASTRINGENCY (± 1 STANDARD ERROR) FOR THE 10 COMPOUNDS
FIG. 5.
MEAN PERCEIVED SOUR AND SPICY SENSATIONS (± 1 STANDARD ERROR) FOR THE 10 COMPOUNDS
Astringent aftertaste showed a significant replication effect (F2,12 = 12.82, P < 0.001), perhaps not surprising because astringency will tend to build up over time (Guinard et al. 1986). There were no effects of session. Panelists' differences were significant for bitterness (F6,13 = 8.94, P < 0.05), bitter aftertaste (F6,5 = 6.61, P < 0.05) and metallic aftertaste (F6,29 = 4.72, P < 0.05). Individual differences in sensitivity to bitterness are well-known and represent a common barrier to panel calibration. Among the 10 attributes showing sample differences, individual differences were also evident in significant panelist by sample interactions for all scales except spicy and astringency. For the remaining eight scales, interaction effects were small (F54,90 between 1.90 and 2.86) except for umami aftertaste (F54,90 = 4.48, P < 0.0001) and metallic aftertaste (F54,90 = 5.09, P < 0.0001). These differences may reflect the tendency of later sensations to persist versus disperse from the mouth as a function of individual physiological factors such as salivary flow (Fischer et al. 1994).
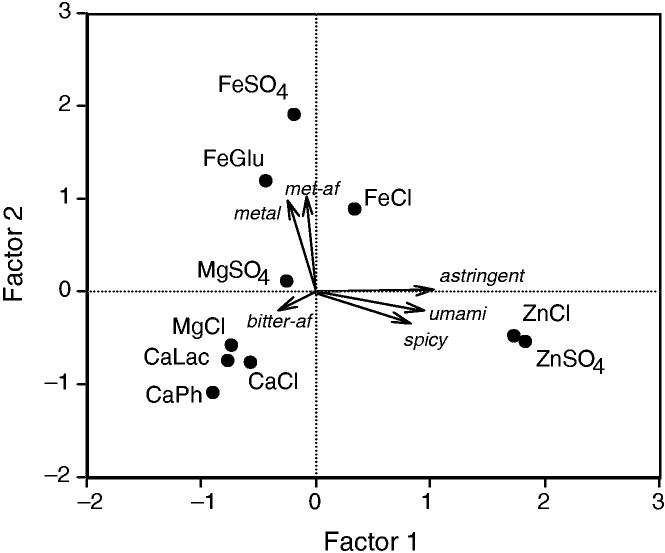
Principal components analysis revealed three factors with Eigenvalues greater than one after rotation. Figure 6 shows the plot of factor scores for the 10 compounds on the first two factors with vectors corresponding to the scales projected into the space based on their loadings. The first factor loaded heavily on astringency, astringent aftertaste, umami, umami aftertaste and the spicy scale (loadings >0.8) and differentiated the zinc compounds from the others. The second factor loaded heavily on the metallic and metallic aftertaste scales (loadings >0.9) and differentiated the iron compounds from the others. The third factor loaded only on the bitter aftertaste scale (-0.86) and differentiated magnesium sulfate. These factors were consistent with a PCA carried out on individual data (rather than means) which found three factors, one related to astringency and umami and differentiating zinc compounds from other metals, a second factor related to bitterness and a third factor related to metallic taste which differentiated the iron compounds from others (Yang 2004).
FIG. 6.
THE FIRST TWO PRINCIPAL COMPONENTS WITH THE 10 STIMULI PLOTTED FROM FACTOR SCORES AND THE CORRELATED ORIGINAL SCALES PROJECTED AS VECTORS BASED ON THEIR LOADINGS. ASTRINGENT AFTERTASTE AND UMAMI AFTERTASTE VECTORS ARE NOT SHOWN AS THEY COINCIDED CLOSELY WITH THE ASTRINGENCY AND UMAMI TASTE, RESPECTIVELY
DISCUSSION
Divalent salts stimulate complex oral and retronasal sensations. The current results show diverse properties but some consistency within salts having the same cations. As found previously, zinc salts are characterized by astringency (Keast 2003). Calcium and magnesium salts are often bitter (Lawless et al. 2003) and magnesium sulfate has often been used in psychophysical studies as a prototypical bitter tastant (Delwiche et al. 2001; Keast and Breslin 2002). Although metallic taste responses do occur for various divalent salts, iron compounds are particularly effective in evoking metallic sensations (Lawless et al. 2004). This is consistent with the use of ferrous sulfate solutions as a recommended reference standard (Civille and Lyon 1996). A new finding by this panel is that some divalent salts, particularly those of zinc, can evoke oral sensations reminiscent of glutamate salts, although weak ratings for “savory” taste were also found by Keast (2003). The consensus vocabulary development in descriptive analysis may facilitate the formation and use of an umami concept, as opposed to the imposed vocabulary found in psychophysical work. In the current study, this panel found monosodium glutamate to be an adequate reference standard for the umami concept. However, in psychophysics, terms are rarely defined or demonstrated by reference standards. The doctrine of four basic taste qualities is strong in that tradition and studies often restrict responses to the traditional taste words, albeit with allowance for an “other” category (e.g., van der Klaauw and Smith 1994).
One important property of some divalent salts is a metallic sensation. Metallic taste or metallic flavor is commonly reported as a defect in many food items (Borocz-Szabo 1980). Development of off-flavors presents a challenge to food scientists who attempt to fortify food with iron compounds (Hurrell 2002). Metallic tastes arise from contact with metal food packaging and processing equipment (Hunzinger et al. 1929; Zacharias and Tuorila 1979; Bodyfelt et al. 1988). Metallic sensations are reported in Burning Mouth Syndrome (Grushka 1987), during taste distortions in pregnancy (Nordin et al. 2004) and from anodal electrical stimulation of the tongue (Frank and Smith 1991). For divalent salts such as calcium and magnesium, one adjective used to describe these compounds is “metallic” (Lawless et al. 2003). The nature of metallic taste was examined by Hettinger et al. (1990) and Lawless et al. (2004). Metallic sensations from ferrous sulfate solutions in the mouth decreased when the external nares were occluded, implying a retronasal olfactory component to the so-called metallic taste from iron salts. This is consistent with the food chemistry literature documenting metallic-smelling compounds that are lipid oxidation products (Guth and Grosch 1990; Hinterholzer and Schieberle 1998; Hinterholzer et al. 1998). Iron compounds may catalyze a rapid lipid oxidation in the mouth, giving rise to a retronasally perceived metallic smell.
Follow-up to this work might examine the effect of nasal occlusion and elimination of retronasal smell. As noted above, nasal occlusion decreases the retronasal metallic sensation from ferrous sulfate (Hettinger et al. 1990; Lawless et al. 2004). One might predict, for example, that iron compounds would fall closer to calcium and magnesium compounds in a perceptual map when evaluated with the nose closed. Although nasal occlusion was explored during later stages of training, this panel felt the metallic concept was best learned from abstracting the common experiences from the entire training set with the nose open. Regarding reference standards, there are two general approaches in panel training. One is to find a compound with a unitary sensory property that typifies the sensation in question and lacks side tastes or other characteristics. An example is using alum as an astringency reference (Drobna et al. 2004). An alternate approach is to facilitate a process of abstraction and generalization (O'Mahony et al. 1990) in which multiple examples of a concept are given. Each example may not be simple, pure or prototypical for the characteristic, but the panel discovers their commonalities. The analogy here would be to children learning color names by being given many diverse examples. The literature on categorization suggests that a multiexample approach leads to more accurate and well-retained learning (Civille and Lawless 1986). If the approach of using a single reference for metallic sensations is taken, the obvious candidate from this set of stimuli would be ferrous sulfate, with its distinct metallic character and lower bitterness compared to ferrous chloride.
A second issue needing study is the question of reproducibility. Training could be repeated with a separate group and different panel leader, a procedure that is rarely done except in the case of interlaboratory cross-validation studies (e.g., Drake et al. 2002). This seems warranted in the current case due to the small final panel size and the individual differences as seen in the panelists by sample interactions. The difficulty we encountered due to panelist attrition may be a challenging problem when attempting to train panels in an academic setting over long periods of time. Availability is likely to change across semesters. Other issues for further study should include concentration effects and time-related changes. Both of these may reasonably be expected to affect the qualitative profile of divalent salts. A recent time-intensity study showed that for iron salts, metallic sensations have rapid onset and strong persistence, astringency has slow onset and persistence, and other taste properties fade rapidly (Yang 2004; Yang and Lawless 2005).
The sourness of ferrous gluconate is probably due to the gluconate anion contributing its own taste properties. When dissolving, a reaction could occur such as the following:
In other words the formation of ferrous hydroxide and gluconic acid with a dissociable hydrogen ion. The formation of ferrous hydroxide and gluconic acid is consistent with the observation of an immediate color change and a drop in pH upon dissolution (pH 4.7 ± 0.036). Sourness from acids depends both on pH and titratable acidity, the latter implying a contribution of the undissociated acid (Plane et al. 1980).
In summary, a trained descriptive panel was able to characterize the oral sensory properties of divalent salts of nutritional importance. Although some of these compounds have been profiled in food, data on their basic sensory properties in simple aqueous solutions is lacking outside the psychophysical literature. Descriptive analysis showed that properties such as bitterness of MgSO4 were consistent with the psychophysical literature based on the judgments of untrained observers. However, more comprehensive detail can be achieved with a systematic approach to vocabulary building which occurs in descriptive analysis training. In this approach, no presuppositions are made as to the acceptable or appropriate vocabulary. This can result in the inclusion of terms which might be omitted in studies of basic taste psychophysics, such as metallic and umami. In descriptive analysis, the crucible of intensive vocabulary development, including ongoing discussion and inspection of potential reference standards, provides a mechanism for insuring inclusion of all important oral sensations and alignment of individual's concepts.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grant RO1-DC006223 to H. Lawless. The authors thank Kathryn W. Chapman for her technical assistance.
REFERENCES
- BODYFELT FW, TOBIAS J, TROUT GM. Sensory Evaluation of Dairy Products. Van Nostrand/AVI Publishing; New York: 1988. [Google Scholar]
- BOROCZ-SZABO M. The influence of iron contamination on the sensory properties of liquid foods. Acta Aliment. 1980;9:341–356. [Google Scholar]
- CIVILLE GV, LAWLESS HT. The importance of language in describing perceptions. J. Sens. Stud. 1986;1:203–215. [Google Scholar]
- CIVILLE GV, LYON BG, editors. Aroma and Flavor Lexicon for Sensory Evaluation. ASTM; West Conshohocken, PA: 1996. [Google Scholar]
- DELWICHE JF, BUTELIC Z, BRESLIN PAS. Covariation in individuals' sensitivities to bitter compounds: Evidence supporting multiple mechanisms. Percep. Psychophys. 2001;63:761–776. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- DRAKE MA, GERARD PD, WRIGHT S, CADWALLER KR, CIVILLE GV. Cross-validation of a sensory language for cheddar cheese. J. Sens. Stud. 2002;17:215–227. [Google Scholar]
- DROBNA Z, WISMER WV, GOONEWARDENE LA. Selection of an astringency reference standard for the sensory evaluation of black tea. J. Sens. Stud. 2004;19:119–132. [Google Scholar]
- FISCHER U, BOULTON RB, NOBLE AC. Physiological factors contributing to the variability of sensory assessments: Relationship between salivary flow rate and temporal perception of gustatory stimuli. Food Qual. Prefer. 1994;5:55–64. [Google Scholar]
- FRANK ME, SMITH DV. Electrogustometry. In: Getchell TV, Bartoshuk LM, Doty RL, Snow JB, editors. Smell and Taste in Health and Disease. Raven Press; New York: 1991. pp. 503–514. [Google Scholar]
- GACULA MC., Jr . Descriptive Sensory Analysis in Practice. Food & Nutrition Press; Trumbull, CT: 1997. [Google Scholar]
- GRUSHKA M. Clinical features of burning mouth syndrome. Oral Surg. 1987;63:30–36. doi: 10.1016/0030-4220(87)90336-7. [DOI] [PubMed] [Google Scholar]
- GUINARD JX, PANGBORN RM, LEWIX MJ. The time-course of astringency in wine upon repeated ingestion. Am. J. Enol. Vitic. 1986;39:184–189. [Google Scholar]
- GUTH H, GROSCH W. Comparison of stored soya-bean and rapeseed oils by aroma extract dilution analysis. Lebensm. Wiss. U-Technol. 1990;23:59–65. [Google Scholar]
- HETTINGER TP, MYERS WE, FRANK ME. Role of olfaction in perception of non-traditional ‘taste’ stimuli. Chem. Senses. 1990;15:755–760. [Google Scholar]
- HINTERHOLZER A, LEMOS T, SCHIEBERLE P. Identification of key odorants in raw French beans and changes during cooking. Zeits. Lebensm. Untersuch. Forsch. A. 1998;207:219–222. [Google Scholar]
- HINTERHOLZER A, SCHIEBERLE P. Identification of the most odour active volatiles in fresh, hand-extracted juice of valencia late oranges by odour dilution techniques. Flav. Frag. J. 1998;13:49–55. [Google Scholar]
- HUNZINGER OF, CORDES WA, NISSEN BH. Metals in dairy equipment. Metallic corrosion in milk products and its effect on flavor. J. Dairy Sci. 1929;12:140–181. [Google Scholar]
- HURRELL RF. Fortification: Overcoming technical and practical barriers. J. Nutri. 2002;132:806S–812S. doi: 10.1093/jn/132.4.806S. [DOI] [PubMed] [Google Scholar]
- KEAST RSJ. The effect of zinc on human taste perception. J. Food Sci. 2003;68:1871–1877. [Google Scholar]
- KEAST RSJ, BRESLIN PAS. Cross adaptation and bitterness inhibition of 1-tryptophan, 1-phenylalanine and urea: Further support for shared peripheral physiology. Chem. Senses. 2002;27:123–131. doi: 10.1093/chemse/27.2.123. [DOI] [PubMed] [Google Scholar]
- VAN DER KLAAUW NJ, SMITH DV. Taste quality profiles for fifteen organic and inorganic salts. Physiol. Behav. 1994;58:295–306. doi: 10.1016/0031-9384(95)00056-o. [DOI] [PubMed] [Google Scholar]
- LAWLESS HT. Descriptive analysis of complex odors: Reality, model or illusion. Food Qual. Prefer. 1999;10:325–332. [Google Scholar]
- LAWLESS HT, RAPACKI F, HORNE J, HAYES A. The taste of calcium and magnesium salts and anionic modifications. Food Qual. Pref. 2003;14:319–325. [Google Scholar]
- LAWLESS HT, SCHLAKE S, SMYTHE J, LIM J, YANG H, CHAPMAN K, BOLTON B. Metallic taste and retronasal smell. Chem. Senses. 2004;29:25–33. doi: 10.1093/chemse/bjh003. [DOI] [PubMed] [Google Scholar]
- LOOKER AC, DALLMAN PR, CARROLL MD. Prevalence of iron deficiency in the United States. J. Am. Med. Assoc. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- MEILGAARD M, CIVILLE GV, CARR BT. Sensory Evaluation Techniques. CRC Press; Boca Raton, FL: 1991. pp. 135–200. [Google Scholar]
- MURPHY CL, CARDELLO AV, BRAND JG. Tastes of fifteen halide salts following water and NaCl: Anion and cation effects. Physiol. Behav. 1981;26:1083–1095. doi: 10.1016/0031-9384(81)90213-4. [DOI] [PubMed] [Google Scholar]
- NORDIN S, BROMAN DA, OLOFSSON JK, WULFF M. A longitudinal descriptive study of self-reported abnormal smell and taste perception in pregnant women. Chem. Senses. 2004;29:319–402. doi: 10.1093/chemse/bjh040. [DOI] [PubMed] [Google Scholar]
- O'MAHONY M, ROTHMAN L, ELLISON T, SHAW D, BUTEAU L. Taste descriptive analysis: Concept formation, alignment and appropriateness. J. Sens. Stud. 1990;5:71–103. [Google Scholar]
- PLANE RA, MATTICK LR, WEIRS LD. An acidity index for the taste of wine. Am. J. Enol. Vitic. 1980;31:265–268. [Google Scholar]
- TORDOFF MG. Some basic psychophysics of calcium salt solutions. Chem. Senses. 1996;21:417–424. doi: 10.1093/chemse/21.4.417. [DOI] [PubMed] [Google Scholar]
- WILLIAMS S. Essentials of Nutrition and Diet Therapy. Mosby; St. Louis, MO: 1999. [Google Scholar]
- YANG H. Descriptive profile of divalent compounds using a trained panel and time-intensity study of iron compounds. Cornell University; Ithaca, NY: 2004. MSc Thesis. [Google Scholar]
- YANG H, LAWLESS HT. Time-intensity characteristics of iron compounds. Food Qual. Prefer. 2005 in press. [Google Scholar]
- YIP R. Iron. In: Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. ILSI Press; Washington, DC: 2001. pp. 311–328. [Google Scholar]
- ZACARIAS I, YANEZ CG, ARAYA M, ORAKA C, OLIVARES M, UAUY R. Determination of the taste threshold of copper in water. Chem. Senses. 2001;26:85–89. doi: 10.1093/chemse/26.1.85. [DOI] [PubMed] [Google Scholar]
- ZACHARIAS R, TUORILA H. Der Reiz- und Erkennungsschwellenwert für Metallverbindungen in verscheidenen Prüfmedien [Taste thresholds for metallic salts in different media] Lebens. Wiss. U-Technol. 1979;12:36–40. [Google Scholar]