Abstract
Butyrate inhibits colonic cell proliferation in vitro but reportedly has an opposite effect in vivo. Because lactulose feeding decreases cecal cell proliferation, an effect attenuated by prefeeding inulin, we hypothesized that lactulose feeding would decrease colonic luminal synthesis of butyrate, and that prefeeding and cofeeding inulin would prevent this effect. Piglets (n = 31) were catheterized and randomly assigned to 1 of 4 groups: Control formula (C); control formula + lactulose (L); control formula + lactulose + inulin (L + I); and control formula + inulin (I). At 6 and 7 d postsurgery, the rate of cecal synthesis of butyrate, cecal cell proliferation and apoptosis, and cecal and distal colon butyrate concentration were measured. In groups C, L, L + I, and I, the rates of synthesis of butyrate (mean ± SEM) were 10.6 ± 3.2, 23.3 ± 4.5, 12.4 ± 3.6, and 14.6 ± 4.0 μmol/min, respectively (Group Effect, P = 0.1; C vs. L, P = 0.03; L vs. L + I, P = 0.06). The cecal butyrate concentrations did not differ among the 4 groups and were 8.7 ± 3.2, 2.4 ± 0.8, 3.4 ± 1.9, and 2.0 ± 0.7 μmol/g dry wt, respectively. The total cecal cell proliferation index was higher in C than in L (P = 0.008) or I (P = 0.026) and was higher in L + I than in L (P = 0.013) or I (P = 0.046). The increased supply of butyrate to the cecum was associated with decreased cell proliferation, but cecal butyrate concentration did not reflect synthesis.
Keywords: butyrate, lactulose, inulin, swine, colon
Butyric acid is a product of the bacterial fermentation of carbohydrate in the rumen of multigastric animals and in the colon of omnivores such as swine or humans (1). In cultured, colonic neoplastic cells, butyrate arrests the cell cycle and stimulates terminal cellular differentiation (2,3). The mechanisms for these effects have been studied at length. In cultured mammalian cells, butyrate inhibits histone deacetylase activity, which leads to histone hyperacetylation and transcriptional activation of the p21Waf1/Cip1 gene; this in turn leads to inhibition of cyclin-dependent kinase 2 activity and arrest of the cell cycle (4–6). In contrast, the literature suggests that butyrate infused into the colonic lumen increases cell proliferation, although its stimulatory effects on cell proliferation may be confined to the crypt base (lower 40%) rather than the surface, the primary location of new cancers (7–11). Cell culture media concentrations of butyrate and glucose used in some studies, the use of neoplastic cells, and the artificial nature of in vitro studies (lack of a mucin barrier to absorption of substrates) could limit the clinical relevance of in vitro studies (3,12). However, one also could question the relevance of some in vivo studies if they are not based on known physiologic rates of entry of butyrate into the lumen because pharmacologic doses of butyrate, like other organic acids, may be toxic to the colonic mucosa (13–15). The main purpose of the present study was to assess the rate of colonic luminal butyrate production in an in vivo model of lactulose fermentation previously shown to decrease cecal cell proliferation and to determine whether abnormal synthesis of butyrate is associated with the changes observed in the proliferation of colonocytes (16,17).
Previously, we used graded doses of lactulose, an indigestible disaccharide of fructose and galactose, to study fermentation and cecal cell proliferation and apoptosis in piglets (16–18). In 2 earlier studies (16,17), chronic feeding of lactulose at large doses sufficient to cause osmotic diarrhea did not affect weight gain or apparent dietary energy utilization but caused abnormally decreased cecal cell proliferation. In one study (17), prefeeding inulin, a fructooligosaccharide, then stopping this supplement and feeding lactulose, prevented the decline in cell proliferation. Based on the assumption that butyrate stimulates colonic cell proliferation in vivo, our main hypothesis was that lactulose feeding sufficient to cause diarrhea would impair butyrate synthesis. However, based on the results of our previous study (17), we also tested an additional hypothesis that pre-feeding and concurrent feeding of inulin would prevent this decrement in butyrate synthesis, possibly by stimulating compensatory bacterial fermentation (16,17).
MATERIALS AND METHODS
Animals, feedings, and design
Standard Yorkshire/Hampshire piglets (n = 31) were studied at The University of Texas Medical Branch, whose Institutional Animal Care and Use Committee approved the research protocol. On ~d 12 of life, the piglets were transported from the pig farm to the laboratory. They were then orally fed a sow’s milk substitute formula (Control formula, C; SPF Lac, Sterile milk replacer, PetAG). Based on actual analysis (Covance Laboratories), the macronutrient composition was as follows: energy, 3.5 MJ/L; protein, 44.1 g/L; fat, 52.3 g/L; total carbohydrate, 47.2 g/L; and lactose, 26 g/L. The formula was further supplemented with lactose (66 g/L).
The piglets were randomly assigned to 1 of 4 formula study groups: 1) the Control group (C) received only sterile milk replacer for 14 d (n = 8). 2) The experimental group, I, was fed sterile milk replacer and inulin (3 g/L) for 14 d (n = 8). 3) The experimental group (L), was fed sterile milk replacer for 7 d, and then lactulose (66 g/L) was added to the formula for 7 d (n =8) . 4) The experimental Group, L + I, was fed sterile milk replacer and inulin (3 g/L) for 14 d, and then lactulose (66 g/L) was added for the last 7 d (n = 9) . During the study, body weight, formula intake, and stool characteristics were monitored. Diarrhea was quantified by computing the fraction of the observation period when diarrhea was observed, as previously described (16,17).
After 6 d of feeding, the piglets underwent a surgical procedure for the insertion of catheters into the stomach or duodenum for feeding, the portal vein for blood drawing during tracer studies, and a cannula into the cecum for tracer infusion. Lactulose was introduced into the diet on d 2 or 3 after surgery in groups L and L + I.
Details of the surgical procedures were described previously (19–22). Anesthesia was induced with a combination of telazol: ketamine:xylazine administered i.m. (telazol, 4 mg/kg; ketamine, 2 mg/kg; xylazine, 2 mg/kg). General anesthesia was then maintained with isoflurane via an endotracheal tube using a pediatric breathing circuit. A catheter (23 gauge; Silastic) was inserted into the portal vein for isotopic measurements (21,22). This line was maintained via a constant infusion of heparinized saline, while attached to a pressure transducer. In 2 piglets (both in group C), a gastric cannula was inserted for feeding as originally described (19), but in the rest of the piglets, a catheter for feeding was inserted into the proximal duodenum (22,23). Finally, a cecal cannula was inserted (19).
Feedings were restricted for 24–48 h postoperatively and then resumed. Piglets were allowed oral formula feeding if the feeding catheter did not function for the entire study period, but data are reported here only for the pigs in Groups L, L + I, and I that received a mean daily dose of lactulose or inulin equal to at least 75% of that intended (120 and 5.4 g, respectively). The feeding goal, postsurgery, was 1.8 L formula/d.
At 6–7 d postsurgery, we conducted a butyrate tracer study as described previously (20), but in this study, the piglets were awake and had access to formula. [1-13C]-Butyrate was infused via the cecal cannula at a mean rate of 0.17 μmmol/(kg·lmin) (Prime/Minute Infusion Rate = 20/1). Blood was sampled from the portal vein before the isotope infusion and at 60, 90, 100, 110, and 120 min after the beginning of the infusion; plateau isotopic enrichment was established before analysis of the data for each study.
To quantify the rate of flow of butyrate in the left colon (24), after the completion of the tracer study, polyethylene glycol (PEG4 4000) (a nonabsorbable marker, 8.2 g/L; Sigma-Aldrich) was administered in the formula for the next 24 h.
On the day after the tracer study, the piglets were anesthetized with telazol:ketamine:xylazine, and bromodeoxyuridine (25 mg/kg) was injected into a jugular vein (16). After 2 h, the piglets were reanesthetized with 0.5 mL of a 50 g/L solution of sodium pentobarbital (i.v.), which was readministered to maintain general anesthesia.
Before collecting tissues and fluids, breath was collected from the trachea for determination of H2, CH4, and CO2 concentrations (Quintron Instrument), and the concentrations of H2 and CH4 were expressed as ppm/% CO2. Cecal pH then was measured (Model Omega PHH-253, Omega Technologies). Cecal tissue was obtained for histology, and determination of cell proliferation and apoptosis, and cecal and distal (left) colon fluid was obtained for determination of concentrations of PEG and short-chain fatty acids.
Tracer model and calculations
We utilized a previously described single isotope dilution model for assessing the rate of synthesis (production) of butyrate (butyric acid, BA) in the colonic lumen (20,25). The rate of dilution of the isotope is equated to the rate of production of butyric acid (BA) (Ra BA) according to the following equation:
| (1) |
where d equals the measured molar isotope infusion rate and IE BA is the plateau isotopic enrichment of BA in the portal vein during the quasi-steady state from 60–120 min (20). IE BA, expressed as moles fraction excess in the equation, is equal to mol % excess divided by 100.
Estimation of distal (left) colon BA entry rate and calculation of net cecal BA Uptake
The rate of flow (entry) of BA in the left colon (LC BA Flow, mmol/min) was estimated from the concentration of BA in the left colon (LC BA, expressed as mmol/L), the concentration of PEG in the left colon (PEG LC, g/L), the concentration of PEG in the formula (PEG formula, 8.2 g/L), and the formula intake (L/min):
| (2) |
Net cecal BA uptake by the cecal mucosa was then calculated using Equation (3).
| (3) |
We assumed that there is negligible production of BA in the distal colon that might be drained by systemic veins, which also could contribute to the “flow” of BA in the distal colon. Thus, in theory, Net Cecal BA Uptake may be underestimated if this fraction of BA production is not quantified by the tracer experiment. However, as noted below, this underestimation must be quite small because Net Cecal BA Uptake is very high when expressed as a fraction of Ra BA.
Analysis of BA enrichment in blood
The isotopic enrichment of BA was measured using a previously described assay (26).
Analysis of the concentration of butyrate and acetate in cecal and colonic fluid
The cecal concentrations of butyrate and acetate were measured using a modification of a previously described method for preparing the samples (27). GC was performed using a Hewlett Packard 5890 Series II Gas Chromatograph with flame ionization detector, equipped with a 30-m, 0.53 mm i.d., HPFFAP-TPA capillary column (Agilent Technologies) and a 1-m, 0.53 mm i.d. deactivated glass capillary precolumn (Supelco 19095F-123, Sigma-Aldrich).
Histological damage assessment
Slides of paraffin-embedded cecal tissue were stained with hematoxylin-eosin for histologic assessment of injury or inflammation using a previously published scoring system (28): Grade O, no abnormalities; 1, mild damage/inflammation (small, focal, or widely dispersed areas of inflammation and/or fibrosis above the muscularis mucosa); 2, moderate damage/inflammation (multifocal or locally extensive and contained inflammation or fibrosis extending into the submucosa); and 3, severe damage/inflammation (inflammatory cells extending into the muscularis propria).
Cell proliferation index
Unstained, paraffin-embedded slides were deparaffinized and then stained for bromodeoxyuridine labeling (Cell Proliferation Kit, Amersham Life Science, No. RPN 20). Then, as described previously (17), we assessed the fractional proliferation index (PI) for the total cecal crypt and for the lower 40% of the crypt (stained cells/total cells); for the upper 40% of the crypt, however, we estimated the φh value (labeled cells in the upper 40% of the crypt divided by total labeled cells in the crypt)(10).
Assessment of apoptosis
DNA fragmentation (apoptosis) was assessed using the TUNEL technique (Tdt-FragEL DNA Fragmentation Detection Kit; CAT QIA33, Oncogene Research Products) (29). The fraction of apoptotic cells was then quantified (total stained cells in crypt/total cells in crypt), again in ~10 crypts.
Measurement of PEG concentration
We used a previously described colorimetric method for analyzing PEG (30)
Data analysis and statistics
During histologic examination and other data analyses, the investigators were unaware of the treatment group for each piglet. We mainly used 1-way ANOVA to determine the effect of diet (C, I, L, L + I), and, in general, when the P-value for the F-test was ≤0.05, we then used the Least Square Difference (LSD) test to compare means between specific groups (SPSS Base 10.0). However, in examining the effect of diet on Ra BA (μmol/min) or cecal butyrate concentration, we accepted a P-value for the F-test of 0.1, to highlight group differences that would have been observed (via t test) if we had confined our study to examining only our major hypothesis concerning the effects of L on the outcome variables. For some variables, t tests were conducted. We also employed both linear (Pearson) and rank (Spearman) correlation analyses. All results are expressed as means ± SEM.
RESULTS
Diarrhea, cecal pH, and breath H2 concentration
The rate of weight gain from the day of surgery until the tracer study was 27.3 ± 5.1 g/(kg·d) (grand mean) and did not differ among the diet groups. Weight gain per unit formula intake also did not differ among the groups and was 0.11 ± 0.02 g/mL. The diet fed affected the proportion of days with diarrhea (P < 0.001), and diarrhea was less frequent (P ≤ 0.001) in the C (0.23 ± 0.08) than in the I (0.58 ± 0.08), L (0.86 ± 0.04), or L + I (0.78 ± 0.06) groups and also more frequent in the L than in the I (P = 0.006) and in the L + I than in I group (P = 0.035). The cecal wet-to-dry weight ratio, an index of the fluidity of cecal fluid, did not differ among the 4 groups and was 5.8 ± 1.0. Breath concentrations of H2 (7.7 ± 2.8 ppm/% CO2) and methane (10.4 ± 2.7 ppm/% CO2) did not differ among the groups. Diet affected cecal pH (P = 0.004); it was lower in the L + I than in the C or L groups (P < 0.003) and also tended to be lower than in the I group (P = 0.057) (Table 1).
TABLE 1.
Cecal pH, BA concentration, cell proliferation and apoptosis in piglets administered C, I, L, or L + I formulas1
| C | I | L | L + I | |
|---|---|---|---|---|
| Cecal pH | 6.8 ± 0.2a | 6.5 ± 0.2ab | 6.7 ± 0.1a | 6.0 ± 0.2b |
| BA, mmol/L | 2.7 ± 0.6 | 1.9 ± 0.6 | 1.1 ± 0.3 | 1.1 ± 0.4 |
| BA, mmol/kg dry wt | 8.7 ± 3.2* | 2.0 ± 0.7 | 2.4 ± 0.8 | 3.4 ± 1.9 |
| PI, Total crypt | 0.24 ± 0.03a | 0.14 ± 0.02b | 0.12 ± 0.03b | 0.22 ± 0.03a |
| PI, Lower 40% crypt | 0.41 ± 0.06a | 0.24 ± 0.03bc | 0.17 ± 0.05c | 0.34 ± 0.06ab |
| φh, Upper 40% crypt | 0.25 ± 0.05 | 0.22 ± 0.04 | 0.25 ± 0.08 | 0.40 ± 0.06 |
| Fractional apoptosis | 0.29 ± 0.13 | 0.14 ± 0.04 | 0.35 ± 0.13 | 0.09 ± 0.03 |
Values are means ± SEM. n = 4–9/group, except *n = 3, group C, BA, mmol/kg dry weight. Means in a row without a common letter differ, P < 0.05.
The rate of cecal BA synthesis (Ra BA) and concentrations of butyrate and acetate
Piglet weight on the day of the tracer study correlated with Ra BA (μmol/min) (Spearman rank r = 0.42, P = 0.031). However, Ra BA also correlated with weight gain (Spearman rank r = 0.40, P = 0.041). Therefore, although we analyzed group differences statistically with Ra BA expressed as μmol/(kg·min), we are emphasizing the differences based on μmol/min because it seems likely from the data presented above that the relation between Ra BA and weight relates mainly to group differences in body mass accretion not to the size of the colonic bacterial mass. Diet tended to affect Ra BA (μmol/min) (P = 0.1). However, there were only 2 values in Group C that were as high as any in Group L. When we conducted the post hoc multiple comparison test, the Ra BA differed between groups C and L (P = 0.03; Fig 1). When expressed as μmol/(kg·min), Ra BA did not differ among the groups (grand mean, 2.2 ± 0.3). Because our primary hypothesis was that lactulose feeding would decrease (not increase) the rate of butyrate synthesis, we also compared the C and L groups using a 2-sample t test. Based on that analysis, the rates differed [μmol/min (P = 0.04) and μmol/(kg·min) (P = 0.05)].
FIGURE 1.
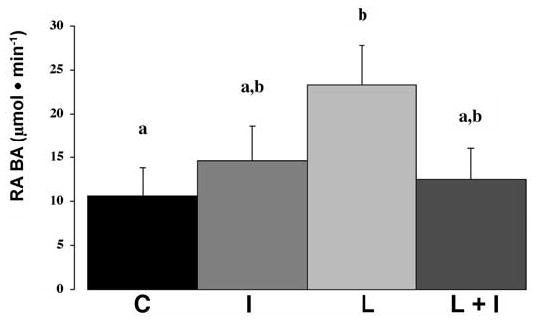
The rate of cecal luminal synthesis of butyric acid (Ra BA, μmol/min) in piglets administered C, I, L, or L + I formulas. Diets tended to affect Ra BA (P = 0.1) and the LSD test was performed. Values are means ± SEM, n = 6–8/group. Means without a common letter differ, P = 0.03.
For 20 piglets, there were sufficient data to estimate Net Cecal BA Uptake (μmol/min) (n = 4, groups C and L + I, and n = 6 for L and I); this variable correlated with Ra BA (μmol/min) (Pearson r = 1.0, P <0.001 and Spearman r = 0.998, P < 0.001). As a proportion of Ra BA, Net Cecal BA Uptake was 0.97 ± 0.01 (range: 0.77–1.00). Diet did not affect Net Cecal BA Uptake (grand mean, 14.0 ± 2.4). LC BA Flow was expressed as a ratio to Ra BA to assess relative appearance (excretion) of butyrate in the distal colon in proportion to that formed in the cecum; this variable did not differ among the 4 groups (grand mean, 0.03 ± 0.01).
Diet tended to affect the cecal butyrate concentration expressed as mmol/L (P = 0.09) or as mmol/kg dry weight (P = 0.1). We conducted the post hoc test and found that for both per L and per kg dry weight, the concentration in group C was greater than that in the L group (P = 0.03) (Table 1). Cecal acetate concentrations did not different among the diet groups and were (grand means) 3.0 ±0.3 mmol/L or 14.8 ± 1.7 mmol/kg dry weight.
Cecal inflammation, cell proliferation, and apoptosis
There was little evidence of cecal inflammation in any of the groups. In the C group, the injury score was 0.5 ± 0.5, but the score was zero in the other groups. Diet affected the crypt cell proliferative index for the total crypt (P =0.015); the index was higher in the C group compared with the I (P = 0.026) or L (P = 0.008) groups and was higher in the L + I group compared with the L (P = 0.013) or I (P = 0.046) groups (Table 1). Diet also affected this index for the lower 40% (P = 0.03). The index was higher in group C than in group I (P = 0.048) or L (P = 0.007) and was higher in group L + I than in group L (P = 0.031) (Table 1). Diet did not affect the φh value or fractional apoptosis (Table 1).
DISCUSSION
As in our 2 previous studies (16,17), we observed that lactulose malabsorption to a degree capable of causing diarrhea, also causes decreased cell proliferation in the colon. However, in contrast to our hypothesis, which was predicated on previous theories about the in vivo effects of butyrate on the colon, we did not observe a decrease in the rate of entry of butyrate into the colonic lumen, but rather, an increase. However, the statistical significance of this effect was borderline using ANOVA and the relatively liberal LSD multiple comparison test.
Differences among groups in the concentration of butyrate in the cecal lumen, also only tended to be significant, but overall, lactulose appeared to have opposite effects on cecal production and concentration of butyrate. Therefore, in our model at least, the luminal concentration of butyrate may not be an accurate index of its entry into the colonic mucosa. This is potentially important because colonic cell proliferation is an important risk factor for colon cancer (6,31), and butyrate, via its inhibition of histone deacetylase may decrease colonic cell turnover (4,6). However, if the effects of butyrate in vivo are mediated by its uptake by colonocytes, and this in turn is related primarily to the rate of production and not the apparent luminal concentration, then further understanding of how dietary fiber may alter the risk of colon cancer in susceptible individuals may be hindered by undue reliance on data correlating fiber intake with butyrate concentration in the colon lumen or feces. Indeed, butyrate and other short-chain fatty acids are readily absorbed by the mammalian colon (32) and what is left in the colonic lumen may be a small proportion of what is actually synthesized or even be a misleading indication of production if there is defective colonic absorption. As evidence of this, our estimates of cecal butyrate uptake, based on the rate of appearance of butyrate into the distal colon, suggest that most of the butyrate produced in the cecal lumen was indeed taken up by the cecal mucosa.
The luminal production rate of butyrate can be more safely and, certainly, more practically manipulated with feedings than absorption can be altered with cathartics or drugs toxic to the colonic mucosa. Using data on physiologic ranges in butyrate production rate, it may be more productive to investigate how butyrate causes changes in both cell proliferation and apoptosis, without relying on the potentially misleading, if more easily obtained, indicators of butyrate production such as luminal or fecal concentration. In this regard, previous studies by others suggested that butyrate in vivo caused increased colonic cell proliferation via unknown mechanisms (7–11,15). It appears that in some of these studies, the infusion rate or perfusion amount was much higher than the mean rates we measured in any of our groups (8,9,15). In other studies, the colon was perfused (7–9,33) or human colon biopsies were incubated (11) with solutions of butyrate at concentrations much higher than we measured, even per unit dry weight of cecal contents. Butyrate at high concentrations may cause colonic injury or inflammation (13,15), and it is plausible that inflammatory processes might cause some of the in vivo stimulatory effects of butyrate on colonic cell proliferation. It is of interest that we did not observe evidence of increased inflammation or injury in the piglets in any of the feeding groups. We do not know whether, in our model, larger production rates of butyrate, even if unphysiological, would cause increased cecal cell proliferation; however, we clearly can refute the hypothesis that we proposed previously (16) that osmotic diarrhea induced by lactulose malabsorption would cause decreased cecal cell proliferation because of “butyrate deficiency.”
We were interested in how severe carbohydrate malabsorption affects gut function in patients who exhibit diarrhea in response to feeding because of primary limitations in intestinal absorption or because of ill-defined effects of stress (16,34). Thus, our observations regarding the interrelations of cecal cell turnover, luminal butyrate concentration, and butyrate production apply strictly to the conditions under study. The rates of postoperative weight gain were 40.2 and 35.7 g/(kg·d) in the lactulose- and inulin-treated groups, respectively; these rates are comparable to that of sow-reared pigs for the same age range [47 g/(kg·d)] (35). The piglets in the L or I groups also did not manifest cecal mucosal damage; thus, lowered cell turnover with lactulose or inulin feeding did not seem to be due to protein-energy malnutrition per se or to colitis. Nevertheless, secondary bile acids such as deoxycholic acid increase cell proliferation in the colon (10,36,37); thus, we speculate that osmotic diarrhea caused by severe lactulose malabsorption might lower cell proliferation via depletion from the lumen of compounds such as deoxycholic acid, which stimulate colonic cell proliferation. We did not measure luminal bile acid concentrations, but the L + I group, which exhibited normal cecal cell proliferation, did not differ from the L group in the frequency of diarrhea or in the wet weight/dry weight ratio of cecal contents. Lupton et al. (37) used magnesium sulfate to produce osmotic diarrhea without lowering cecal pH; they showed that cecal pH was inversely correlated with the cecal cell proliferative index, but transit time had no apparent effect. Thus, osmotic diarrhea per se probably did not cause abnormal cell proliferation in the L group. We contend that these results are physiologically relevant because carbohydrate malabsorption with secondary osmotic diarrhea occurs relatively frequently in the course of normal variations in healthy as well as in diseased states. For example, osmotic diarrhea such as that produced in piglets in the L group, might be observed in healthy humans with lactose intolerance or undergoing laxative therapy with lactulose, in patients with inflammatory states such as burns being treated with formula diets, or in patients with short bowel syndrome (34). However, under such conditions, there would be other effects of carbohydrate malabsorption in addition to abnormal production of fermentation metabolites, and the relation between the entry rate of butyrate into the lumen and cell turnover in the colon might differ from that produced by exogenous infusions of butyrate or from endogenously formed butyrate during fermentation of dietary fiber.
Our series of studies was stimulated by an interest in the beneficial vs. adverse effects of persistently feeding carbohydrate (particularly disaccharides) at an intake level that exceeds the capacity for full small intestinal digestion and absorption. An intact colon capable of salvaging diet carbohydrate-derived energy plays an important nutritional role in patients with short bowel syndrome (38). Whether lowered colonic cell proliferation affects colonic mucosal mass and overall colonic function was not evaluated specifically in this study. However, the rate of “flow” of butyrate into the distal colon as a fraction of butyrate production did not differ among the groups.
We had anticipated that feeding inulin would increase, not decrease, cecal cell proliferation based not only on its effect when fed with lactulose (17) but also based on a previous study by others (39). However, there also are data suggesting that inulin treatment of healthy mice or humans does not affect cell proliferation in the colon (40,41). Because butyrate synthesis was not significantly elevated in the I group compared with the controls, we cannot attribute the decrease in cell proliferation in this group to higher butyrate availability to the mucosa.
In this particular study, we were not able to obtain satisfactory measurements of the colonization of the cecum by bacteria such as Clostridia and Bifidobacteria; thus, these data were not reported and we are unable to address at this time whether the effects of lactulose and inulin on butyrate production are mediated by changes in the colonization of the cecum by Clostridia, which plays a major role in butyrate synthesis in the colon lumen (42). Nevertheless, it is reasonable to point out how indigestible carbohydrates might alter colonic bacterial flora, fermentation pathways, and thus cell proliferation (43). Inulin feeding may stimulate the growth of bifidobacteria at the expense of Clostridia (44–46), and there is some evidence that fructooligosaccharides may diminish toxicity from overproduction of butyrate by reducing colonization with some Clostridial species (42,46–49). However, Clostridial species can ferment fructooligosaccharides (50), and one human study suggested that inulin did not change Clostridial colonization (41). In contrast, we suggest that when fed with lactulose, inulin may stimulate the growth of bifidobacteria, at the expense of Clostridia, but also lower cecal pH with the net result of lowered butyrate production and increased cell proliferation (13,39,42,43,45,47–49,51).
In summary, feeding an indigestible, fructose-galactose disaccharide, lactulose, appeared to cause increased cecal luminal synthesis of butyrate and decreased cecal cell proliferation compared with normally fed piglets. Pre- and cofeeding of a fructooligosaccharide, inulin, seemed to prevent both the increase in butyrate production and the reduction in cell proliferation. These data provide the first evidence that when butyrate production is abnormally increased by feeding a fermentable carbohydrate, cell proliferation is decreased; thus, the apparent effects of butyrate synthesized by colonic bacteria seem to mirror those previously described in cultured colonic adenoma or carcinoma cells. We did not detect a significant increase in butyrate production in piglets fed the control formula plus inulin, and this group also manifested decreased cecal proliferation. Clearly, there are unknown factors determining how cell proliferation in the colon is affected by carbohydrate fermentation. However, the present data can be used to construct new studies aimed at applying ranges in butyrate entry rate into the intact colon, which have been actually observed in response to carbohydrate malabsorption, to determine further the mechanisms by which butyrate may alter colonic nutritional status.
Acknowledgments
We are grateful to John R. Salsbury for technical assistance, to Drs. Barbara Stoll and Doug Burrin at Baylor College of Medicine for consultation with surgical techniques, to Joanne Lupton and her laboratory at Texas A & M for advice on assays for short-chain fatty acid concentration, and to Jann Bunn, Ph.D., University of Vermont, for statistical assistance.
Footnotes
Presented in part at Experimental Biology 04, April 2004, Washington, DC [Kien, CL. The rate of production of butyric acid (BA) (Ra BA) in the colon: effects of lactulose (LACTUL) or inulin (abstract). FASEB J. 2004;18:A475]; at the Pediatric Academic Societies’ Annual Meeting, May 2004, San Francisco, CA [Kien CL. Inulin (IN) feeding decreases diarrhea associated with disaccharide malabsorption possibly via attenuation of accelerated production of butyric acid (BA) (abstract). Pediatr Res. 2004;55:187A]; at the Digestive Disease Week 2004, May 2004, New Orleans, LA [Kien CL, Frankel, WL. In vivo colonic synthesis of butyric acid (BA) and colonic mucosal cell proliferation (CP) in pigs fed lactulose (LACTUL) and/or inulin (IN) (abstract). Gastroenterology 2004;126:A-400]; and at Experimental Biology 05, April 2005, San Diego, CA [Kien CL, Frankel WL. Lactulose (LAC) and inulin (IN) fermentation: inter-relationships of the colonic concentration (CON) and rate of butyric acid (BA) synthesis (RA BA), cell proliferation (CP) and apoptosis (APOP) (abstract). FASEB J. 2005;19(5):A1696].
Supported by National Institutes of Health grant R01 DK061775, Ross Products Division of Abbott Laboratories, Columbus, OH (provided the piglet formula), and by Grant 8450, Shriners of North America, which supported the Large Animal Intensive Care Unit, UTMB.
Abbreviations used: BA, butyric acid; C, Control formula; I, formula supplemented with inulin; IE BA, plateau isotopic enrichment of butyric acid in the portal vein; L, formula supplemented with lactulose; LC BA, concentration of BA in the left colon; LC BA Flow, rate of flow (entry) of BA in the left colon; L + I, formula supplemented with lactulose and inulin; PEG, polyethylene glycol; PEG formula, concentration of PEG in the formula; PEG LC, concentration of PEG in the left colon; φh value, labeled cells in the upper 40% of the crypt divided by total labeled cells in the crypt; PI, fractional cell proliferative index, stained cells/total cells; Ra BA, rate of production of butyric acid.
References
- 1.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–79. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassig CA, Tong JK, Schreiber SL. Fiber-derived butyrate and the prevention of colon cancer. Chem Biol. 1997;4:783–9. doi: 10.1016/s1074-5521(97)90111-3. [DOI] [PubMed] [Google Scholar]
- 3.Singh B, Halestrap A, Paraskeva C. Butyrate can act as a stimulator of growth or inducer of apoptosis in human colonic epithelial cell lines depending on the presence of alternative energy sources. Carcinogenesis. 1997;18:1265–70. doi: 10.1093/carcin/18.6.1265. [DOI] [PubMed] [Google Scholar]
- 4.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–93. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 5.Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982;42:65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- 6.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012–7. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 7.Sakata T, von Engelhardt W. Stimulatory effect of short chain fatty acids on epithelial cell proliferation in rat large intestine. Comp Biochem Physiol A. 1983;74:459–62. doi: 10.1016/0300-9629(83)90631-x. [DOI] [PubMed] [Google Scholar]
- 8.Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: a possible explanation for trophic effects of fermentable fibre, gut microbes and luminal tropic factors. Br J Nutr. 1987;58:95–103. doi: 10.1079/bjn19870073. [DOI] [PubMed] [Google Scholar]
- 9.Frankel WL, Zhang W, Singh A, Klurfield DM, Don S, Sakata T, Modlin I, Rombeau JL. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106:375–80. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez OC, Zhou D, Seto RW, Jabbar A, Choi J, Lederer HM, Rombeau JL. In vivo crypt surface hyperproliferation is decreased by butyrate and increased by deoxycholate in normal rat colon: associated in vivo effects on c-Fos and c-Jun expression. JPEN J Parenter Enteral Nutr. 1996;20:243–50. doi: 10.1177/0148607196020004243. [DOI] [PubMed] [Google Scholar]
- 11.Bartram HP, Scheppach W, Schmid H, Hofmann A, Dusel G, Richter F, Richter A, Kasper H. Proliferation of human colonic mucosa as an intermediate biomarker of carcinogenesis: effects of butyrate, deoxycholate, calcium, ammonia, and pH. Cancer Res. 1993;53:3283–8. [PubMed] [Google Scholar]
- 12.Hague A, Singh B, Paraskeva C. Butyrate acts as a survival factor for colonic epithelial cells: further fuel for the in vivo versus in vitro debate. Gas troenterology. 1997;112:1036–40. doi: 10.1053/gast.1997.v112.agast971036. [DOI] [PubMed] [Google Scholar]
- 13.Butel MJ, Roland N, Hibert A, Popot F, Favre A, Tessedre AC, Bensaada M, Rimbault A, Szylit O. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. 1998;47:391–9. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 14.Argenzio RA, Meuten DJ. Short-chain fatty acids induce reversible injury of porcine colon. Dig Dis Sci. 1991;36:1459–68. doi: 10.1007/BF01296816. [DOI] [PubMed] [Google Scholar]
- 15.Sakata T, Tamate H. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J Dairy Sci. 1978;61:1109–13. doi: 10.3168/jds.S0022-0302(78)83694-7. [DOI] [PubMed] [Google Scholar]
- 16.Kien CL, Murray RD, Qualman SJ, Marcon M. Lactulose feeding in piglets: a model for persistent diarrhea and colitis induced by severe sugar malabsorption. Dig Dis Sci. 1999;44:1476–84. doi: 10.1023/a:1026672306929. [DOI] [PubMed] [Google Scholar]
- 17.Kien CL, Chang JC, Cooper JR, Frankel WL. Effects of prefeeding a prebiotic on diarrhea and colonic cell proliferation in piglets fed lactulose. JPEN J Parenter Enteral Nutr. 2004;28:22–6. doi: 10.1177/014860710402800122. [DOI] [PubMed] [Google Scholar]
- 18.Kien CL, Cooper JR, Frankel WL. Moderate disaccharide malabsorption does not affect weight gain and cecal cell proliferation in piglets. JPEN J Parenter Enteral Nutr. 2003;27:323–6. doi: 10.1177/0148607103027005323. [DOI] [PubMed] [Google Scholar]
- 19.Kien CL, Ailabouni AH, Murray RD, Powers PA, McClead RE, Kepner J. Technical note: pig model for studying nutrient assimilation by the intestine and colon. J Anim Sci. 1997;75:2161–4. doi: 10.2527/1997.7582161x. [DOI] [PubMed] [Google Scholar]
- 20.Kien CL, Chang JC, Cooper JR. Quantitation of colonic luminal synthesis of butyric acid in piglets. J Pediatr Gastroenterol Nutr. 2002;35:324–8. doi: 10.1097/00005176-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Ebner S, Schoknecht P, Reeds P, Burrin D. Growth and metabolism of gastrointestinal and skeletal muscle tissues in protein-malnourished neonatal pigs. Am J Physiol. 1994;266:R1736–43. doi: 10.1152/ajpregu.1994.266.6.R1736. [DOI] [PubMed] [Google Scholar]
- 22.Reeds PJ, Burrin DG, Jahoor F, Wykes L, Henry J, Frazer EM. Enteral glutamate is almost completely metabolized in first pass by the gastrointestinal tract of infant pigs. Am J Physiol. 1996;270:E413–8. doi: 10.1152/ajpendo.1996.270.3.E413. [DOI] [PubMed] [Google Scholar]
- 23.Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, Reeds PJ. Substrate oxidation by the portal drained viscera of fed piglets. Am J Physiol. 1999;277:E168–75. doi: 10.1152/ajpendo.1999.277.1.E168. [DOI] [PubMed] [Google Scholar]
- 24.Flourie B, Briet F, Florent C, Pellier P, Maurel M, Rambaud JC. Can diarrhea induced by lactulose be reduced by prolonged ingestion of lactulose? Am J Clin Nutr. 1993;58:369–75. doi: 10.1093/ajcn/58.3.369. [DOI] [PubMed] [Google Scholar]
- 25.Kien CL, Chang JC, Cooper JR. Butyric acid is synthesized by piglets. J Nutr. 2000;130:234–7. doi: 10.1093/jn/130.2.234. [DOI] [PubMed] [Google Scholar]
- 26.Powers L, Osborne MK, Yang D, Kien CL, Murray RD, Beylot M, Brunengraber H. Assay of the concentration and stable isotope enrichment of short-chain fatty acids by gas chromatography/mass spectrometry. J Mass Spectrom. 1995;30:747–54. [Google Scholar]
- 27.Deschner EE, Ruperto JF, Lupton JR, Newmark HL. Dietary butyrate (tributyrin) does not enhance AOM-induced colon tumorigenesis. Cancer Lett. 1990;52:79–82. doi: 10.1016/0304-3835(90)90080-h. [DOI] [PubMed] [Google Scholar]
- 28.Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–12. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 29.McKay BC, Ljungman M, Rainbow AJ. Persistent DNA damage induced by ultraviolet light inhibits p21waf1 and bax expression: implications for DNA repair, UV sensitivity and the induction of apoptosis. Oncogene. 1998;17:545–55. doi: 10.1038/sj.onc.1201963. [DOI] [PubMed] [Google Scholar]
- 30.Meeroff JC, Go VL, Phillips SF. Gastric emptying of liquids in man. Quantification by duodenal recovery marker. Mayo Clin Proc. 1973;48:728–32. [PubMed] [Google Scholar]
- 31.Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313:1381–4. doi: 10.1056/NEJM198511283132203. [DOI] [PubMed] [Google Scholar]
- 32.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–7. [PubMed] [Google Scholar]
- 33.Moreau NM, Champ MM, Goupry SM, Le Bizec BJ, Krempf M. Nguyen PG, Dumon HJ, Martin LJ. Resistant starch modulates in vivo colonic butyrate uptake and its oxidation in rats with dextran sulfate sodium-induced colitis. J Nutr. 2004;134:493–500. doi: 10.1093/jn/134.3.493. [DOI] [PubMed] [Google Scholar]
- 34.Thakkar K, Kien CL, Rosenblatt JI, Herndon DN. Diarrhea in severely burned children. JPEN J Parenter Enteral Nutr. 2005;29:8–11. doi: 10.1177/014860710502900108. [DOI] [PubMed] [Google Scholar]
- 35.Zijlstra RT, Whang KY, Easter RA, Odle J. Effect of feeding a milk replacer to early-weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J Anim Sci. 1996;74:2948–59. doi: 10.2527/1996.74122948x. [DOI] [PubMed] [Google Scholar]
- 36.Lupton JR. Butyrate and colonic cytokinetics: differences between in vitro and in vivo studies. Eur J Cancer Prev. 1995;4:373–8. doi: 10.1097/00008469-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Lupton JR, Coder DM, Jacobs LR. Influence of luminal pH on rat large bowel epithelial cell cycle. Am J Physiol. 1985;249:G382–8. doi: 10.1152/ajpgi.1985.249.3.G382. [DOI] [PubMed] [Google Scholar]
- 38.Nordgaard I, Hansen BS, Mortensen PB. Importance of colonic support for energy absorption as small-bowel failure proceeds. Am J Clin Nutr. 1996;64:222–31. doi: 10.1093/ajcn/64.2.222. [DOI] [PubMed] [Google Scholar]
- 39.Howard MD, Gordon DT, Pace LW, Garleg KA, Kerley MS. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr. 1995;21:297–303. doi: 10.1097/00005176-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Pajari AM, Rajakangas J, Paivarinta E, Kosma VM, Rafter J, Mutanen M. Promotion of intestinal tumor formation by inulin is associated with an accumulation of cytosolic beta-catenin in Min mice. Int J Cancer. 2003;106:653–60. doi: 10.1002/ijc.11270. [DOI] [PubMed] [Google Scholar]
- 41.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–6. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butel MJ, Waligora-Dupriet AJ, Szylit O. Oligofructose and experimental model of neonatal necrotising enterocolitis. Br J Nutr. 2002;87(suppl 2):S213–9. doi: 10.1079/BJNBJN/2002540. [DOI] [PubMed] [Google Scholar]
- 43.Florent C, Flourie B, Leblond A, Rautureau M, Bernier JJ, Rambaud JC. Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study) J Clin Invest. 1985;75:608–13. doi: 10.1172/JCI111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christl SU, Murgatroyd PR, Gibson GR, Cummings JH. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–77. [PubMed] [Google Scholar]
- 45.Wang X, Gibson GR. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Bacteriol. 1993;75:373–80. doi: 10.1111/j.1365-2672.1993.tb02790.x. [DOI] [PubMed] [Google Scholar]
- 46.Roberfroid M. Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit Rev Food Sci Nutr. 1993;33:103–48. doi: 10.1080/10408399309527616. [DOI] [PubMed] [Google Scholar]
- 47.Danan C, Huret Y, Tessedre AC, Bensaada M, Szylit O, Butel MJ. Could oligosaccharide supplementation promote gut colonization with a beneficial flora in preterm infants? J Pediatr Gastroenterol Nutr. 2000;30:217–9. doi: 10.1097/00005176-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Kiyosawa I, Takase M, Yamauchi K, Ono J, Yaeshima T, Okonogi S. Lactulose and intestinal microflora in infant nutrition. Bifidobacteria Microflora. 1986;5:27–35. [Google Scholar]
- 49.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 50.Roberfroid MB, Van Loo JA, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128:11–9. doi: 10.1093/jn/128.1.11. [DOI] [PubMed] [Google Scholar]
- 51.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–82. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]


